Extraction of Valuable Metals from Titanium-bearing Blast Furnace Slag by Acid Leaching
LIU Yan ,CHEN Xuegui ,MAO Shuaidong ,XIAO Yadong ,LI Jiacong
(1.School of Metallurgy,Northeastern University,Shenyang 110819,China;2.Key Laboratory of Ecological Metallurgy of Multi-metal Intergrown Ores of Ministry of Education,Shenyang 110819,China)
Abstract: To realize the resource utilization of the valuable metals in the titanium-containing blast furnace slag,the process route of “hydrochloric acid leaching-electrolysis-carbonization and carbon dioxide capture-preparation of calcium carbonate” was proposed.In this study,the influences of process conditions on the leaching rates of calcium,magnesium,aluminum,and iron and the phases of the leaching residue were investigated for the leaching process.The experimental results show that the HCl solution could selectively leach the elements from the titanium-containing blast furnace slag.The better leaching conditions are the HCl solution concentration of 4 mol/L,the leaching time of 30 min,the ratio of liquid volume to solid gas of 10 mL/g,and the stirring paddle speed of 300 r/min.Under the conditions,the leaching rates of calcium,magnesium,aluminum,and iron can reach 85.87%,73.41%,81.35%,and 59.08%,and the leaching rate of titanium is 10.71%.The iron and the aluminum are removed from the leachate to obtain iron-aluminum water purification agents,and the magnesium is removed from the leachate to obtain magnesium hydroxide.The leaching residue phase is dominated by perovskite,followed by magnesium silicate and tricalcium aluminate,and the titaniumrich material could be obtained from the leaching residue by desiliconization.
Key words: titanium-containing blast furnace slag;acid leaching;valuable metals;comprehensive utilization
1 Introduction
There are abundant vanadic titanomagnetite resources in China[1-3],and the titanium-containing blast furnace slag produced by blast furnace smelting can be divided into low-titanium slag (TiO2<10%),medium-titanium slag (TiO2: 10%-15%) and high-titanium slag (TiO2: 20%-25%) according to the TiO2content[4].At present,millions of tons of blast furnace slag are discharged and piled up every year,which still contains titanium,calcium,magnesium,aluminum,and other valuable metal elements,which is a great waste of resources,and meanwhile aggravates the pollution of the natural environment.With the deterioration of the environment and the depletion of resources,people have begun to pay attention to the rational use of blast furnace slag,and a lot of research has been carried out[5-7].
There are two treatment ideas in total for titanium-containing blast furnace slag[8-11],one is the treatment without titanium extraction for direct utilization,and the other is the titanium extraction treatment[12,13].The direct utilization mainly includes: being the raw materials for photocatalytic degradation[14,15],production of slag glass-ceramics and cast stone[16,17],and being the construction materials[18-20],etc.However,the titanium and other high-value-added components have not been extracted and utilized,which is a simple but rough type of utilization,and therefore the economic value of titanium-containing blast furnace slag has not been duly realized.Another is the titanium extraction utilization,the main methods of which mainly include acid treatment for titanium extraction[21-26],alkali treatment for titanium extraction[27,28],preparation of TiC by high-temperature carbonization-low-temperature selective chlorination[29,30],acid-base combined method of titanium extraction[31],and so on.
Using the characteristic of selective leaching of hydrochloric acid on titanium-containing blast furnace slag,combined with previous research and the current national “double carbon” goal,the process route of“hydrochloric acid leaching-electrolysis-carbonization for capturing carbon dioxide-preparation of calcium carbonate” of resource utilization of titanium-containing blast furnace slag is proposed in this study.In the process,calcium,magnesium,aluminum,iron,and other elements can be extracted and separated from the titanium-containing blast furnace slag,so that the titanium-containing blast furnace slag becomes titanium-rich raw materials (titanium source) and calcium carbonate (calcium source),to achieve high-value use of slag at the same time to absorb carbon dioxide[32].In this study,for the leaching process in the process,the leaching rates of Ti,Ca,Si,Al,Mg,and Fe under each single-factor condition were studied,to provide reference and basis for the later industrialization application.
