Update in lean metabolic dysfunction-associated steatotic liver disease
Karina Sato-Espinoza, Perapa Chotiprasidhi, Mariella R Huaman, Javier Díaz-Ferrer
Abstract BACKGROUND A new nomenclature consensus has emerged for liver diseases that were previously known as non-alcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated fatty liver disease (MAFLD). They are now defined as metabolic dysfunction-associated steatotic liver disease (MASLD), which includes cardiometabolic criteria in adults. This condition, extensively studied in obese or overweight patients, constitutes around 30% of the population, with a steady increase worldwide. Lean patients account for approximately 10%-15% of the MASLD population. However, the pathogenesis is complex and is not well understood.AIM To systematically review the literature on the diagnosis, pathogenesis, characteristics, and prognosis in lean MASLD patients and provide an interpretation of these new criteria.METHODS We conducted a comprehensive database search on PubMed and Google Scholar between January 2012 and September 2023, specifically focusing on lean NAFLD, MAFLD, or MASLD patients. We include original articles with patients aged 18 years or older, with a lean body mass index categorized according to the World Health Organization criteria, using a cutoff of 25 kg/m2 for the general population and 23 kg/m2 for the Asian population.RESULTS We include 85 studies in our analysis. Our findings revealed that, for lean NAFLD patients, the prevalence rate varied widely, ranging from 3.8% to 34.1%. The precise pathogenesis mechanism remained elusive, with associations found in genetic variants, epigenetic modifications, and adaptative metabolic response. Common risk factors included metabolic syndrome, hypertension, and type 2 diabetes mellitus, but their prevalence varied based on the comparison group involving lean patients. Regarding non-invasive tools, Fibrosis-4 index outperformed the NAFLD fibrosis score in lean patients. Lifestyle modifications aided in reducing hepatic steatosis and improving cardiometabolic profiles, with some medications showing efficacy to a lesser extent. However, lean NAFLD patients exhibited a worse prognosis compared to the obese or overweight counterpart.CONCLUSION MASLD is a complex disease comprising epigenetic, genetic, and metabolic factors in its pathogenesis. Results vary across populations, gender, and age. Limited data exists on clinical practice guidelines for lean patients. Future studies employing this new nomenclature can contribute to standardizing and generalizing results among lean patients with steatotic liver disease.
Key Words: Lean; Non-obese; Non-alcoholic fatty liver disease; Metabolic dysfunction-associated fatty liver disease; Metabolic dysfunction-associated steatotic liver disease; Guidelines; Diagnosis; Management; Pathogenesis; Treatment
INTRODUCTION
In 1980, Ludwig, Viggiano, McGill, and Oh introduced the term non-alcoholic fatty liver disease (NAFLD), defining the disease as the presence of hepatic fat in the absence of significant alcohol intake. It was characterized as hepatic steatosis observed through imaging or histology, excluding other causes of chronic liver disease and steatosis, such as substantial alcohol consumption, prolonged use of steatogenic medication, or hereditary monogenic disorders[1]. By utilizing this exclusionary criterion, the differential diagnosis of NAFLD was formed. In 2020, the concept of metabolic dysfunctionassociated fatty liver disease (MAFLD) emerged, encompassing individuals previously excluded due to alcohol consumption or other liver diseases[2]. This represented a shift towards a "positive" diagnosis, moving away from an exclusory approach. However, even with this new terminology, patient stigmatization persisted due to the continued use of the term "fatty." Consequently, a collaborative effort involving the following groups: American Association for the Study of Liver Disease, European Association for the Study of the Liver, and Latin American Association for the Study of the Liver, utilizing the Delphi method, led to the development of a novel nomenclature metabolic dysfunction-associated steatotic liver disease (MASLD)[3]. The recent consensus reclassified NAFLD and MAFLD[4,5] to MASLD[3]. To meet the new MASLD criteria, individuals must exhibit at least 1 of 5 cardiometabolic risk factors linked to insulin resistance (IR). MASLD constitutes approximately 30% of the global population, and its prevalence is steadily increasing worldwide[6]. Despite this condition being extensively researched in overweight and obese individuals, 10%-15% of MASLD patients will exhibit normal weight and are classified as either lean or non-obese[7]. The categorization depends on ethnicity; the World Health Organization (WHO) categorizes a normal body mass index (BMI) for the general population with a cutoff of 25 kg/m2and 23 kg/m2for the Asian population[8]. Most studies have predominantly focused on BMI when investigating patients with lean MASLD. However, BMI has been proven to be an imperfect marker of adiposity[9-13]. Vilarinhoet al[14] have proposed a classification system for patients with lean MASLD, distinguishing two phenotypes based on epidemiological characteristics, natural history, and prognosis. Type 1 includes individuals with visceral adiposity and insulin resistance. While type 2 comprises of those with hepatic steatosis resulting from monogenic diseases, this requires a nuanced understanding of the pathophysiology.
