Synthesis of Core-shell ZSM-5@ Ordered Mesoporous Silica by Tetradecylamine Surfactant
MA Kuoyan ,ZHAO Pengxian ,YI Hongyu ,YU Haijun* ,ZHOU Moxi ,ZHANG Lingling ,LIU Yupu
(1.Chongqing Key laboratory of Scientific Utilization of Tobacco Resource,Chongqing 400060,China;2.Department of Chemistry,Laboratory of Advanced Materials,Fudan University,Shanghai 200433,China)
Abstract: A core-shell composite consisting of ZSM-5 zeolite as the core and ordered mesoporous silica as the shell was prepared by a surfactant-controlled sol-gel process and using tetradecylamine (TDA) as the template and Tetraethylorthosilicate (TEOS) as the silica precursor.The pores of the silica shell were found to be ordered and perpendicular to the crystal faces of the zeolite core.The thickness of the shell in the coreshell structured composite can be adjusted in the range of 20-90 nm,while the surface morphology and the pore size distribution were modified by changing the mass ratio of TEOS to zeolite.The composite molecular sieves have higher surface area for capturing molecules than ZSM-5,and with the increase of mesoporous shell layer,the ZSM-5@SiO2-x composites show stronger adsorption capacity of butyraldehyde.However,when the shell thickness exceeds 90 nm,the adsorption capacity of butyraldehyde decreases instead.The composites have a huge potential for environmental applications.
Key words: core-shell;composite;tetradecylamine;surfactant;adsorption
1 Introduction
The zeolite composite with core-shell structures possess encapsulation properties,high specific surface area,and similar fabrication techniques,making it a promising candidate for overcoming the limitation of traditional zeolites[1-5].Specifically,the zeolite composite with core-shell structures could be more suitable for multi-stage catalysis[6].Moreover,it has demonstrated effectiveness in entrapping hazardous substances in its internal cavities for use as adsorbents[7].To date,enormous efforts have been made to synthesize porous core-shell structured materials.Zhao[8-10]carried out the synthesis of ZSM-5@MCM-41,Y@MCM-41 and A@MCM-41 using cetyltrimethylammonium bromide (CTAB) under alkaline conditions,while the silicalite@SBA-15[11]was prepared by using the triblock copolymer Pluronic P123 under acidic conditions.Zhao[12]described the synthesis of ZeoA@MesoS,a gradient porous material obtained by a micellar dynamic assembly strategy.Yu[13]synthesized zeolite-based core-shell monolithic catalysts with interconnected honeycomb structure,in which the hydrophilic noncompact silica served as shell and Cu-SSZ-13 zeolite acted as core,using a facile coaxial 3D printing strategy.ZSM-5@HMS[14]was synthesized utilizing dodecylamine (DDA),with the composites’ shell demonstrating wormhole-like pores of around 3.5 nm in diameter.To our understanding,tetradecylamine (TDA) has not been used to prepare core-shell materials without controlling the pH.The World Health Organization (WHO) defines VOCs(volatile organic compounds) as organic compounds with boiling temperatures at ambient temperatures ranging from 50 to 260 ℃.Most VOCs are toxic,including aromatic hydrocarbon,aldehydes,alcohols,ethers,esters,etc.Some VOCs pose a health hazard to humans.There is an increasing demand for various types of highly efficient adsorbents to help protect the environment[15,16].
Therefore,this study obtained core-shell structures of ZSM-5@SiO2with TDA surfactant,and investigated their efficacy as adsorbents for harmful gases.It has been discovered that the pores of the silica shell were ordered and perpendicular to the crystal faces of the zeolite core.Additionally,the shell thickness and pore size distribution were altered by adjusting the mass ratio of TEOS to zeolite.
2 Experimental
2.1 Materials
Tetradecylamine (TDA),tetrapropylammonium hydroxide (TPAOH),sodium hydroxide (NaOH),solidum aluminate (NaAlO2),aluminum isopropoxide(iPrO)3Al,Tetraethylorthosilicate (TEOS) were purchased from Aladdin Chemical Company.All chemicals were used without further purification.Deionized water was used for all experiments.