2 Experimental
2.1 Raw materials
The experimental raw materials were air-cooled titanium-containing blast furnace slag provided by a factory in Heilongjiang Province.After being ground in the rod mill and passing the 100-mesh sieve,the slag with particle size 150 μm was taken and put in the thermostatic drying oven undergoing drying treatment for use.The X-ray fluorescence spectrometer (XRF)was used to analyze the chemical composition of titanium-containing blast furnace slag,and the analysis results are shown in Table 1.

Table 1 Composition of titanium-bearing blast furnace slag/wt%
As can be seen from Table 1,in the titanium-containing blast furnace slag,the CaO content is the highest,followed by SiO2,Al2O3,MgO,TiO2,and Fe2O3.
The X-ray diffractometer (XRD) and scanning electron microscope (SEM) were used to analyze the phase composition and surface morphological characteristics of titanium-containing blast furnace slag,and the results are shown in Fig.1 and Fig.2.

Fig.1 XRD patterns of titanium-bearing blast furnace slag
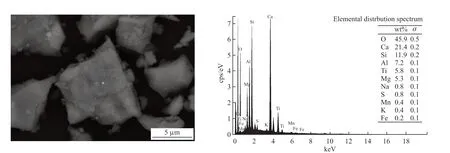
Fig.2 SEM-EDS analyses of titanium-bearing blast furnace slag
Combined with the XRF,XRD,and SEM results of the titanium-containing blast furnace slag,it is known that the main phases of the slag are perovskite Ca(TiO3),gehlenite Ca2Al(AlSi)O7,akermanite Ca2MgSi2O7,and variants of diopside [Ca2(Mg0.25Al0.75)(Si1.25Al0.75O7) and Ca4Mg0.42Al3.08Si2.48O14].
2.2 Process route and reaction principles
2.2.1 Process route
The process flow chart of acid leaching of titanium-containing blast furnace slag for extracting valuable metals is shown in Fig.3.Through the appropriate process conditions,the extraction and separation of elements of Ti,Ca,Si,Al,Mg,and Fe from the titanium-containing blast furnace slag can be realized,so that the blast furnace slag can be reasonably utilized.
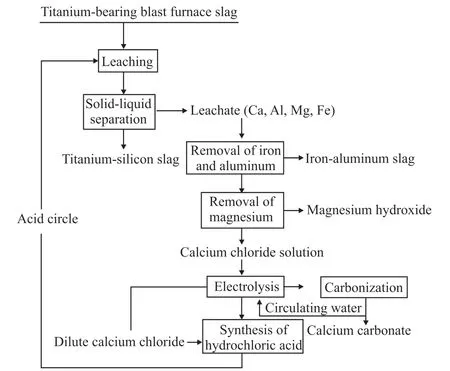
Fig.3 Comprehensive utilization of titanium-bearing blast furnace slag
After acid leaching purification and electrolytic carbon sequestration treatment,the titanium-containing blast furnace slag was mainly composed of titanium-containing sources and produced by purification,aluminum-iron slag,magnesium hydroxide slag,and high-value calcium carbonate.Titanium and most of the silicon were enriched in the slag,and by dint of desiliconization the titanium-rich material could be obtained;aluminum-iron slag can be made into Fe-Al water purifier after re-reaction treatment;magnesium hydroxide can be processed into a flame retardant,or be roasted into the magnesium oxide;the final product of calcium carbonate can be directly used in the market.
2.2.2 Reaction principles
The whole process is divided into two parts:leaching purification and electrolytic carbonization.This study focuses on exploring the leaching process,and the reaction formulae of the leaching process are shown as follows:
Since TiO2is an amphoteric oxide with weaker acidity and alkalinity,its corresponding titanate and titanium salt are easily hydrolyzed:
Therefore,theoretically,titanium finally enters the slag system in the form of metatitanic acid,and titanium does not consume hydrochloric acid in the whole process,which can realize the purpose of titanium enrichment.
The calcium oxide (or calcium hydroxide) was added to the solution,and the pH of the solution was adjusted to a specific value so that the iron ions and the aluminum ions in the solution were precipitated in the form of hydroxide;after filtration,the calcium oxide(or calcium hydroxide) was added to the filtrate,and the pH of the solution was adjusted to another specific value so that the magnesium ions in the solution were precipitated in the form of hydroxide.The reaction formulae for the purification process are shown as follows:
2.3 Experimental methods and testing
The experiments were conducted to investigate the optimal conditions for leaching of each element in titanium-containing blast furnace slag by changing the initial concentration of hydrochloric acid,the ratio of liquid volume to solid mass,the reaction time,the reaction temperature,and the stirring paddle speed.