The pathophysiology of MASLD is intricate and diverse. The clinical spectrum of this disease ranges from simple steatosis to cirrhosis and is influenced by diverse factors, including the overconsumption of carbohydrates and dietary sugars such as fructose, sucrose, and glucose[15]. Dysbiosis, bacterial translocation, and pro-inflammatory factors in the liver also contribute to its complexity[16]. It is proposed that the disease phenotype arises from intricate interactions between genetic and environmental factors[17]. Despite the various potential mechanism proposed, the literature supports that IR and lipotoxicity play a key role in the pathogenesis[18]. This interplay results in a chronic elevation of plasma levels of non-esterified fatty acids, which are ectopically deposited in the liver, promoting the development of steatosis. Additionally, triglycerides (TG) within hepatocytes further increase the accumulation of toxic lipids, such as ceramides and diacylglycerols, intensifying IR and activating inflammatory pathways. Furthermore, it has been reported that lean MASLD patients experience increased concentrations of serum bile acids and elevated farnesoid X receptor (FXR) activity as an initial metabolic response[16-19].
Genes have been identified as modulators of insulin sensitivity and regulators of the intracellular flow of fatty acids, TG, oxidative stress, endotoxin response, cytokine activity, and the development of fibrosis[18]. The most studied single nucleotide polymorphisms (SNPs) associated with steatosis across diverse ethnicities are rs58542926 in theTM6SF2gene (transmembrane 6 superfamily member 2)[20] and rs738409 in thePNPLA3gene (patatin-like phospholipase domaincontaining protein 3)[21]. The I148M polymorphism ofPNPLA3disrupts triglyceride lipolysis in lipid droplets[22]. Polymorphism inTM6SF2plays a pivotal role in hepatic and cholesterol metabolism[20]. Additionally,MBOAT7influences phospholipid metabolism[23].
Regarding the diagnosis of steatotic liver disease in lean patients, it is typically conducted through[18,24] imaging modalities such as abdominal ultrasound (US)[25,26], computed tomography (CT)[27,28], or magnetic resonance imaging (MRI)[29]. Additionally, FibroScan, assessing the controlled attenuation parameter (CAP)[30-32] and liver stiffness measurement (LSM)[31,33], is employed. However, liver biopsy is usually reserved for patients with an unclear diagnosis. Conversely, non-invasive scores are also utilized for diagnosis, which will be discussed later in this review.
The development of the new MASLD nomenclature consensus has been proven helpful for accurately classifying patients with liver steatosis, allowing individuals previously classified as "lean NAFLD" to be categorized as lean MASLD, facilitating uniform studies in the future, particularly for those presenting with cardiometabolic risk[34,35]. These new approaches broaden the focus regarding the metabolic pathogenesis of the disease. However, individuals not meeting these criteria and have no known cause of liver disease have been classified as having cryptogenic steatotic liver disease[3]. This distinction is significant because some patients previously labeled as NAFLD are now reclassified as cryptogenic steatotic liver disease. Discussing this reclassification is important because this new approach does not imply that other causes of steatosis should not be considered, and it also allows for a more in-depth characterization of fibrosis severity using a non-invasive test. Due to the homogenization of the concept of steatotic liver disease, this has been a significant step forward in understanding and addressing this complex disease. As establishing a consensus on how to categorize these patients is essential for future studies, ensuring that results are comparable across different research endeavors.
Considering the significant implication of this complex disease, we intended to conduct a systematic review of the literature pertaining to the diagnosis, pathogenesis, characteristics, and complications associated with lean MASLD patients. Additionally, our goal is to provide an interpretation of this new criteria.