2.2 Synthesis
Zeolite ZSM-5: ZSM-5 single crystals were hydrothermally synthesized[17]using a typical procedure.A mixture containing TEOS,TPAOH,(iPrO)3Al,and deionized water was stirred for 3 hours to obtain a gel composition of 1.0SiO2:0.21(TPAOH):103.2H2O:0.005Al2O3,which was then transferred to a Teflon-lined autoclave and crystallized at 150 ℃ for 72 hours.The zeolite products were thoroughly washed three times with large volumes of water and then calcined at 540 ℃ for 6 hours.
ZSM-5@SiO2: The composites with core-shell structure were prepared by a surfactant-controlled sol-gel process using TDA as the template and TEOS as the silica source.First,0.20 g of ZSM-5 zeolite particles were combined with a mixture of 0.23 g TDA,45 mL water,and 65 mL ethanol.The solution was then sonicated for 30 minutes to create a suspension of zeolite particles.The resulting mix was then treated ultrasonically for 30 minutes.Following that,TEOS was added slowly and stirred for 48 hours at 25 ℃.To regulate the thickness of mesoporous silica shells,the mass ratios of TEOS/Zeolite were modified ranging from 0.75 to 1.50.The core-shell-structured composites produced were obtained through centrifugation,followed by washing with water and ethanol,and ultimately air-drying at 100 ℃.The core-shellstructured composites were acquired post-calcination at 550 ℃ for 5 h,and identified as ZSM-5@SiO2-x(wherexspecifies the thickness of the mesoporous silica shell).
2.3 Characterization techniques
X-ray diffraction (XRD) measurement was carried out using a Bruker D8 Advance diffractometer with CuKα radiation.Sample morphology and size were observed using SEM (Hitachi S-4800,Japan) at 10 kV acceleration voltage and TEM (JEOL 2011F,Japan) at 200 kV.N2adsorption-desorption isotherms were obtained using a Micromeritics ASAP 2020 analyzer at 77 K.Before each measurement,the samples were degassed at 300 ℃ in vacuum for 15h.The BET method was used to calculate the specific surface areas,while the t-plot method was used to calculate the surface area and micropore volume.From the adsorption branches,the NLDFT (Nonlocal Density Functional Theory) method was employed to calculate the pore size distribution.The total pore volumes(Vt) were evaluated from the amount adsorbed at a relative pressure of approximately 0.995.Gravimetric adsorption equilibrium analyses of butyraldehyde and benzene were conducted using a fully automated digital microbalance,which was equipped with a vapor generator and connected to a high-vacuum system(Hiden Isochema Instrument,model IGA-002).Prior to measurements,all the samples were degassed in vacuum at 300 ℃ overnight.
3 Results and discussion
3.1 Characterization of materials
The wide range X-ray pattern of the pristine ZSM-5 (Fig.1(a)) shows typical diffraction peaks that are characteristic of the MFI zeolite structure[17].The diffraction peaks of the core-shell composites are the same,indicating that the coating process has little impact on framework of the zeolites when using TDA template.However,as the thickness of the shell increases in the core-shell composites (Figs.1(b)-1(e)),there is a gradual reduction in the intensity of the diffraction peak.This suggests that the silica shells play a shielding role and the zeolite is diluted after coating.

Fig.1 XRD patterns of (a) ZSM-5,(b) ZSM-5@ SiO2-20,(c) ZSM-5@SiO2-40,(d) ZSM-5@ SiO2-70,and (e) ZSM-5@ SiO2-90
SEM images show that the good dispersive ZSM-5 (Figs.2(a)-2(b)) with average particle size of 300 nm are of pseudo-hexagonally prismatic shape and the particle surfaces are relatively coarse (Fig.2(b)),which are with the typical characteristic of crystalline MIF zeolites.After constructing mesoporous silica shells using TDA as a template,the composite with core-shell structure (ZSM-5@SiO2-x) became rougher in surface and more rounded in shape relative to the pristine ZSM-5 molecular sieves (Fig.2(c)),but still maintained good dispersion and homogeneity (Fig.2(d)).Additionally,when the TEOS/ZSM-5 zeolite is less than 1.2:1,the wrinkles on the surface of the composites decrease gradually with the increase of TEOS,resulting in a rounded surface.However,when the TEOS/ZSM-5 ratio is 1.5:1,the composite demonstrates the wrinkled morphology again,with the appearance of a significant number of dispersed pores.