All leaching experiments were carried out in a 500 mL beaker,which was heated and kept warm using a water bath and stirred by a mechanical stirring paddle.At the beginning of an experiment,a certain concentration of HCl solution was prepared in a volumetric flask,and then a certain amount was taken and placed in a 500 mL beaker.After heating to the experimental temperature using a water bath,the stirring paddle was turned on,and 20 g of titanium-containing blast furnace slag was added to the reactor as the rotational speed was stable,and meanwhile,the timing was started.At the end of the reaction,a small part of the solution was filtered and the filtrate was used for quantitative analysis of the leached ions,and most of the solution was filtered using a vacuum filter,and the filter residue was washed with deionized water.The washed leaching residues were placed in an oven at 70 ℃ for drying.
The ion concentration in the leachate was analyzed by an inductively coupled plasma emission spectrometer (ICP).The X-ray fluorescence spectrometer(XRF) and the X-ray diffractometer (XRD) were used to analyze the chemical composition and phase composition of the leaching residue.
The leaching conditions of the titanium-containing blast furnace slag are as follows: the concentration of HCl solution of 1-6 mol/L,the temperature range of 20-95 ℃,the reaction time of 10-60 min,the stirring speed of 200-600 r/min,the ratio of liquid volume to solid mass of 6-14 mL/g,and the particle size of about 150 μm.
The concentration of an ion in the leaching filtrate was measured by ICP to calculate the leaching rateη.The formula is shown below:
where,msis the total mass of slag used for the reaction,g;ωthe the mass fraction of oxide of the ion in the slag;Cmthe concentration of the ion in the leachate,mol/L;Vlthe volume of the leachate,L;Mrthe relative molecular mass of the oxide.
3 Results and discussion
3.1 Acid leaching process of the titaniumcontaining blast furnace slag
3.1.1 Effect of the concentration of HCl solution on the leaching rate of each element
Fixed experimental conditions: the ratio of liquid volume to solid mass of 8 mL/g,reaction temperature of 20 ℃,reaction time of 30 min,and stirring speed of 200 r/min.The changes in the leaching rate of each element were investigated when the concentration of the HCl solution was varied,and the results are shown in Fig.4.
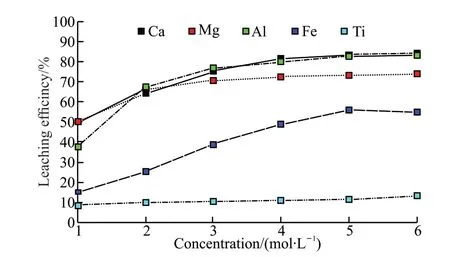
Fig.4 The leaching rates of each element at different concentrations of HCl solution
As can be seen from Fig.4,the leaching rates of calcium,magnesium,aluminum,and iron increased with the increasing concentration of HCl solution and then tended to level off.And titanium would also have some into the liquid phase,but in the concentration range,titanium basically did not change with the increase in the concentration of HCl solution,and the titanium leaching rate was maintained at about 10%.
When the concentration of HCl solution was 1-4 mol/L,the leaching rate of calcium,magnesium,aluminum,and iron increased significantly with the increase of HCl solution concentration,and when the concentration of HCl solution reached 4 mol/L,the leaching rates of calcium,magnesium,aluminum,and iron reached 81.53%,72.45%,79.97%,and 48.92%,respectively,and thereafter the change was gentle with the increase of hydrochloric acid concentration.At this time,increasing the concentration of HCl solution not only made the acid consumption too large,decreasing the effective utilization rate of hydrochloric acid,but also made the formation of silicic acid colloid increase,which affected the purification of the leachate in the next step.Therefore,the better concentration of HCl solution in this experiment was selected as 4 mol/L.