MATERlALS AND METHODS
We conducted a database search on PubMed and Google Scholar, selecting papers published between January 2012 and September 2023 in the English language. The last access to PubMed and Google Scholar occurred on 25 September 2023. The keywords and terms utilized in our search were as follows: (1) NAFLD or non-alcoholic liver disease; (2) MASLD or metabolic dysfunction association steatotic liver disease; (3) guidelines; (4) management; (5) characteristics; and (6) lean. The specific search terms included "non-alcoholic fatty liver disease"[MeSH Terms] OR nafld [All Fields], "guideline"[Publication Type] OR "guidelines as topic"[MeSH Terms] OR "guidelines" [All Fields], "diagnosis"[Subheading] OR "diagnosis"[MeSH Terms] OR diagnosis [All Field], "organization and administration"[MeSH Terms] OR "disease management"[MeSH Terms] OR management[All Field], "therapy"[Subheading] OR "therapeutics"[MeSH Terms] OR treatment [All Field], characteristic[All Fields], and lean[All Fields].
We included original articles that featured patients aged 18 years or older, with BMI categorized by the WHO for both the general and Asian populations. In the general population, BMI was described as normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (> 30 kg/m2). In the Asian population, BMI was described as normal (18.5-22.9 kg/m2), overweight (23-24.9 kg/m2), and obese (> 25 kg/m2). In this review, normal BMI is referred to as lean, non-obese, or normal weight. We included studies that diagnose steatosis liver disease using abdominal US, abdominal CT, or MRI, in conjunction with FibroScan, which incorporates CAP and/or LSM, as well as histological diagnosisviabiopsy. Diagnosis may also involve clinically identifying steatosis liver disease based on elevated liver enzymes, while ruling out other liver diseases.
We excluded systematic reviews, review articles, case reports, poster presentations, conference abstracts, editorials, letters to the editor, studies involving patients under 18 years old, studies which utilizes animals, and studies categorizing BMI differently than the WHO. After removing duplicates and applying our inclusion and exclusion criteria, a total of 85 papers were identified. Refer to Figure 1 for more details.
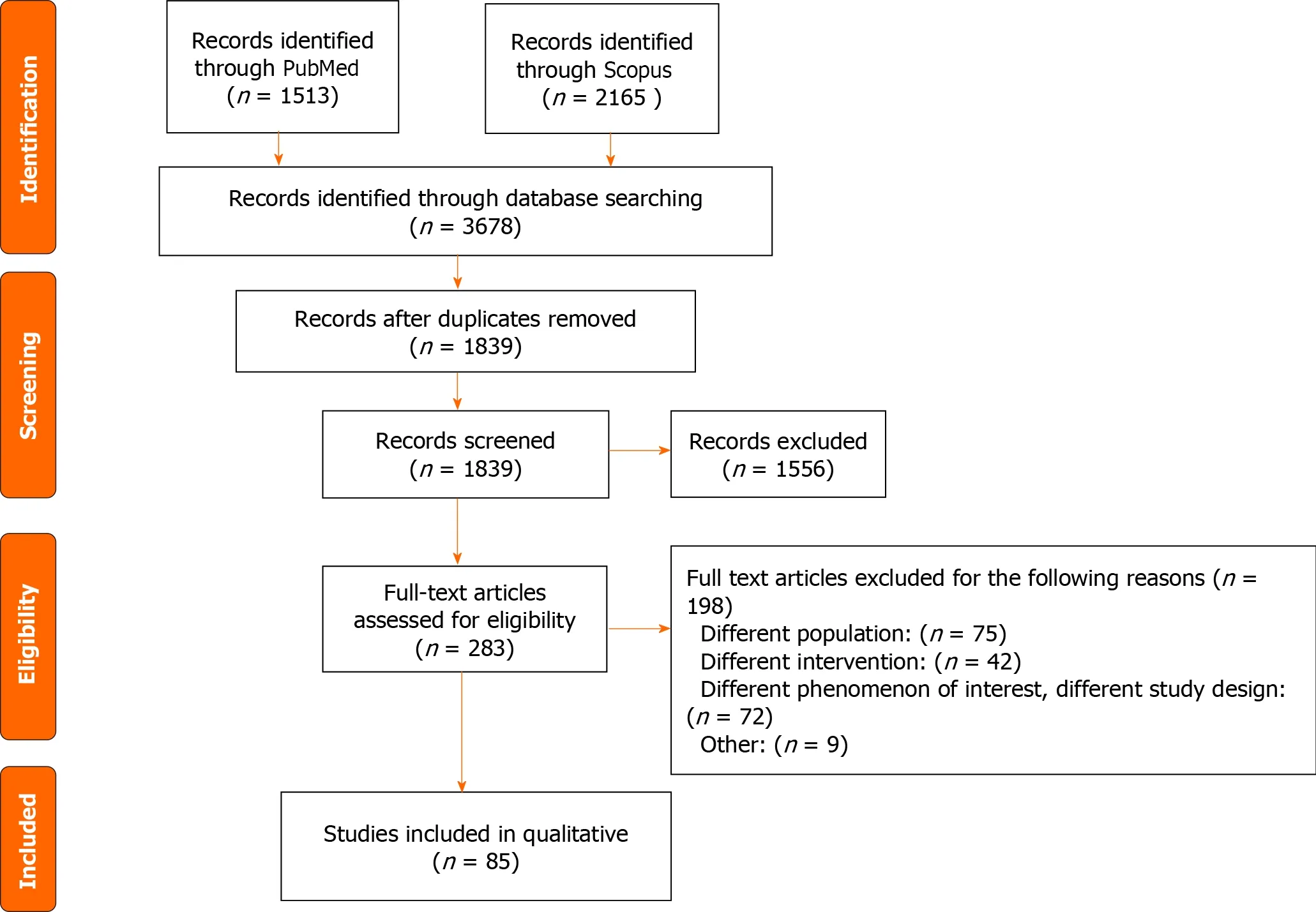
Figure 1 Flow chart of the systematic review. PUBMED: Publication from MEDLINE; Scopus: Society for cutting up of old publications.
RESULTS
Current guidelines
