Nickel Sulfide Modified NiCu Nanoalloy with Excellent Oxygen Evolution Reaction Properties Prepared through Electrospinning and Heat Treatment
LI Tao,LING Shuai,ZHONG Shujie,LOU Qiongyue
(Department of Materials Engineering,School of Mechanical Engineering,Chengdu University,Chengdu 610106,China)
Abstract: Ni2+/Cu2+/SO42-/polyvinyl alcohol precursor fibers with uniform diameters were prepared through electrospinning.Nickel-based composite nanoalloys containing Ni,Cu,and S were prepared through heat treatment in an Ar atmosphere.The experimental results show that the main components of the prepared nanoalloys are NiCu,Ni3S2,Ni,and C.The nanoalloys exhibit fine grain sizes about 200 -500 nm,which can increase with increasing heat treatment temperature.Electrochemical test results show that the nickel sulfidemodified NiCu nanoalloy composites exhibit excellent oxygen evolution reaction properties,and the oxygen evolution reaction properties gradually improve with the increasing heat treatment temperature.The sample prepared at1000℃for 40 min show a low overpotential of 423 mV and a small Tafel slope of 134 mV·dec-1 at acurrent densityof 10mA·cm-2.
Key words: NiCu;nanoalloy;Ni3S2;structure transformation;oxygen evolution reaction
1 Introduction
Since the beginning of industrialization,an increasing number of environmental problems,such as environmental pollution and climate warming,have emerged[1,2].The development and utilization of renewable energy have become an important subject in the international community,and hydrogen energy has emerged as the most promising green energy with a zero emission advantage.Studies are now focused on preparing electrolytic water for “green hydrogen”[3-6].In the research on the electrolysis of water to produce“green hydrogen,” noble metals with excellent electrocatalytic properties,such as Pt,Ru,and Ir,have been used for a long period[4,7-11].The high price and scarcity of these precious metals result in the high cost of electrolysis of water for producing “green hydrogen”,restricting its preparation in large quantities and its wide application in the downstream of industries.
Therefore,the research on hydrogen energy application has started focusing on developing low-cost and high-efficiency catalysts.Transition metals with unique electronic structures show excellent electronic transfer properties,and they have become an inexpensive substitute for noble-metal catalysts.The representative transition metals include iron,cobalt,nickel,copper,and molybdenum[12-14].The modern technology for the use of catalysts involves transforming transition metals into alloys[15-17],oxides[18,19],hydroxides[20,21],and other multielement compound materials[22-24].
In studies on the preparation of transition-metal catalysts,the catalytic performance can be improved by changing the crystal structure,reducing the grain size,and doping to form a heterojunction.The doping of transition-metal alloys with nonmetallic elements,such as P,S,C,and N[22,23,25-28,11],to form the corresponding compounds while generating heterostructures,such as the nickel diphosphide phase junction (c-NiP2/m-NiP2)[27]and Ni3S2/NiS heterojunction[26],can effectively improve the electrocatalytic performance of materials.Studies indicate that the sulfides in transition-metal multielement alloy compounds have inherent advantages,such as evident chemical stability,good conductivity,and special redox characteristics,which play important roles in promoting oxygen evolution reaction activity[28-31].Raniet alsynthesized three types of sulfide nanostructures via a solvothermal method and proposed the electrochemical mechanism of three transition-metal sulfides[28].Xiang Wet aldeveloped a simple one-step hydrothermal synthesis strategy for NiS2microspheres and suggested that Ostwald ripening plays an important role in the growth of NiS2[29].Chen Qet aldesigned the Fe-Co-Ni ternary transition-metal disulfide nanorod array using a one-step hydrothermal method,which exhibited a low overpotential (337 mV)and a small Tafel slope (77 mV·dec-1) at a current density of 50 mA·cm-2[30].Li Tet aldesigned a technology based on electrospinning and atmospheric heat treatment for Ni3Fe/Ni4S3/Ni/C mixed-crystal composites,and it resulted in excellent oxygen evolution reaction properties[31].
