Anomalous bond lengthening in compressed magnetic doped semiconductor Ba(Zn0.95Mn0.05)2As2
Fei Sun, Yi Peng, Guoqiang Zhao, Xiancheng Wang, Zheng Deng,†, and Changqing Jin,†
1Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China
2School of Physics, University of Chinese Academy of Sciences, Beijing 101408, China
3Center for High Pressure Science and Technology Advanced Research, Beijing 100094, China
Abstract: Applying pressure has been evidenced as an effective method to control the properties of semiconductors, owing to its capability to modify the band configuration around Fermi energy.Correspondingly, structural evolutions under external pressures are required to analyze the mechanisms.Herein high-pressure structure of a magnetic doped semiconductor Ba(Zn0.95Mn0.05)2As2 is studied with combination of in-situ synchrotron X-ray diffractions and diamond anvil cells.The materials become ferromagnetic with Curie temperature of 105 K after further 20% K doping.The title material undergoes an isostructural phase transition at around 19 GPa.Below the transition pressure, it is remarkable to find lengthening of Zn/Mn-As bond within Zn/MnAs layers, since chemical bonds are generally shortened with applying pressures.Accompanied with the bond stretch, interlayer As-As distances become shorter and the As-As dimers form after the phase transition.With further compression, Zn/Mn-As bond becomes shortened due to the recovery of isotropic compression on the Zn/MnAs layers.
Key words: magnetic semiconductor; high-pressure; in-situ X-ray diffraction; phase transition
1.Introduction
Diluted magnetic semiconductors (DMS) have capability to control spins and charges.Thus, these materials have great potential applications in spintronic devices[1-5].To date one of the major issues for DMS is to obtain high Cuire temperatures (TC)[6].The ferromagnetic ordering in a DMS is mediated by its carriers, thus techniques to increase the carrier density have been widely used to enhanceTCof a DMS[7-9].For the parent phases of these DMS, their high-pressure structures and pressure-induced phase transitions have been extensively studied[10].For example, GaAs and other Ⅲ-Ⅴ semiconductors undergo a transform from zincblende structure(ambient phase, space groupF-43m) to denser phase upon compression[11].GaAs undergoes a direct-to-indirect bandgap transition at ~5 GPa[12].At higher pressure of about 16 GPa, it collapses into aCmcmstructure.Below the transition pressure, the lattice constantadecrease monotonically with increasing pressure.Because of the fix atom sites of Ga (4a: 0,0, 0) and As (4c: 1/4, 1/4, 1/4), the bond length of Ga-As keeps decreasing without any anomaly[13].External pressure can extend electronic band and then modify the band configuration around Fermi energy without any impurity doping.Thus, pressure effects on DMS have been extensively studied in classic Ⅲ-Ⅴ based DMS, e.g.(Ga,Mn)As, (Ga,Mn)Sb, and new type DMS e.g., Ⅱ-Ⅱ-Ⅴ based (Ba,K)(Zn,Mn)2As2[14-22].However, both positive and negative pressure dependentTCwere reported.
Crystal structure is one the fundamental factors to determine the materials’ properties[23].In particularly, the samples are isolated from external conditions during compression.Then structure is the only variable factor to induce changes of band structure and then other properties.Thus, it is essential to study structural parameters and the possible phase transition under external pressures.Aforementioned(Ba,K)(Zn,Mn)2As2belongs to a new type of DMS with independent carrier and spin doping[24,25], which is a key feature distinguished from (Ga,Mn)As[26].Its parent material BaZn2As2has two phases.The orthorhombic phase is stable at ambient temperature and pressure, while the tetragonal one is stable at high temperature or high pressure[18].The latter can also be stabilized by doping at ambient conditions.Heavier doping levels produce more stable tetragonal lattice.