Only one expert review on clinical practice updates for lean MASLD patients was found in the literature[24]. The review offered practical advice for physicians. The evaluation of MASLD patients should include routine assessments for hypertension (HTN), type 2 diabetes mellitus (T2DM), dyslipidemia, and a comprehensive alcohol consumption history. Regarding screening lean patients, only patients older than 40 years old with T2DM require recommended evaluation. It is essential to investigate and rule out alternative causes of liver steatosis, starting with non-invasive methods such as serum scores or imaging; liver biopsy should be reserved for undetermined diagnosis. NAFLD fibrosis score (NFS) and fibrosis-4 score (FIB-4) were the two non-invasive scores recommended. The recommended imaging modalities were transient elastography (FibroScan) and magnetic resonance elastography. While no specific treatment exists for lean patients, it is recommended that lifestyle modifications advocating a modest weight loss of 3%-5% (less than in overweight or obese patients) be pursued. Surveillance for liver cancer is crucial, and it involves employing abdominal ultrasound, with or without alpha-fetoprotein, in patients with cirrhosis.
DlSCUSSlON
Pathogenesis
Genetic variants and epigenetic modifications have been correlated in lean NAFLD patients. However, the precise mechanisms have yet to be fully elucidated, and in some cases, have produced contradictory results. Zenget al[36] described that in the Chinese population, there was no significant difference in SNPs in theSIRT1,APOC3,PNPLA3,AGTR1, andPPARGC1Agenes between lean patients with and without NAFLD. They concluded that metabolic factors played a vital role in the occurrence and progression of NAFLD rather than genetic factors.
On the other hand, Weiet al[37] found that a SNP inPNPLA3(rs738409) had a higher prevalence in non-obese patients compared to obese patients with NAFLD. Carrying the GG allele inPNPLA3(rs738409) increases the risk of NAFLD in the general population, especially in patients without metabolic syndrome (MetS). This SNP appeared to be independent of dietary factors or metabolic conditions[38]. Despite these contradictory results, the GG variant of patatin-like phospholipase domain 3 (PNPLA3), encodes adiponutrin and plays a crucial role in lipid metabolism. It has been identified as an independent variable, and it has been associated with a higher risk of NAFLD and significant fibrosis in lean patients[37-39].