The above discussion indicates that in nickel-based catalyst systems[26-29],the formation of sulfide heterojunction through doping of sulfur on the basis of alloying design effectively improves the electrocatalytic performance.Cu is also an effective alloying element in nickel-based catalyst systems.NiCu alloy composite nanomaterials also exhibit a good electrocatalytic performance in Ni-based alloy catalysts[15,18],but the reports on NiCu alloy nanomaterials are few.Thus,a study of NiCu alloy composite nanocatalysts promotes the expansion of the application prospects of nonnoble metal catalysts.
Herein,polyvinyl alcohol (PVA) was used as a colloidal-forming agent mixed with nickel acetate and copper sulfate.NiCu nanocrystalline composite fibers modified with nickel sulfide were prepared through electrospinning and atmospheric heat treatment.The oxygen evolution reaction properties of Ni,Cu,and S multielement composite fibers were studied,and the results provide a technical reference for hydrogen production based on water electrolysis.
2 Experimental
2.1 Materials
Experimental raw materials,including nickel acetate (Ni(CH3COO)2),copper sulfate (CuSO4),and PVA,were all commercially available chemical reagents.Deionized water was made in the laboratory using a reverse-osmosis ultrapure water machine.
2.2 Apparatus
The experimental apparatus comprised the following: a high-temperature furnace (KMTF-1100-S-50-220),electrochemical workstation(CS350M),an electrothermal drying oven (DHG-9030A),an electrospinning machine (DP30),a reverse-osmosis water purifier (UPT-Ⅰ),a water bath(DK·98·Ⅱ),an auto stirrer (D25-2F),glassware.
2.3 Preparation process
The NiCu composite nanoalloy was prepared through the process depicted in Fig.1.First,11.11 g PVA powder was added to 100 mL deionized water to prepare a 10% mass fraction PVA aqueous solution,which was allowed to stand overnight to release bubbles.Then,3.584 g nickel acetate and 0.899-g copper sulfate were added to a 30 mL PVA aqueous solution with constant stirring in an 80 ℃ water bath until they dissolved and formed a spinning precursor sol.A total of 5 mL of the mixed solution was aspirated using a syringe equipped with a steel needle.Then,the syringe was fixed on the feeding device of the electrospinning machine.Electrospinning was conducted under the following conditions: voltage controlled at 25 kV,spinning distance of 15 cm,temperatures of 30-50 ℃,and feeding speed controlled at 0.001 mm/s.The as-prepared composite precursor fibers were collected using a steel plate and dried at 95 ℃ for 12 h.
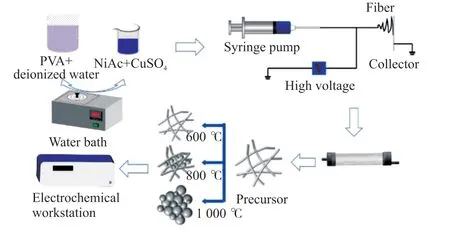
Fig.1 Preparation process of the NiCu nanoalloy via electrospinning and heat treatment
Ni2+/Cu2+/SO42-/PVA precursor fibers were obtained and placed in a quartz glass crucible,which was then placed at the center of the tubular furnace.Then,high-purity argon gas was fed into the furnace,and the argon gas flow rate was controlled at 200 mL/min.After 10 min of argon gas ventilation,the tube furnace was heated up from 25 ℃ at the heating rate of 5 ℃/min.The heat treatment temperatures of the samples were controlled at 400,600,800,and 1 000 ℃ and kept for 40 min.The heat-treated samples were naturally cooled to 300 ℃ and then rapidly cooled to room temperature by flowing air.Composite nanoalloys were obtained and respectively labeled as S-400,S-600,S-800,and S-1000.
After the prepared samples were ground into powder,the catalyst material ink comprising 5 mg sample powders,a 30 µL Nafion solution,200 µL ethanol,and 600 µL deionized water was ultrasonically stirred for 10 min.Then,4.6 µL catalyst material ink was evenly coated onto the platinum-carbon electrode,and the working electrode was formed from the mixed-crystal composite sample after baking and drying.