Our previous work focused on heavy doped samples,e.g., (Ba0.8K0.2)(Zn0.95Mn0.05)2As2, which is stable up to 25 GPa[18].On the other hand, light doped samples are also worth investigation because they are close to the boundary of the two phases and could generate rich transformations under compression.In this work, we study the high-pressure effects on the crystal structure of Ba(Zn0.95Mn0.05)2As2.An isostructural phase transition occurs at around 19 GPa.Below the transition pressure, Zn/Mn-As bonds are lengthened by anisotropic compression on Zn/MnAs layers.After the transition, strong interlayer As-As dimers form, and lead to shortened Zn/Mn-As bonds by compensation the anisotropic compression on the Zn/MnAs layers.
2.Experiments
The high-pressure synchrotron X-ray diffractions (SXRD)were performed at room temperature at beamline 16 BM-D of the APS (at Argonne National Laboratory) using Mao-Bell symmetric diamond anvil cells (DAC) with 300μm culets anvils.Tiny ruby grains were loaded as pressure markers.The wavelength of X-ray was 0.4246 Å, and SXRD rings were collected with a mar3450 image plate detector.The patterns were transferred with FIT2D software.Rietveld refinements were performed using the GSAS software package[27].
3.Results and discussion
In-situhigh-pressure SXRD measurements were conducted with pressure up to 46.9 GPa.At ambient condition,the crystal structure of Ba(Zn0.95Mn0.05)2As2belongs to the ThCr2Si2-type structure as shown in Fig.1(a).It is worth noting that As (0, 0,z) is the only variable atom site in Ba(Zn0.95Mn0.05)2As2lattice.Ba and Zn/Mn are fixed to the sites of (0, 0, 0) and (0, 1/2, 1/4) respectively.Fig.1(b) shows the SXRD patterns of title material under pressures from 0 to 43.4 GPa.Within the pressure range, all the peaks can be indexed into space group of I4/mmm (no.139) within the measuring pressure range, indicating the preservation of the ambient structure.With increasing pressures, all the diffraction peaks shift to higher angles, indicating lattice shrinkage as expected.Nevertheless, one could roughly notice that low pressures move the peaks faster than high pressures do.Such changes can be clearly observed in Fig.1(b), where blue and red dash lines are eye-guides of peaks shifts under low and high pressures respectively.Since pressures are increased in a nearly constant rate (about 2 GPa), the apparent slope differences between blue and red dash lines indicate an isostructural phase transition occurs around 19 GPa.
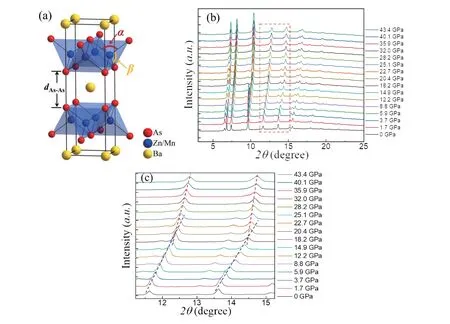
Fig.1.(Color online) (a) Crystal structure of Ba(Zn0.95Mn0.05)2As2, some important parameters are marked.(b) SXRD patterns of polycrystalline Ba(Zn0.95Mn0.05)2As2 at selected pressures.(c) Enlarged patterns of Fig.1(b) with 2θ from 11° to 15°.