Alharthiet al[16] described an alteration in adaptive metabolic response characterized by elevated concentrations of serum bile acids and increased activity of the FXR in lean NAFLD patients. Models of metabolic maladaptation loss have been proposed for these patients[16,19]. The Western diet may alter intestinal permeability, increase exposure to bacterial products, and lipopolysaccharides. In lean patients with NAFLD, this could lead to higher endotoxemia, increased expression of macrophage TLR4, and higher production of inflammatory cytokines compared to healthy thin individuals.
Characteristic
The prevalence of lean NAFLD exhibits a wide range, varying from 3.8% to 34.1%[7,40-56]. Refer to Table 1, for more details.

Table 1 Characteristics of lean non-alcoholic fatty liver disease patients
Many studies have indicated that lean NAFLD occurs to people that are older than 40 years old[40,41,46,47,53,55-57]. However, conflicting findings exist, with some studies suggesting that patients are younger than 40 years old[7,42,58,59]. While other studies report patients being older than 60 years old[45,60]. One study demonstrated, by stratifying the prevalence of lean NAFLD by age and sex, that males under 50 years old have an increased likelihood of developing the lean NAFLD phenotype; however, beyond 50 years old, no significant differences between the sexes were observed[37].
When examining the sexes separately, some studies reported a high prevalence of lean NAFLD in males[40,41,45,46,59,61], while others indicated a higher prevalence in females[7,50,58,62]. Nevertheless, there are studies reporting no significant differences in prevalence between females and males[42,51,52,57,60,63].
These variations highlight the heterogeneity of lean NAFLD prevalence in different cohorts and across distinct populations.
Risk factors
Studies have compared lean patients with and without NAFLD. These studies have demonstrated that lean NAFLD patients are at a higher risk of atherogenic dyslipidemia[40,64], MetS, T2DM[41,46], dyslipidemia, and cardiovascular complications[46]. Additionally, these patients manifest elevated cardiovascular and all-cause mortality rates[65]. When laboratory values were compared, this revealed elevated levels of TG, total cholesterol, and fasting blood glucose (FBG) for patients with lean NAFLD[41]. Regarding anthropometric measurements, the studies showed higher waist circumference (WC)[40,41,44,46] and BMI[41] in lean NAFLD patients compared to those without NAFLD.
When comparing lean patients with NAFLD and overweight/obese patients with NAFLD, studies reported that lean NAFLD patients have a lower prevalence of T2DM[7,37,50,58,60-62,66], dyslipidemia[7,50,58,60] , HTN[7,49,50,52,56-58,60,63,66,67], MetS[49,52,62,66], cardiovascular disease[60], and cirrhosis[60,62]. Laboratory values were compared, indicating lower levels of aspartate aminotransferase (AST)[7,53,57,59,62,63,67], alanine aminotransferase (ALT)[7,53,57,59,62,63,67], platelet count[7,66], FBG[53,58,63], TG[53,57,58,61,62], homeostatic model assessment for insulin resistance (HOMA-IR)[57,63,68], and total cholesterol[57,58,61-63], as well as higher levels of high density lipoproteins (HDL)[56,61-63,66,69]. Regarding anthropometric measurements, the studies reported lower WC[52,56,63,66], BMI[63,70], and waistto-hip ratio (WHR)[63,70] in lean NAFLD compared to overweight/obese counterparts.
In studies where BMI was compared, lean NAFLD patients exhibited a lower prevalence of comorbidities and a more favorable laboratory profile when compared to overweight or obese patients with NAFLD. Conversely, in studies comparing individuals with and without NAFLD, lean NAFLD patients displayed a worse profile with the highest rates of comorbidities and adverse laboratory values compared to healthy lean individuals without NAFLD. This consideration holds significant importance in the interpretation and application of risk factor concepts in clinical practice. These heterogeneous results underscore the need for regular monitoring in patients who are lean and have NAFLD, given the elevated risk of metabolic diseases compared to those who are lean and do not have NAFLD.
Histological characteristics and diagnosis scores
Patients with NAFLD are at risk of progressing to non-alcoholic steatohepatitis (NASH) and developing other complications[71]. We will now present literature that has evaluated and characterized NASH patients, refer to Table 2 for more details. The most used score in studies diagnosing NASH in patients is the NAFLD Activity Score (NAS), which has been proposed and validated by the NASH Clinical Research Network[72]. This score assesses three characteristics in liver histology: Steatosis Grade, Lobular Inflammation, and Hepatocellular Ballooning. The score ranges from 0 to 8, with a score < 3 correlating with not-NASH, and a score > 5 correlating with a diagnosis of NASH.