3.1.2 Effect of the ratio of liquid volume to solid mass on the leaching rate of each element
Fixed experimental conditions: the concentration of HCl solution of 4 mol/L,reaction temperature of 20℃,reaction time of 30 min,and stirring speed of 200 r/min.The changes in the leaching rate of each element were investigated when the ratio of liquid volume to solid mass was changed,and the results are shown in Fig.5.
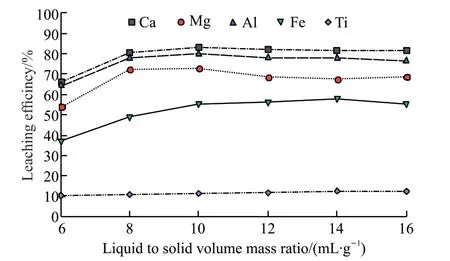
Fig.5 The leaching rates of each element at different ratios of liquid volume to solid gas
As can be seen from Fig.5,the leaching rates of calcium,magnesium,aluminum,and iron basically showed an increasing trend with the increase of the ratio of liquid volume to solid mass,and titanium remained unchanged.The leaching rates of calcium,magnesium,aluminum,and iron were 83.03%,72.72%,79.82%,and 55.29%,respectively,when the ratio of liquid volume to solid mass was 10 mL/g.After exceeding 10 mL/g,the leaching rates changed gently.Therefore,the subsequent experiments were carried out under the liquid-solid ratio of 10 mL/g.
3.1.3 Effect of stirring speed on the leaching rate of each element
Fixed test conditions: the concentration of HCl solution was 4 mol/L,the ratio of liquid volume to solid mass was 10 mL/g,the reaction temperature was 20℃,and the reaction time was 30 min.The changes in the leaching rate of each ion were investigated when the stirring speed was varied,and the results are shown in Fig.6.

Fig.6 Leaching rates of each element at different stirring speeds
As can be seen from Fig.6,the effect of stirring speed on the leaching rate of calcium,magnesium,aluminum,and iron increased with the increase of stirring speed,titanium was not greatly affected under this condition and still maintained a very low leaching rate of about 10%.Calcium,magnesium,aluminum,and iron to reached better leaching rates at the stirring speed of 300 r/min,when the leaching rate of each element were 86%,73.65%,82.97%,and 59.40%.When the stirring speed was greater than 300 r/min,the leaching effect decreased,because the increase in stirring speed promoted the acidolysis of diopside and accelerated the coagulation and precipitation of silicic acid colloid,producing a white gel-like precipitate,which adhered to the surface of the particles and caused agglomeration,causing the later reactions and the decrease of the leaching rate.Therefore,the subsequent experiments were carried out at the stirring speed of 300 r/min.
3.1.4 Effect of reaction time on the leaching rate of each element
Fixed test conditions: the concentration of HCl solution was 4 mol/L,the ratio of liquid volume to solid mass was 10 mL/g,the reaction temperature was 20℃,and the stirring speed was 300 r/min.The changes in the leaching rate of each element were investigated when the reaction time was varied,and the results are shown in Fig.7.
As can be seen from Fig.7,the leaching rates of calcium,magnesium,aluminum,and iron increased with the prolongation of the reaction time,and titanium did not change much and remained at about 10%.In the first 30 min,the leaching rate of each element increased significantly with the increasing reaction time,especially calcium,magnesium,and aluminum,and at this time,the corresponding leaching rate of each ion were 85.64%,73.37%,81.01%,58.43%.However,when the reaction time exceeded 30 min,the changes in the leaching rate of each ion tended to flatten out,and there was no obvious linear change.Therefore,the subsequent experiments were carried out under the reaction time of 30 min.
3.1.5 Effect of reaction temperature on the leaching rate of each element
Fixed test conditions: the concentration of HCl solution was 4 mol/L,the ratio of liquid volume to solid mass was 10 mL/g,the reaction time was 30 min,and the stirring speed was 300 r/min.The changes in the leaching rate of each element were investigated when the reaction temperature was changed,and the results are shown in Fig.8.