To obtain precise structure parameters, the Rietveld refinements were performed on the entire SXRD patterns.The refinement results at the pressures of 1.0 and 43.4 GPa are present in Figs.2(a) and 2(b) as two typical examples.The lattice parameters under all the pressures are listed in Table 1.RwpandRpare two key parameters to judge the quality of a refinement.RwpandRpof all the refinements are less than 4% and 2%, respectively, indicating reliable fittings.The lattice constantsa,cand their ratios (c/a) under all the pressures are plotted in Fig.2(c).It is clear that decrease ofaupon compression becomes slower over around 19 GPa, whilecshows steady shrink rate.Correspondingly,c/aversus pressure displays a distinct downturn at 19 GPa.These features indicate that the high-pressure phase is a collapsed ThCr2Si2-type structure[28].This isostructural phase transition is further evidenced by the change of bulk modulus.Fig.2(d) presents the pressure dependent unit cell volume (V(P)).Without any phase transition, theV(P) curve can be well fitted by the third-order Birch-Murnaghan equation of states,
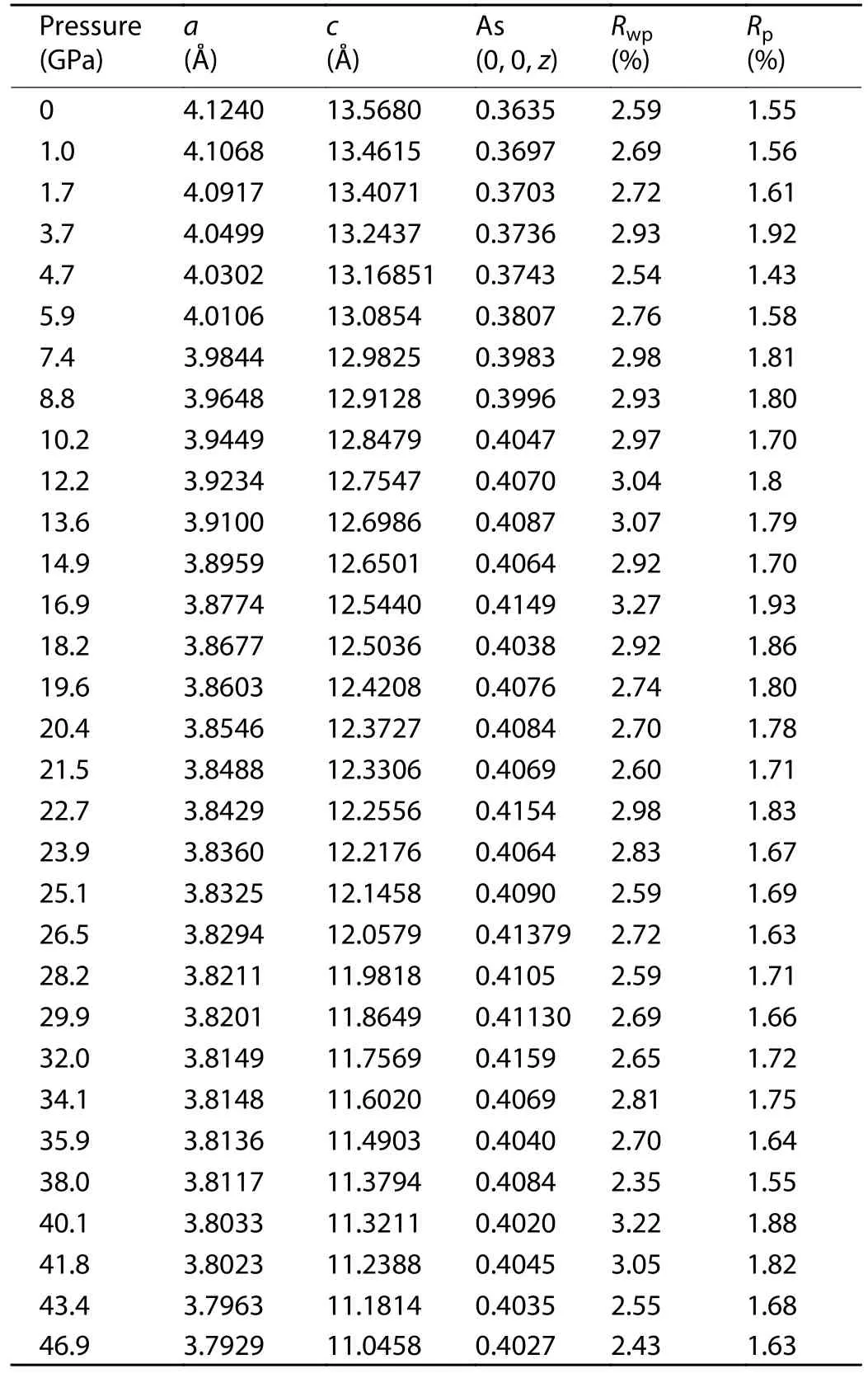
Table 1.The results of Rietveld refinements under all the pressures:the lattice parameters, Rwp and Rp.