2.4 Characterization
The physical phase and crystal structure of the as-prepared samples were examined using an X-ray diffractometer (DX-2700B type) at a voltage/current of 40 kV/30 mA and a step angle of 0.06°.The morphology,microstructure,and elemental distribution of samples were characterized via scanning electron microscopy(SEM,Zeiss,Ultra 55).The characteristics of carbon components of the samples were examined through laser Raman spectroscopy (Invia),and the electrochemical properties of the samples were tested using an electrochemical workstation (CS350M).
3 Results and discussion
3.1 X-ray diffraction analysis
The X-ray diffraction (XRD) patterns of the samples are shown in Fig.2.Fig.2 indicates that all the samples had an evident diffraction convex packet at approximately 26°,which is the diffraction characteristic of amorphous carbon,indicating that the samples contained more amorphous carbon.Fig.2(a) shows that when the heat treatment temperature was approximately 400 ℃,according to PDF #04-0850 and PDF 04# 0836 card,the diffraction peaks exhibited strong diffraction peaks of metallic Ni and metallic Cu,but no evident diffraction peak of NiCu alloy was observed,which means a less NiCu alloying reaction.Figs.2(b)-2(d) show that the samples had strong and sharp diffraction peaks at 2-theta diffraction angles of 44.30°,51.55°,and 75.95°.According to PDF #04-006-6386 diffraction card,the prepared samples contained considerable amounts of NiCu alloy.The diffraction peaks at 2-theta diffraction angles of 44.30°,51.55°,and 75.95° corresponded to the typical (111),(200),and(220) crystal planes of the NiCu alloy,respectively.

Fig.2 XRD patterns of the as-prepared samples
Figs.2(c) and 2(d) reveal that the samples contained a certain amount of Ni3S2,according to the raw material formula and PDF#99-000-1494 diffraction card.However,the samples displayed in Figs.2(a) and 2(b) did not reveal substantial diffraction peaks of Ni3S2,which indicates that Ni3S2could not be effectively generated after the samples were treated at lower temperatures of 600 and 400 ℃.XRD analysis showed that the carbon-based nanoalloys primarily comprised amorphous carbon and compound grains of NiCu,Ni3S2,and Ni.Effective production Ni3S2is feasible only at high temperatures and in the presence of a large amount of carbon.The amorphous carbon formed from the decomposition of organic components in the system is an important reducing agent.Ni3S2was formed by the comprehensive reaction of carbon with some SO3and Ni,which were produced by the decomposition of reactants,with the chemical reaction process designed as follows:
The XRD data of the full width at half maximum (FWHM) and integral strength of the (111),(200),and (220) planes of the samples at different heat treatment temperatures are presented in Tables 1 and 2,respectively.Table 1 shows that the XRD FWHM gradually decreased with increasing heat treatment temperature,and the range of reduction became more evident from 600 to 1 000 ℃.Table 2 reveals that the integral strength of the XRD peak gradually increased with increasing heat treatment temperature.The results indicate the heat treatment temperature considerably influenced the crystallization degree of the NiCu alloy samples prepared via thermal decomposition.