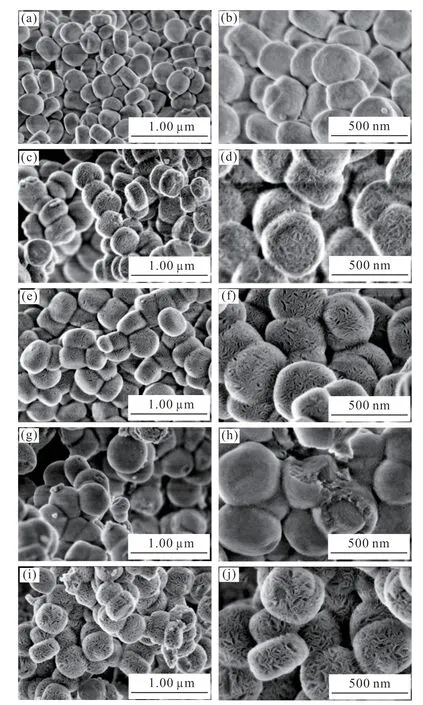
Fig.2 SEM images of (a) (b) ZSM-5,(c) (d) ZSM-5@ SiO2-20,(e)(f) ZSM-5@SiO2-40,(g) (h) ZSM-5@ SiO2-70,and (i) (j)ZSM-5@ SiO2-90
High-resolution TEM clearly demonstrates that ZSM-5@SiO2possesses a well-defined core-shell architecture (Figs.3(a)-3(h)).Furthermore,the thickness of the mesoporous silica shell can be tuned between 20 (Fig.3(b)) and 90 nm (Fig.3(h)) by adjusting the proportion of TEOS/ZSM-5.This suggests that composites were successfully synthesized by using TDA as a templating agent,and facile regulation of the shell thickness is achievable.When the ratio of TEOS to ZSM-5 is less than 1.2:1,the high-resolution TEM images demonstrate that the mesoporous silica shell exhibits well-distributed pores that are nearly perpendicular to the surfaces of ZSM-5.When TEOS/ZSM-5 is 1.5:1,divergent pores appear on the surface of the ZSM-5,with curved channels and a broader distribution of pore sizes.However,the pores within the shell remain perpendicular to the crystal facets of ZSM-5.The representative HRTEM images (Figs.3(d),3(f),and 3(h)) also show that the MIF framework and the mesoporous silica shells grow closely together at the shell-core interface.Therefore,the open connectivity between the mesopore and the micropore is of great importance for the transport of gas molecules in the core-shell structure.

Fig.3 TEM images of (a) (b) ZSM-5@SiO2-20,(c) (d) ZSM-5@SiO2-40;(e) (f) ZSM-5@ SiO2-70,and (g) (h) ZSM-5@SiO2-90
The N2sorption isotherm of pristine ZSM-5 shows a sharp uptake (typical type I curves) at low relative pressure (P/P0),which is a typical micropore structure.After coating the mesoporous silica shell,ZSM-5@SiO2-xshows a similar type I curve as ZSM-5 at relatively low pressure and another capillary condensation change (typical IV curve) atP/P0=0.3-0.5,and the formation of this H2 hysteresis loop is caused by mesopores.This bimodal-pore property,ranging from micropores to mesopores,aligns with the core-shell structure of the TEM images.
Moreover,the core-shell composites (Fig.4(c))have a similar curve change as the shell thickness increases to 70 nm,but the hysteresis loops become more pronounced at 0.3-0.5,which further suggests that the dual-porosity structure of the material and the thickness of the mesoporous shell are intensifying.Differently,when the shell thickness is increased to 90 nm,the curve of the core-shell composites (Fig.4(e))changes significantly,and the hysteresis loop shifts to 0.4-0.9,which suggests that the material has larger pores.
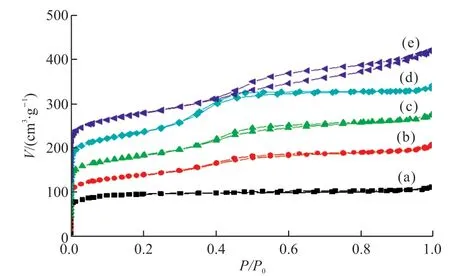
Fig.4 N2 sorption isotherms of (a) ZSM-5,(b) ZSM-5@SiO2-20,(c)ZSM-5@ SiO2-40;(d) ZSM-5@ SiO2-70,and (e) ZSM-5@SiO2-90
The pore size distribution (PSD) is calculated via the non-local density functional theory (NLDFT)method.The PSD of pristine ZSM-5 illustrates a discernible micropore centered at 0.56 nm and very little mesopores,indicating the true microporous property.