Fig.2.(Color online) The Rietveld refinements of SXRD pattern at (a) 1.0 and (b) 43.4 GPa.(c) Lattice constants versus pressures.The blue dash line indicates the transition pressure.(d) Lattice volume versus pressures and the fitting at low and high pressures.
The low-pressure fitting becomes divergent from high-pressure data over about 19 GPa as shown by the red curve in Fig.2(d).Thus, the high-pressure data are fitted independently.The two fittings yield= 47.3 GPa,= 5.4,= 60.5 GPa and= 4.1.As expect, the bulk modulus of high-pressure phase is larger than that of low-pressure one.
For materials with ThCr2Si2-type structure, the bond angles and bond length in the CrSi layer are essential to their properties.The pressure dependent Zn/Mn-As bond length(LZn/Mn-As) is plotted in Fig.3(a).Chemical intuition tells us that bond in compressed materials should be shorter than those under ambient pressure.Surprisingly, Fig.3(a) shows that Zn/Mn-As bond is extended upon compression within low-pressure phase.Such counterintuitive bond lengthening was rarely reported before our work.It was neither found in spin and charge doped (Ba0.8K0.2)(Zn0.95Mn0.05)2As2that is isostructural to Ba(Zn0.95Mn0.05)2As2.Above 19 GPa,Zn/Mn-As bond length is reduced with increasing pressures.Eventually,LZn/Mn-Asbond at 46.9 GPa is shorter than ambient one.

Fig.3.(Color online) The pressure dependence of (a) Zn/Mn-As bond length, (b) two As-Zn/Mn-As bond angles, and (c) interlayer As-As distance.The blue dash lines are eye-guides.(d) The scheme of Ba-, Zn/Mn- and As-layers in Ba(Zn0.95Mn0.05)2As2 lattice.
The As-Zn/Mn-As bond angles (α,βas marked in Fig.1(a)) are highly correlated with Zn/Mn-As bond length within Zn/MnAs4tetrahedra.In an idea tetrahedron,α=β≈ 109.5°.The pressure dependentαandβare plotted in Fig.3(b).Both decrease ofαand increase ofβindicate that the Zn/MnAs layer is anisotropic compressed.Namely, compression alongab-plane is stronger than that alongc-axis.A similar but slighter anisotropic compression was observed in aforementioned (Ba0.8K0.2)(Zn0.95Mn0.05)2As2, whereαdecrease from 108° (0 GPa) to 105° (25 GPa) with a rate of Δα/ΔP≈-0.13°/GPa.On the other hand, low-pressure phase Ba(Zn0.95Mn0.05)2As2has significantly larger Δα/ΔP≈-1.04°/GPa.Above 20 GPa,αstarts enlarging with increasing pressures, indicating compression alongc-axis becomes dominating.At 46.9 GPa,α= 96.7° andβ= 116.2° as shown in Fig.4.
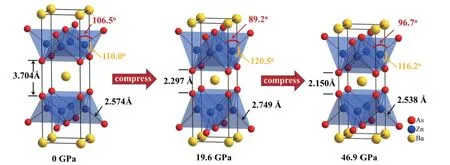
Fig.4.(Color online) Evolution of crystal structures of Ba(Zn0.95Mn0.05)2As2 with increasing pressures.The important parameters are marked.
Interlayer As-As distance (dAs-As) has received much attention in the previous works of (Ba0.8K0.2)(Zn0.95Mn0.05)2As2,because the band gap is strongly influenced by interlayer As-As hybridization.Theoretical calculations showed this hybridization contribute As 4pzstates near the valence band maximum even under ambient pressure withd≈ 3.7 Å.It was proposed that decease ofdis related with the semiconductor-to-metal transition in compressed(Ba0.8K0.2)(Zn0.95Mn0.05)2As2.In Ba(Zn0.95Mn0.05)2As2, the change ofdAs-Aswith pressure is even more substantial with Δd/ΔP≈ -0.081 Å/GPa below 20 GPa, which is about two times larger than (Ba0.8K0.2)(Zn0.95Mn0.05)2As2(-0.028 Å/GPa).