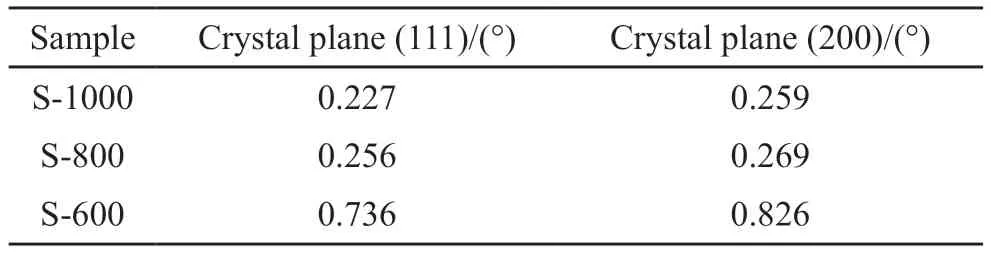
Table 1 FWHM data of the as-prepared samples
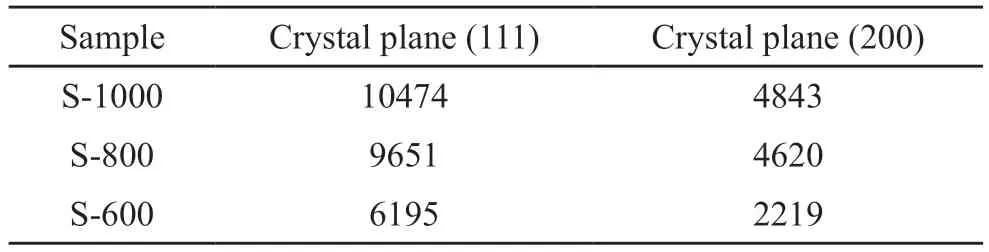
Table 2 Integrated intensity of the as-prepared samples
The variation in the crystal plane space of the(111) and (200) crystal planes of NiCu alloy are shown in Table 3.Table 3 shows that NiCu alloy was effectively formed,and the crystal plane spacesdNiCu(111)anddNiCu(200)increased slightly relative todNi(111)anddNi(200)for metallic Ni but decreased slightly relative to the dCu(111)and dCu(200)for metallic Cu.As the atomic radius of Ni is smaller than that of Cu,when the NiCu alloy continuous solid solution was formed,the crystal plane space of nickel atoms was increased,but that of copper atoms was decreased.
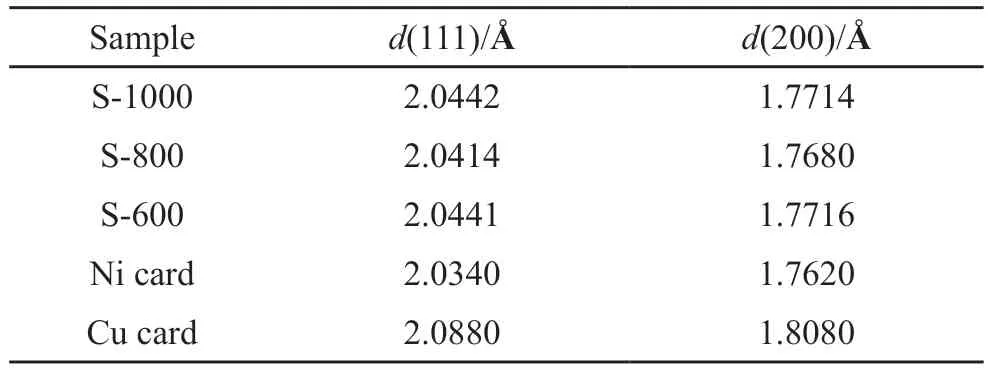
Table 3 Crystal plane space of the as-prepared samples
Based on the XRD diffraction peaks of the samples prepared at different temperatures,the grain size was calculated using the Debye-Scherer formula:
where,the Debye-Scherer constantkis 0.89,the XRD wavelengthλis 0.154 nm,andβisthe FWHM (radians).NiCu alloy was not formed efficiently of sample S-400,so the grain size was not calculated.With (111)and (200) crystal planes of S-600,S-800,and S-1000 samples as target peaks,the average grain sizes were 11.038 (S-600),32.77 (S-800),and 35.513 (S-1000)nm,respectively.The calculation results show that the fine grain sizes of the prepared samples.In addition,the grain size increased gradually with the increase in heat treatment temperature,which indicates that the crystallinity increased gradually with the increase in heat treatment temperature.
3.2 SEM and energy-dispersive spectroscopy analysis
The SEM image of precursor fibers is shown in Fig.3(a).The SEM images of samples S-600,S-800,and S-1000 are shown in Figs.3(b)-3(d),respectively.