The core-shell composites with different silica shell thicknesses show two pore distributions:micropores at 0.56 nm and mesopores at 3.4 nm(Figs.5(b)-5(d)),which further demonstrates the coexistence of micropores and mesopores,with a more homogeneous distribution of the corresponding pore sizes;whereas (Fig.5(e)) the composite shows a wider distribution of mesopores,with a pore size distribution at 5-30 nm in addition to the main peak at 3.4 nm.The above results indicate that the coreshell structure can be obtained by using TDA and TEOS/ZSM-5 modulation,and the mesopores in the shell are perpendicular to the molecular sieves,with a varied distribution of pore sizes.The ZSM-5 has a specific BET surface area of 335 m2·g-1and a total pore volume of 0.21 cm3·g-1,of which approximately 74.3% (249 m2·g-1) and 71.4% (0.15 cm3·g-1) are microporous.After a 20 nm mesoporous silica coating,the specific BET surface area and total pore volume increase to 447 and 0.32 cm3·g-1,respectively.Most significantly,62.4% (279 m2·g-1) and 78.1% (0.25 cm3·g-1) come from mesopores.By changing the shell thickness to 40 nm,both the BET specific surface area and the total pore volume increase to 474 m²·g-1and 0.37 cm3·g-1respectively.The majority of the additional specific surface area (336 m²·g-1) and total pore volume (0.32 cm3·g-1) is due to the increased proportion of mesopores.Following coating with a 70 nm mesoporous silica shell layer,the specific surface area and pore volume exhibit a significant increase to 534 and 0.40 cm³·g-1(refer to Table 1).As the shell thickness increases to 90 nm,the specific surface area increases to 528 m2·g-1,demonstrating a decline from the 70 nm shell.The pore volume continues to increase to 0.47 cm3·g-1,with mesopores contributing most of the increase in specific surface area (443 m2·g-1) and pore volume (0.45 cm3·g-1) (see Table 1),consistent with the pore size distribution above.The above findings indicate that modifying the thickness of the shell layer can alter the mesoporous/microporous porosity of the composites.The decrease in microporous porosity with thicker shell layers is mainly due to the zeolite fraction reduction in the composites.
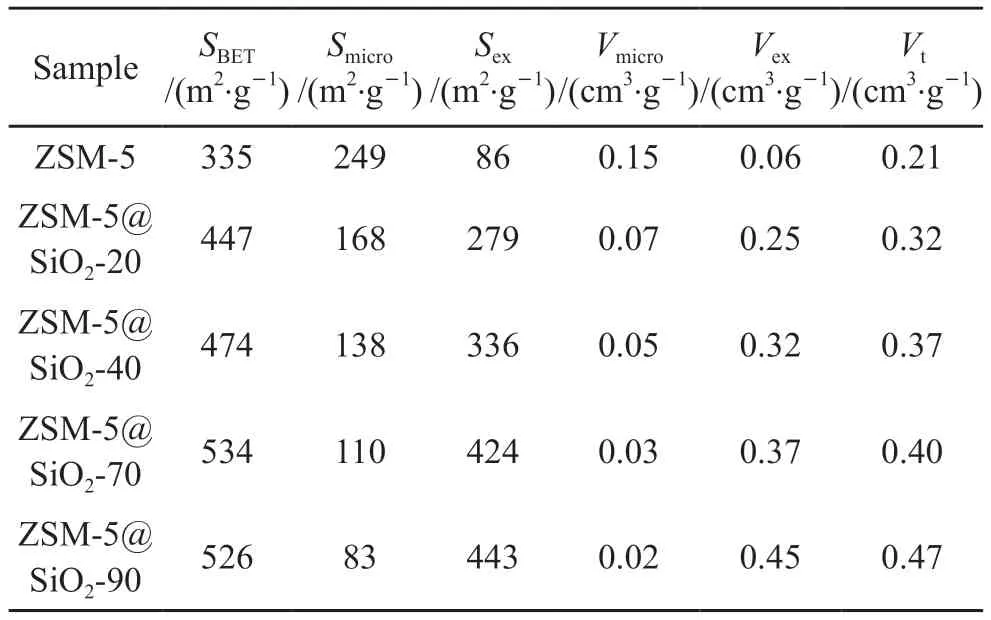
Table 1 Textural properties of the pristine zeolite ZSM-5 and the core-shell structured composite molecular sieve ZSM-5@SiO2-x

Fig.5 Pore size distribution of (a) ZSM-5,(b) ZSM-5@SiO2-20,(c)ZSM-5@ SiO2-40,(d) ZSM-5@ SiO2-70,and (e) ZSM-5@SiO2-90
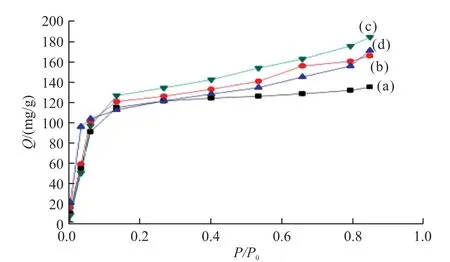
Fig.6 The butyraldehyde adsorption isotherms of (a) ZSM-5,(b)ZSM-5@SiO2-40,(c) ZSM-5@SiO2-70,and (d) ZSM-5@SiO2-90
3.2 Adsorptive property