Leunget al[73] reported that non-obese patients with NASH exhibited lower NAS due to reduced steatosis and hepatocyte ballooning, along with lower liver stiffness. Furthermore, Iwakiet al[74] observed a low grade of lobular inflammation and fibrosis stage, with no significant differences in steatosis, ballooning, and overall NAS in non-obese compared to obese patients. Additionally, Kimet al[75] found that lean patients displayed a low grade of steatosis and overall NAS, but a higher stage of fibrosis compared to their obese counterparts with NAFLD.
On the contrary, Denkmayret al[76] identified a higher proportion of lobular inflammation and hepatocellular ballooning, with a notable prevalence of cirrhosis in lean patients. However, the degree of steatosis was similar across the groups. Also, Rastogiet al[77] found a high proportion of hepatocyte ballooning but a high prevalence in none/earlystage fibrosis.
The results of histology in different studies are inconclusive. They indicate that histological characteristics could vary, showing either worse or better outcomes in leanvsoverweight or obese individuals. However, this emphasizes the importance of careful evaluation for lean patients, similar to the rest of the population. These contradictory results may be influenced by the different types of patients undergoing liver biopsy. Leung, Kim, and Denkymar assessed histology in the following types of patients: those exhibiting abnormal liver enzyme levels, those with suspected NAFLD, and those with a confirmed diagnosis of NAFLD through non-invasive tools. In contrast, Iwaki examined the histology in a tertiary center where referrals were received, particularly for patients with more severe liver conditions. Moreover, the differences in study designs, including prospective, retrospective, and cross-sectional approaches, complicate the comparison of results. A limitation noted across all the studies was the relatively small sample size in the lean group compared to the overweight/obese groups.
In the context of interpreting non-invasive tools in lean patients with NAFLD or NASH, a critical consideration is the selection of the most suitable scoring system or algorithm for clinical application. We will now present literature that has evaluated accuracy of those scores, refer to Table 2 for more details.
The accuracy of FIB-4 and NFS was compared in patients who underwent liver biopsy[78]. FIB-4 assessed age, levels of AST, ALT, and platelets, while NFS considered age, BMI, impaired fasting glucose or diabetes, levels of AST, ALT, platelets, and albumin. In a study by Erenet al[79], it was observed that both FIB-4 and NFS were ineffective in discriminating against advanced fibrosis in both lean and morbidly obese patients. Contrastingly, a study by Parket al[80] revealed that the diagnostic performance of FIB-4 and NFS in identifying advanced hepatic fibrosis was comparable, irrespective of BMI. The sensitivity of NFS in lean patients was inferior to that of FIB-4. In addition to comparing FIB-4 and NFS, Fuet al[81] included AST-to-platelet, BARD score, and the AST-to-ALT ratio in the comparison. They found that all non-invasive scores performed equally for both obese and non-obese patients. The negative predictive value (NPV) was higher in non-obese patients due to the lower prevalence of advanced fibrosis. Moreover, Liet al[82] compared 8 NAFLD-related algorithms, finding that WHR and Fatty Liver Index exhibited diagnostic accuracy for NAFLD in both lean and overweight/obese populations, but Zhejiang University Index and Hepatic Steatosis Index demonstrated exclusively positive associations in lean patients.
In summary, the review of accuracy and performance across different non-invasive tools in patients with NAFLD revealed that FIB-4 outperformed NFS in this specific population. However, it is crucial to note that this result was observed in only one study. Nonetheless, this finding does hold significance, considering that the only clinical guideline for lean MASLD recommends FIB4 and NFS equally. Thus, it is imperative that new studies compare these non-invasive tools in patients with MASLD due to the updated guidelines.
Treatment
Clinical trials were conducted to explore potential treatments for NAFLD. In the literature reviewed, we found two types of treatment: pharmacological and non-pharmacological.