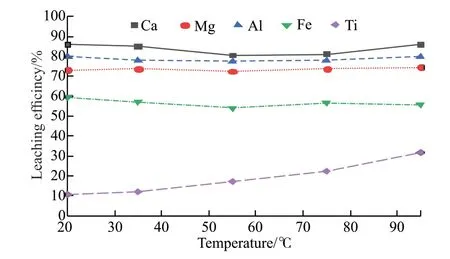
Fig.8 Leaching rate of each element at different reaction temperatures
As can be seen from Fig.8,the leaching rate of calcium,magnesium,aluminum,iron,and titanium changed significantly with the increase in reaction temperature.The leaching rate of titanium was greatly improved with the increase in temperature,and the leaching rate of titanium increased to 31.66% when the reaction temperature reached 95 ℃,while the leaching rate of calcium,magnesium,aluminum,and iron decreased when the temperature was higher than 35℃,and then recovered after the reaction temperature reached 95 ℃.This was because the elevated temperature would make the electrons and protons more active in the silicic acid molecules,increasing the possibility of collision with neighboring molecules,which was conducive to the agglomeration of silicic acid colloid attached to the blast furnace slag surface and hindering the leaching reaction,but when the temperature was too high,the leaching rate had risen due to the decomposition of silicic acid colloid.Therefore,the subsequent experiments were carried out at a room temperature of 20 ℃.
3.2 Treatment of leaching residues
Under the selected preferred process parameters of HCl solution concentration of 4 mol/L,the ratio of liquid volume to solid mass of 10 mL/g,reaction time of 30 min,reaction temperature of room temperature 20 ℃,and stirring speed of 300 r/min,the synthetical leaching rates of calcium,magnesium,aluminum,and iron reached 85.87%,73.41%,81.35%,and 59.08%,respectively,and the titanium leaching rate was 10.71%.
At the end of the test,the leaching residues were washed with deionized water,and the washed leaching residues were dried in an oven at 70 ℃.The XRD analysis was carried out.Compared with the XRD result of the original slag,the analysis results are shown in Fig.9.
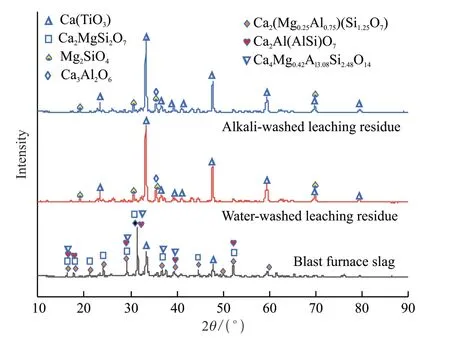
Fig.9 Comparison results of XRD before and after leaching
It can be seen that the phase of the leaching residue was dominated by perovskite Ca(TiO3),followed by magnesium silicate Mg2(SiO4) and tricalcium aluminate Ca3Al2O6,while the phases of gehlenite Ca2Al(Al-Si)O7,akermanite Ca2MgSi2O7,and variants of diopside[Ca2(Mg0.25Al0.75) (Si1.25Al0.75O7) and Ca4Mg0.42Al3.08Si2.48O14]disappeared,indicating that calcium,magnesium,aluminum,and iron were well leached,and the vast majority of titanium was still retained in the slag phase.Combined with the composition of slag before and after the leaching in Table 2,it shows that the selective leaching effect of hydrochloric acid on calcium,magnesium,and aluminum was very good.Under this condition,calcium,magnesium,and aluminum were leached,and titanium had been enriched at the same time.Subsequently,titanium-rich materials can be obtained by dint of desiliconization.