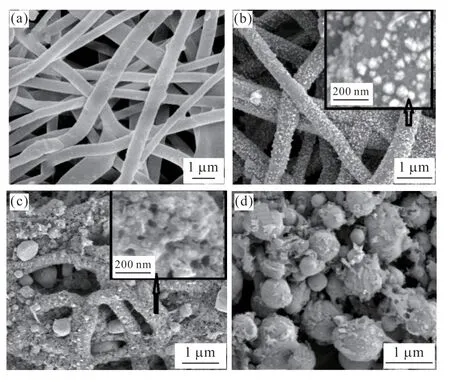
Fig.3 SEM images of the samples prepared via heat treatment: (a)precursor fibers,(b) S-600,(c) S-800,and (d) S-1000
Fig.3(a) indicates that the precursor fibers had a uniform diameter (0.5-1 µm) and a smooth fiber surface.Fig.3(b) reveals that sample S-600 maintained an evident fiber shape after the precursor was heat treated at 600 ℃.The fiber diameter was uniform at approximately 500 nm,and the fiber surface was very rough.The image embedded in a small window showed that many fine particles were attached to the fiber surface,and their sizes were in the range of 30-50 nm.Fig.3(c)shows that the morphology of the original fibers changed considerably after heat treatment at 800 ℃.Some fibers maintained a good morphology,and some did not.Many smooth spherical particles and coarse particles were observed on the surface of the fiber body.The inset image indicates that numerous micropores were generated on the surface of fibers with pore sizes of approximately 20-50 nm.Fig.3(d) reveals that after the precursor fibers were heat treated at 1 000 ℃,the original fiber shape no longer existed.All particles became spherical,the sizes were not uniform,and some debris attachments were observed on the surface of the spherical particles.
The changes in the carbon content of the samples was preliminarily determined via energy-dispersive spectroscopy (EDS).The mass percentage contents were 45.85% (S-600),29.82% (S-800),and 15.05% (S-1000).The data show that the carbon content decreased rapidly with the increase in heat treatment temperature.Comprehensive analysis of EDS data and SEM images revealed that the carbon content had an important effect on the integrity of fiber morphology.
The images of EDS and mappings of Ni,Cu,and S are shown in Figs.4(a)-4(c),respectively.The image of EDS and mappings of sample S-600 are shown in Fig.4(a).Fig.4(a1) shows that the ratio of Ni/Cu was approximately 3.35.Figs.4(a2)-(a5) reveal that Ni,Cu,and S were uniformly distributed in the fibers.Figs.4(b1) and 4(b2) display that the ratios of Ni/Cu were approximately 3.62 in the coarse particles and 3.60 in the body fibers,respectively.Figs.4(b3)-4(b6) reveal that Ni,Cu,and S were also uniformly distributed in the fibers and coarse particles.Fig.4(c1)shows that the ratio of Ni/Cu was approximately 3.77.Figs.4(c2)-4(c5) illustrate that the precursor fibers were transformed into spherical particles,and Ni,Cu,and S were still evenly distributed in the spherical particles.Figs.4(a1),(b1),(b2),and (c1) indicate that the ratio of Ni/Cu gradually increased and eventually approached the proportion of formula design of 4.0.
The results of SEM,EDS,XRD,and mapping analyses indicate that the morphology of PVA precursor fibers rich in Ni,Cu,and S gradually evolved from fibrous to granular with the increase in heat treatment temperature and were uniformly distributed in the sample,forming a composite crystal with multiple coexistence of NiCu,Ni3S2,Ni,and C.
3.3 Raman analysis
The Raman properties of samples were detected using a laser Raman spectrometer at the detection wavelength of 514.5 nm,and the detection results are shown in Fig.5.As shown,two evident peaks appeared at 1 350 and 1 600 cm-1and were labeled as D and G bands,respectively.The D band corresponds to the peak band induced by structural defects,and the G band refers to the structural characteristic peak caused by carbon sp2hybridization.TheID/IGratio is an important parameter used to estimate the degree of carbon material defects[32].TheID/IGratios of samples S-600,S-800,and S-1000 were 0.61,0.71,and 0.87,respectively.The results indicate that the carbon in the samples tended to be disordered as the heat treatment temperature increased,the degree of defects increased with temperature due to the influence of generation,and grain structural changes in metallic elements and sulfides gradually increased again.