Pharmacological:In a one-year follow-up study involving 8 lean patients with NAFLD, half received ursodeoxycholic acid, and the other half received 10 mg of the Niemann-Pick C1 Like 1 (NPC1L1) inhibitor, ezetimibe. The findings revealed that patients treated with ezetimibe for 12 months experienced decreased levels of AST and low-density lipoprotein, but no significant changes were observed in HDL, TG, HOMA-IR, or liver fat attenuation in abdominal US[83]. In another study involving 50 patients, 25 received a synbiotic capsule, and 25 received a placebo capsule. Both groups received advice on maintaining a balanced diet and engaging in physical activity. After 28 wk of treatment and follow-up, both groups exhibited reduced hepatic steatosis and inflammatory markers, with the synbiotic group having a higher mean reduction in FBS, TG, and AST[84].
Pemafibrate, a selective peroxisome proliferator-activated receptor-αmodulator, dosed at 0.1 mg twice daily was studied. The first study by Shinozakiet al[85] treated 71 patients for 6 months, finding that lean patients experienced a greater reduction in ALT and serum mac-2 binding protein glycosylation isomer than obese patients. The second study by Suzukiet al[86] treated 38 patients for 12 months and found a strong association in the decrease of ALT, AST, hepatic steatosis, and fibrosis in both lean and obese patients. Canagliflozin at a dosage of 100 mg once daily was evaluated in 20 patients with T2DM and NAFLD, but due to only one patient being lean, the results were inconclusive in this population[87].
Various pharmacological treatments and interventions have been investigated in patients with lean MASLD, demonstrating some degree of efficacy in improving the metabolic profile or reducing hepatic steatosis. However, longitudinal clinical trials with large study populations are still warranted to identify a promising drug for treating both lean MASLD and MASH. On the other hand, the literature supports that lifestyle modification is an effective therapy in lean patients with MASLD, similarly to overweight/obese patients.
Non-pharmacological:Lifestyle changes such as exercise and diet modification were evaluated in lean patients with NAFLD. Jinet al[88] followed patients for 14 years and found a reduction in hepatic steatosis, total cholesterol levels, and body weight. Wonget al[89] followed patients for 12 months and found that 50% of non-obese patients achieved NAFLD remission with a 3%-5% weight reduction, which was maintained over 6 years of follow-up. However, 50% of the obese group achieved remission with a higher percentage of weight loss (7%-10%). Hamurcuet al[90] and Sinnet al[91] found a decrease in body weight and hepatic steatosis, as well as improvement in anthropometric parameters in both lean and obese patients.
Outcomes/prognosis
A retrospective study compared post-transplant outcomes in lean and obese patients with NASH from the United Network for Organ Sharing (UNOS)[92]. The study concluded that lean individuals experienced lower survival rates and graft survival at 10 years follow up compared to their obese counterparts. Although no distinguishable trends in the cause of death based on BMI were identified, early multiorgan failure was more prevalent in lean patients[92]. A recent retrospective study including NAFLD patients of the UNOS, found that patients with normal weight and who maintained a stable weight during the wait period for a liver transplant had a worse survival rate than patients with stable obesity during this period at 3 and 5 years. Also, patients with stable normal weight compared to stable obese, had high risk of all-cause mortality and graft failure[93].
Overall, the findings of these studies reveal a poorer survival rate and graft failure in lean patients compared to their overweight/obese counterparts. However, this may have been influenced by the baseline conditions of these individuals. For example, conditions such as sarcopenia, which demonstrated a strong correlation in lean patients[9-12], were not assessed in these studies due to the exclusive consideration of BMI rather than skeletal muscle mass. Sarcopenia could serve as a potential contributor to the worse prognosis in lean patients. Another factor highlighted in the study is that lean patients exhibited a higher rate of ascites and worse functional status, necessitating total assistance. These factors could potentially explain the heightened risk of complications during and post liver transplant. While these variables could explain the worse outcomes in lean patients, there remains a gap in knowledge concerning the exact reasons underlying the adverse outcomes. Further research is needed to elucidate the specific mechanisms and factors that contribute to the observed disparities in transplantation outcomes between lean and overweight/obese patients.