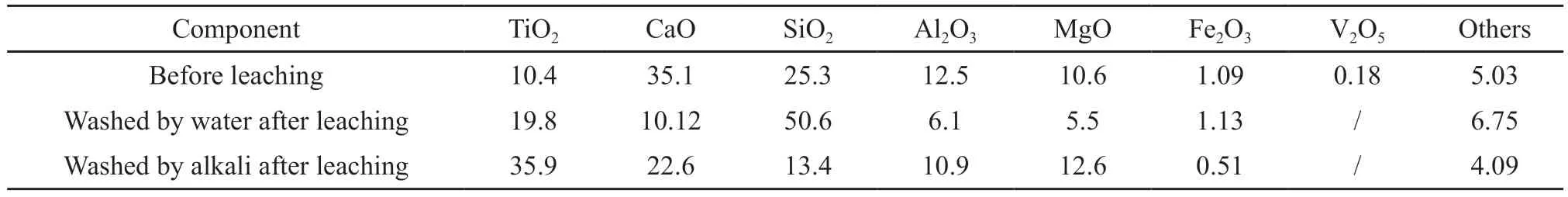
Table 2 Composition of slag before and after leaching/wt%
3.3 Treatment of leachate
3.3.1 Leachate purification by precipitation
The purification solution was treated according to the order of formation of hydroxide precipitation of various metal ions.As there was a certain amount of silicon dissolved into the leachate in the reaction process,the first step in the process of purification of the leachate was to remove the silicon from the leachate.The stability of the silicic acid colloid is related to the pH.The pH value which is too high or too low will make the colloid unstable,and the silicic acid colloid is generally stable between pH 2-10,but the specific range needs to be determined based on the different experimental conditions and the specific requirements of colloid stability.Through repeated tests,it was obtained that part of the silicon in the liquid phase formed the silicic acid colloid near pH of 2.3,so the silicon was controlled to precipitate at pH=2.3 during the experiment.The precipitated solids were characterized,and Table 3 and Fig.10 show the composition and XRD diagram of the product obtained after precipitation,respectively.Through the composition table and XRD diagram of the product precipitated at pH=2.3,the phases of the precipitate were mainly NaCl,SiO2,Ca2SiO4,etc.,which indicates that the silicon in the leachate was well precipitated,providing a good condition for the subsequent precipitation reactions.
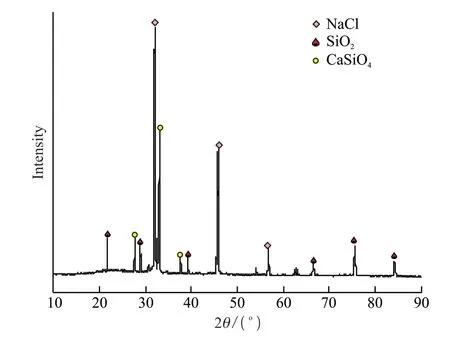
Fig.10 XRD pattern of the precipitated product at pH=2.3

Table 3 Precipitation components at pH=2.3/wt%
Iron ions are mainly in the form of trivalent iron in this leachate,and in the leachate,there are very few iron ions,the precipitation of which will coincide with the precipitation of aluminum.Therefore,it is difficult to precipitate the iron ions alone,and the two ions were precipitated at the same time during the experiment,with the precipitated product subsequently treated.At pH=8,the precipitates were detected and the result of XRF and XRD are shown in Table 4 and Fig.11.The phases of the precipitates were mainly Al(OH)3,Fe(OH)3,NaCl,and CaCl2,which indicated that the aluminum and the iron in the leachate were well precipitated.The precipitated products could be processed to prepare the iron-aluminum water purification agents.
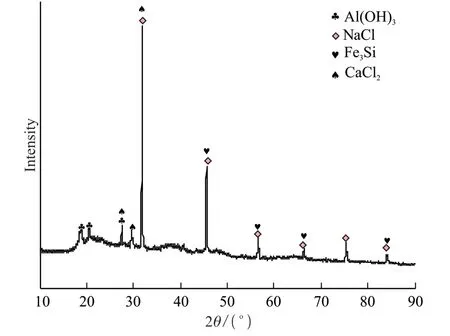
Fig.11 XRD pattern of the precipitated product at pH=4.8

Table 4 Precipitation components at pH=4.8/wt%
Continuing to adjust the pH value of the solution,the magnesium in the solution was completely precipitated at pH=11.2,and the precipitates were detected and the result of XRF and XRD are shown in Table 5 and Fig.12.The main phases of the precipitates were Mg(OH)2,NaCl,Ca5.62Mg0.38C6O18.The high-purity magnesium hydroxide could be obtained after treatment.Magnesium hydroxide is a compound that is very commonly used in a wide range of applications,such as medicine,pyrotechnics,industry,food,and many other fields.