Fig.5 Raman spectra of the prepared samples
3.4 Analysis of electrocatalytic properties
A standard three-electrode system with a solution of 1.0-M KOH was used to test the electrocatalytic performance of NiCu alloy composite.The work electrodes were prepared in accordance with the electrode fabrication process using the multiphase composite materials of S-600,S-800,and S-1000.
LSV was used to test the changes in the current density with potential during the catalytic process of electrode materials,and the results are shown in Fig.6.Fig.6(a) shows that the LSV curves of sample S-1000 had an oxidation reaction peak near 1.45 V,which exhibited a small oxidation current,indicating the reaction of nickel to the higher valence state.Meanwhile,the S-1000 sample showed the best oxygen evolution reaction activity among samples.Fig.6(b) reveals that the S-1000 sample had an overpotential of 423 mV at a current density of 10 mV·cm-2,which is lower than those of samples S-800 (475 mV) and S-600 (539 mV).As shown in Fig.6(c),the Tafel slope of S-1000 was 134 mV·dec-1,which is lower than those of S-800 (159 mV·dec-1) and S-600 (160 mV·dec-1).The lower Tafel slope indicates that S-1000 favored the catalyze reaction kinetics in an alkaline medium.Electrochemical impedance spectroscopy was performed to investigate the charge transfer behavior inside S-600,S-800,and S-1000.The radius of the semicircle in the high-frequency region of the Nyquist plot determines the charge transfer resistance (Rct) of catalysts,and a smallRctindicates a fast reaction rate.Fig.6(d) shows that the sample catalyst of S-1000 had a smallRct.
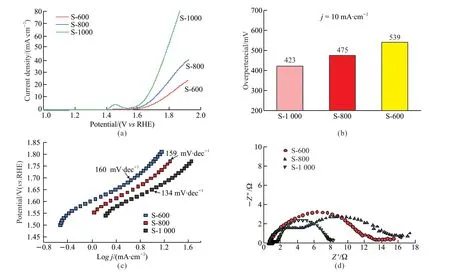
Fig.6 Results of electrochemical performance analysis of the samples: (a) Linear sweep voltammetry (LSV) curves;(b) Overpotential, j=10 mA·cm-2;(c) Tafel curves;and (d) Impedance diagram
4 Conclusions
A single system of multielement in situ composite was designed.The alloying and vulcanization reactions were realized via a one-step precursor atmospheric heat treatment.The composites of NiCu,Ni3S2,Ni,and C were prepared,which provide a simple and reliable technical scheme for the research of transition-metal electrode materials.
The atmospheric heat treatment system has an evident influence on the composition and morphology of composite nanoalloys of NiCu,Ni3S2,Ni,and C.High heat treatment temperatures can make the alloy combination closer and the crystal development complete.With the increase in heat treatment temperature,the uniform fibrous morphology gradually changes to a spherical granular morphology and the spherical particles gradually change from small to large and finally into more uniform spherical composite particles.The transformation mechanism from fibrous structure to spherical particle structure is explored.
The hybrid composite nanomaterials of the as-prepared NiCu alloy,with coexisting Ni3S2,Ni,and C,showed good electrocatalytic activity in the alkaline system of oxygen evolution reaction.At a current density of 10 mA·cm-2,the catalyst of S-1000 showed a relatively low overpotential of 423 mV,small Tafel slope of 134 mV·dec-1,and a small charge transfer resistance.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Fabrication of YAG: Ce3+ and YAG: Ce3+,Sc3+ Phosphors by Spark Plasma Sintering Technique
- Preparation of Modified UiO-66 Catalyst and Its Catalytic Performance for NH3-SCR Denitration
- Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
- Ultraviolet Photodetector based on Sr2Nb3O10 Perovskite Nanosheets
- Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
- Bio-inspired Hydroxyapatite/Gelatin Transparent Nanocomposites