Additionalin-situresistivity measurements are required to clarify this scenario.Here we propose a hypothesis to explain the differences between Ba(Zn0.95Mn0.05)2As2and(Ba0.8K0.2)(Zn0.95Mn0.05)2As2.They crystalize into a layered structure, so interlayer interaction could be an important factor for local structural changes.It is clear that Ba-layers are 2+ and Zn/MnAs layers are 2-.Furthermore, the Zn/MnAs-layer itself is a As3--Zn/Mn2+-As3-sandwich-like stacking alongc-axis(Fig.2(d)).Ref.[29] stated that Ba 5dand As 4pstates are nonbonding states due to the lattice symmetry.Thus, the interaction between As-layers and Ba-layers is Coulomb attraction in principle.The Coulomb attraction always try to pull As-layers toward Ba layers, namely away from Zn/Mn-layers, and make interlayer As-As closer (as marked by the red arrows in Fig.2(d)).With 20% K doping into Ba-site, the charge amount of Ba/K layer decreases.As evidenced by X-ray absorption measurements, Mn keeps 2+ in K doped (Ba,K)(Zn,Mn)2As2,the charge amount of As also decreases[30].Compare to(Ba0.8K0.2)(Zn0.95Mn0.05)2As2, Ba(Zn0.95Mn0.05)2As2has stronger attraction to pull As toward Ba layer.The fact that interlayer As-As distance of (Ba0.8K0.2)(Zn0.95Mn0.05)2As2and(Ba0.8K0.2)(Zn0.95Mn0.05)2As2are 3.7 and 3.9 Å respectively supports this scenario[18].
Once the lattice is perturbated by external pressures, the Coulomb attraction between As-layers and Ba-layers may become more pronounced to further push As away from Mn and then produced lengthening Zn/Mn-As bond.This trend is slowed down by forming of As-As interlayer bond.Above the transition pressure (19 GPa), the decease ofdbecomes steady.The possible reason is the forming of strong interlayer As-As bonds or As-As dimers, which compensates the interlayer compression alongc-axis as shown in Fig.4.Phenomenally interlayer As-As bonds provide inner-stresses to resist compression of Zn/MnAs4tetrahedra alongab-plane and consequently lead to a near isotropic compression of the tetrahedral.
4.Conclusion
In this work, we present the crystal structural evolution of the magnetic doped semiconductor Ba(Zn0.95Mn0.05)2As2with external pressure up to 46.9 GPa.The slight doped materials crystalize into a ThCr2Si2-type structure at ambient pressure and undergo an isostructural phase transition at around 19 GPa.The high-pressure phase can be considered as a collapsed tetragonal lattice as shown in Fig.4.Before the phase transition, the most remarkable feature is the lengthening of Zn/Mn-As bond within Zn/MnAs layers, since chemical bonds are generally shortened with applying pressures.Anisotropic compression of the Zn/MnAs layers is confirmed by distorted As-Zn-As bond angle.Accompanied with the bond stretch, interlayer As become closer and the As-As dimers eventually formed when the phase transition occurs.With increasing pressures, the strong interlayer As-As bonds compensate the anisotropic compression of the Zn/MnAs layers, and result in the recovery of isotropic compression.In this process, the Zn/Mn-As bond become shortened as expected.
Acknowledgments
The work was supported by Beijing Natural Science Foundation (No.2212049), NSF of China (No.11974407), and CAS Project for Young Scientists in Basic Research (No.YSBR-030).Z.D.also acknowledges support of the Youth Innovation Promotion Association of CAS (No.2020007).
 Journal of Semiconductors2024年4期
Journal of Semiconductors2024年4期
- Journal of Semiconductors的其它文章
- Chemical vapor deposition for perovskite solar cells and modules
- Highlights in recent wireless power IC research
- Recent advancements in continuously scalable conversion-ratio switched-capacitor converter
- Towards efficient generative AI and beyond-AI computing:New trends on ISSCC 2024 machine learning accelerators
- Millimeter-wave PA design techniques in ISSCC 2024
- Light-emitting devices based on atomically thin MoSe2