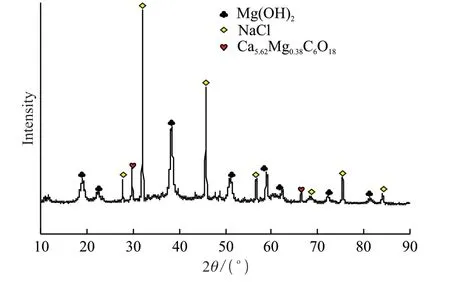
Fig.12 XRD pattern of the precipitated product at pH=11.2

Table 5 Table of precipitation components at pH=11.2/wt%
3.3.2 Preparation of nano calcium carbonate from purified leachate
Eventually,the aforementioned purified liquid was processed by a self-designed jet carbonization reactor to prepare nano-sized calcium carbonate and realize the comprehensive and high-value utilization of the titanium-containing blast furnace slag,and the structure of the jet carbonization reactor is shown in Fig.13.The main body of the reactor consists of the upper cylindrical section and the lower conical section,and the inlet of the reactor is a Venturi jet located in the tangential position of the cylindrical section.During the carbonization process,the purified leachate and CO2gas entered the carbonization reaction device tangentially at a flow rate of 4 and 0.6 L/min,respectively,to form a rotational flow in the device,so that CO2fully reacted with calcium hydroxide to generate light calcium carbonate.During the carbonization process,5% sodium dodecyl sulfate was added as an additive to reduce the particle size of calcium carbonate particles and inhibit the agglomeration between particles.At the end of the reaction the liquid-solid separation was carried out,and the product was put into the 70 ℃ vacuum drying oven for drying to get light calcium carbonate powder.
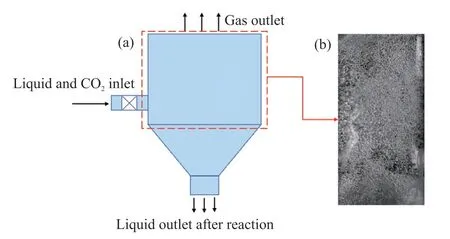
Fig.13 (a) Structure of the carbonization reactor;(b) Bubble distribution in the reaction zone
The SEM test and particle size analysis of the calcium carbonate product obtained from the carbonization reaction of the purified leachate were examined,and the results of crystal morphology of calcium carbonate were obtained as shown in Fig.14,as well as the results of particle size distribution as shown in Fig.15.It was found that the particle size of calcium carbonate prepared by carbonization reaction was between 150 and 500 nm,and the average particle size was 289 nm.Moreover,the particles were well dispersed without obvious agglomeration,and there was no adhesion between the particles.This was because when the direction of the jet was tangential,the fluid in the reactor moved in a spiral,and due to inertia,the solid particles tended to move in a large circle,and the gas was confined to the axial part.This realized the separation of calcium carbonate particles from carbon dioxide gas to some extent,and it was easier to produce smaller calcium carbonate particles.
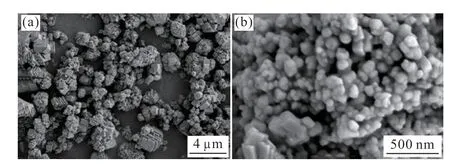
Fig.14 SEM images of CaCO3 prepared from the purified solution of blast furnace slag: (a) 5,000 times,and (b) 60,000 times
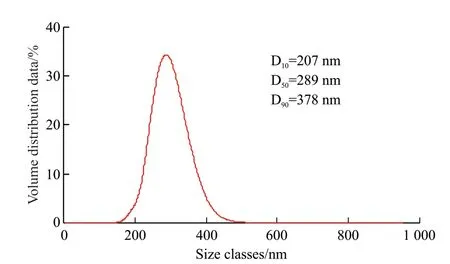
Fig.15 Particle size distribution of CaCO3 prepared from the purified solution of blast furnace slag
Table 6 and Fig.16 show the X-ray fluorescence analysis (XRF) and X-ray diffraction analysis (XRD)diagrams of the CaCO3product prepared from the purified solution of the slag,respectively.It was determined by testing and analysis that the crystalline form of this nano calcium carbonate was calcite,the specific surface area of the sample was 19.08 m2·g-1,and the average particle size calculated by the formula of cubic specific surface area was about 283 nm.Through testing and analysis,it was determined that the purity of the nano calcium carbonate product was as high as 99.2%,which can be used in the production of pharmaceuticals,food,and other high-end fields;the whiteness was as high as 94.2%,which enables it to be widely used in the coatings,rubber,plastics and other industries;and the calcite-form calcium carbonate has a better processing performance,which can satisfy the requirements of the processing technology of different products.