The hazardous butyraldehyde was utilised to evaluate the absorption capability of these substances.The adsorption isotherm of pristine ZSM-5 for butyraldehyde exhibits a distinct increase at lowP/P0(generally displaying type I curves),demonstrating the existence of predominant micropores.For the ZSM-5@SiO2-xcomposite,there is a clear increase in adsorption observed across the tested range of relative pressure(P/P0≤ 0.9),in addition to a sharp uptake at lowP/P0,indicating a combined adsorption behaviour of micropores and mesopores.
The ZSM-5@SiO2-40 composites exhibit greater adsorption capacity for butyraldehyde (165.9 mg/g) compared to pristine ZSM-5 for butyraldehyde(134.7 mg/g),owing to the presence of a mesoporous silica shell.And with the increase of mesoporous shell layer,the ability of the ZSM-5@SiO2-70 composite to adsorb butyraldehyde (184.4 mg/g) increases.However,when the shell thickness exceeds 90 nm,the adsorption capacity of butyraldehyde (172.0 mg/g)decreases instead.This may be due to the larger pore size that is not suitable for adsorbing small molecules of butyraldehyde.
The above results indicate that the adsorptive capacity of ZSM-5 for pollutants is significantly enhanced after being coated with mesoporous silica shells,which is probably due to two reasons.Firstly,the pores of the silica shell are found to be ordered and perpendicular to the crystal faces of the zeolite core,which facilitates the diffusion of molecules from mesopores to micropores.Secondly,the composites exhibit a greater surface area for trapping molecules than the pure ZSM-5.
4 Conclusions
In conclusion,the core-shell structured ZSM-5@SiO2composites were synthesized by using TDA as a template and TEOS as a silica precursor.Pores of the silica shell are observed to be ordered and also perpendicular to the crystal-faces of zeolite cores.The obtained composites have compact meso-/micro-pore junctions to form a hierarchical pore structure and increase the specific surface area and total pore volume.The shell-thickness,the surface morphology and pore size distribution can be tuned by changing the mass ratio of TEOS/zeolite.After coating with mesoporous silica shells,the ZSM-5@SiO2-40 (165.9 mg/g),ZSM-5@SiO2-70 (184.4 mg/g) composites exhibit greater adsorption capacity for butyraldehyde compared to pristine ZSM-5 (134.7 mg/g) for butyraldehyde.However,when the shell thickness exceeds 90 nm,the adsorption capacity of butyraldehyde (172.0 mg/g)decreases instead.This may be due to the larger pore size that is not suitable for adsorbing small molecules of butyraldehyde.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Fabrication of YAG: Ce3+ and YAG: Ce3+,Sc3+ Phosphors by Spark Plasma Sintering Technique
- Preparation of Modified UiO-66 Catalyst and Its Catalytic Performance for NH3-SCR Denitration
- Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
- Ultraviolet Photodetector based on Sr2Nb3O10 Perovskite Nanosheets
- Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
- Bio-inspired Hydroxyapatite/Gelatin Transparent Nanocomposites