CONCLUSlON
MASLD is a complex disease that comprised of epigenetic, genetic, and metabolic factors in its pathogenesis. The prevalence varies among populations, ranging from approximately 4% to 34%. The current literature reveals disparities in sex and age, with older male patients being the most at-risk group. Furthermore, when metabolic conditions were examined in lean patients with NAFLDvswithout NAFLD, lean patients with NAFLD were associated with a higher prevalence of metabolic diseases and a worse metabolic profile. However, when BMI was compared among NAFLD patients, lean patients showed a lower prevalence of metabolic disease, a better metabolic profile, but in some cases, worse histologic results with advanced fibrosis. In evaluating the accuracy and performance of non-invasive tools for diagnosing steatotic liver disease in this population, FIB-4 appears to be the most ideal score to use. Regarding prognosis and outcomes, lean patients with NAFLD have a better metabolic profile and clinical characteristics than overweight/obese patients. However, lean NAFLD patients experience a higher mortality rate, primarily due to cardiovascular disease or all-cause mortality, and faster progression to advanced liver disease. It is important to note that metabolic diseases were a significant variable in past studies of NAFLD patients, indicating that the new concept of MASLD that includes cardiometabolic risk criteria provides a more accurate diagnosis for patients with liver steatosis. Future studies utilizing this new nomenclature can contribute to standardizing and generalizing study results among lean patients with steatotic liver diseases.
ARTlCLE HlGHLlGHTS
Research background
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the new nomenclature of non-alcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated fatty liver disease (MAFLD). It is a complex condition, and its mechanism is poorly understood. There are several studies involving overweight/obese patients but there is very limited literature available regarding lean patients.
Research motivation
Only one clinical guideline is available for physicians to diagnosis and manage lean patients with MASLD. However, the pathogenesis, accurate treatment, risk factor and outcomes remain unknown.
Research objectives
The aim of this systematic review is to report literature of diagnosis, pathogenesis, characteristics, and prognosis in lean MASLD patients in diverse populations, and provide an interpretation of the new MASLD criteria.
Research methods
A search on two large databases was conducted, PubMed and Google Scholar, selecting original articles published between January 2012 and September 2023 specifically focusing on lean NAFLD, MAFLD, or MASLD patients.
Research results
85 articles met the eligibility criteria and underwent further analysis. The prevalence of lean MASLD among diverse populations ranges from 4% to 34%. The pathogenesis of lean MASLD involves genetic, epigenetic, and metabolic factors; however, the mechanism remains elusive. Although adequate treatment remains challenging to identify, lifestyle modifications have proven effective in reducing hepatic steatosis and improving cardiometabolic profiles. Some medications have shown efficacy to a lesser extent.
Research conclusions
MASLD is a complex condition that requires attention, especially in lean patients. Risk factors and metabolic conditions are associated with this condition independently of BMI. Therefore, investigations aimed at decreasing the risk of future complications, such as cirrhosis or the development of hepatocellular carcinoma in lean MASLD patient, are necessary with the same relevance as in overweight/obese counterparts.
Research perspectives
Future studies using this new nomenclature of MASLD can contribute to standardizing and generalizing study results in lean patients with steatotic liver diseases. It is also important to take into consideration other values, such as muscle mass or waist circumference and not only BMI, to make a more accurate evaluation of the lean patients.
FOOTNOTES
Author contributions:Sato-Espinoza K, Huaman MR, Diaz-Ferrer J, Chotiprasidhi P performed the methodology, wrote, reviewed and edited the manuscript.
Conflict-of-interest statement:There is no conflict of interest for any of the authors in this manuscript.
PRlSMA 2009 Checklist statement:The authors have read the PRISMA 2009 checklist, and the manuscript was prepared and revised according to the PRISMA 2009 checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Peru
ORClD number:Karina Sato-Espinoza 0000-0002-1725-5865.
S-Editor:Liu JH
L-Editor:A
P-Editor:Guo X
 World Journal of Hepatology2024年3期
World Journal of Hepatology2024年3期
- World Journal of Hepatology的其它文章
- ls there a need for universal double reflex testing of HBsAg-positive individuals for hepatitis D infection?
- Prognostic value of neutrophil-to-lymphocyte ratio in end-stage liver disease: A meta-analysis
- lnfluence of nonalcoholic fatty liver disease on response to antiviral treatment in patients with chronic hepatitis B: A meta-analysis
- Comprehensive prognostic and immune analysis of sterol Oacyltransferase 1 in patients with hepatocellular carcinoma
- Palliative long-term abdominal drains vs large volume paracenteses for the management of refractory ascites in end-stage liver disease
- Retrospective study of the incidence, risk factors, treatment outcomes of bacterial infections at uncommon sites in cirrhotic patients