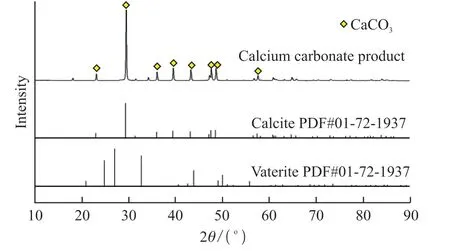
Fig.16 XRD patterns of CaCO3 product prepared from the purified solution of blast furnace slag
4 Conclusions
Utilizing the characteristics of selective leaching of hydrochloric acid,the process route of hydrochloric acid leaching of titanium-containing blast furnace slag is proposed,which can realize the utilization of valuable metals in titanium-containing blast furnace slag and zero discharge.In this study,for the leaching process in the process,the influence of each single factor on the leaching rate of each element was investigated,and the following conclusions were obtained.
a) The method of hydrochloric acid leaching of titanium-containing blast furnace slag can realize the leaching of calcium,magnesium,aluminum,and iron from the titanium-containing blast furnace slag.The increase in hydrochloric acid concentration is conducive to the leaching of each element,but too high a concentration of hydrochloric acid will lead to an increase in the content of the silicic acid colloid,which affects the purification of the leachate in the next step.Under the experimental conditions in this study,the leaching rates of calcium,magnesium,aluminum,and iron increase with the increasing hydrochloric acid concentration,ratio of liquid volume to solid mass,reaction time,and stirring speed.Considering the energy consumption and other factors,the low temperature of 20 ℃,the concentration of HCl solution of 4 mol/L,the ratio of liquid volume to solid mass of 10 mL/g,the reaction time of 30 min,and the stirring speed of 300 r/min were selected as the experimental conditions,and the leaching rate of each ion of calcium,magnesium,aluminum,and iron reached 85.87%,73.41%,81.35%,and 59.08%,and that of titanium was 10.71%,respectively.
b) The leaching slag phase was dominated by perovskite Ca(TiO3),followed by magnesium silicate Mg2(SiO4) and tricalcium aluminate Ca3Al2O6.The phases of gehlenite Ca2Al(AlSi)O7,akermanite Ca2MgSi2O7,and variants of diopside [Ca2(Mg0.25Al0.75)(Si1.25Al0.75O7) and Ca4Mg0.42Al3.08Si2.48O14]in the original slag were destroyed by hydrochloric acid,allowing the leaching of valuable metals.Under the reaction condition,calcium,magnesium,and aluminum were leached at the same time,and titanium was also enriched.By dint of desiliconization,titanium concentrate could be obtained,from which the titanium-rich materials could be further produced,providing an idea for the comprehensive utilization of valuable metals in the titanium-containing blast furnace slag.
c) In the purification of the leachate,the pH value was adjusted to 2.3 to precipitate silicon,to avoid the influence of silicon on the subsequent purification;and then the pH value was adjusted to 4.8 to precipitate silicon precipitate both iron and aluminum,which can be used to prepare the iron-aluminum water purification agents;continuing to adjust the pH value,when pH=11.2,magnesium was precipitated to obtain the precipitation of magnesium hydroxide,which can be used in medicine,pyrotechnics,industry,food,and many other fields.After the filtration the products should be fully washed,otherwise,the entrained NaCl would affect the purity of the products.
d) Calcium carbonate obtained from carbonization treatment of purified leachate possessed purity of 99.2%,whiteness of 94.2%,specific surface area of 19.08 m2·g-1,particle size between 150 and 500 nm,the average particle size of 289 nm,with excellent performance,which can achieve the comprehensive and high-value utilization of the titanium-containing blast furnace slag.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Fabrication of YAG: Ce3+ and YAG: Ce3+,Sc3+ Phosphors by Spark Plasma Sintering Technique
- Preparation of Modified UiO-66 Catalyst and Its Catalytic Performance for NH3-SCR Denitration
- Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
- Ultraviolet Photodetector based on Sr2Nb3O10 Perovskite Nanosheets
- Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
- Bio-inspired Hydroxyapatite/Gelatin Transparent Nanocomposites
