Structural Transformations and Multiferroic Properties of Bi1-xEuxFe0.95Co0.05O3 (x=0.05,0.10,0.15,and 0.20)
LIANG Xiuqian ,LI Yongtao,* ,LIU Liqing ,GUO Aiqian ,LI Jianda ,ZHANG Hongguang
(1.College of Science,Nanjing University of Posts and Telecommunications,Nanjing 210023,China;2.College of Electronic Science and Engineering,Nanjing University of Posts and Telecommunications,Nanjing 210023,China)
Abstract: Bi1-xEuxFe0.95Co0.05O3 (x=0.05,0.10,0.15,and 0.20) nanoparticles were prepared through the sol-gel technique.Its structure,local electronic structure,magnetic and electric properties were systematically investigated.X-ray diffraction data show (104),(110) bimodal alignment and high angular migration,indicating that with the increase of Eu substitution at Bi site,the structure of BFO undergoes a continuous change in crystal structure.The hysteresis loop and the FC/ZFC curve show how magnetism varies with the size of the field and temperature.Finally,the causes of magnetic changes were analyzed by studying SXAS and hysteresis loops.
Key words: magnetic materials;XAS;electric structure;magnetic property
1 Introduction
Multiferroics are an interesting group of materials that exhibit both ferroelectricity and ferromagnetism with coupled electric and magnetic order parameters.These materials have been used in many devices,ranging from large devices like electrical transformers to small devices like sensors,and in integrated circuits and as storage devices,due to their interesting physical,chemical,and mechanical properties[1-4].BiFeO3(BFO) is a widely studied multiferroic material for its above-room-temperature ferroelectric Curie temperature (TC~ 1 103 K) and its antiferromagnetic Néel temperature (TN~ 643 K)[5].BFO is currently the only known ABO3-type simple perovskite that exhibits room-temperature multiferroism.It is therefore considered to be the most promising candidate for practical applications of multiferroics[6].
The magnetism of BFO is a long-standing problem,and various controversial issues have arisen from recent research on this material[7,8].It is reported that BFO has G-type antiferromagnetic (AFM) order,in which each atom is surrounded by six other atoms with opposite spins.The wavelength of the linear spin structure is λ=62 nm and is not matched with the lattice wavelength.This results in low magnetization,high leakage current and low ME coupling[9].None of the systems based on it exhibits strong coupling between electrical polarization (P) and magnetization (M) near room temperature,which is important for practical applications[10-12].Improvement in the magnetization of BFO can help in utilizing the room-temperature multiferroicity of BFO for practical applications[13,14].
Doped BFO can improve its ferromagnetism,for example,BFO nanofibre materials doped Sm have higher dielectric constants and magnetization[15].The results of this research are expected to facilitate the performance enhancement of BFO in applications such as magnetic memory devices and sensors.li16]et alshowed that the magnetic properties of BiFeO3were enhanced by Co doping with good magnetic recovery and stability,which opened a new route for the synthesis of BiFeO3with excellent photocatalytic activity and good magnetic recovery.
There appear to be only a few reports on the codoping effect of Eu and Co in BFO focusing on its local structure and magnetic properties[17-20].In this paper,we report the preparation of Bi1-xEuxFe0.95Co0.05O3multiiron nanoparticles and the effects of co-doping on their structure,local electronic structure and electrical properties,as well as the dependence of magnetic properties on temperature and magnetic field.
2 Experimental
Polycrystalline samples of Bi1-xEuxFe0.95Co0.05O3(BEFC) (x=0.05,0.10,0.15,and 0.20) were prepared by a sol-gel method.Analytical grade Bi2O3,Eu2O3,Fe(NO3)3·9H2O,and Co(NO3)2were used as starting materials.These materials were stirred in diluted nitric acid in a stoichiometric ratio according to the normal compositions until the solution became clear.Tartaric acid was added as a complexing agent.The precursor solution was mixed thoroughly and baked at 120 ℃ for 4 hours,then dried at 80 ℃ in an oven for 24 h,and then pre-sintered at 250 ℃ for 2 hours.Afterward,the pre-sintered powder was annealed at 600 ℃ for 2 hours in air to yield BEFC samples.A homogeneous solution of precursors in the appropriate molar ratio was processed to obtain sample powders.
X-ray diffraction (XRD,D/Max 2000) with Cu-Kαradiation was used for the phase analysis.Magnetic hysteresis loops were recorded on a vibrating sample magnetometer (VSM) integrated in a physical property measurement system (PPMS-9,Quantum Design).The ferroelectric hysteresis loops of the samples were measured using a Precision Premier II (Radiant Technology,USA).The measurement of X-ray absorption spectrum (XAS) of Fe and Co core level in total electron yield mode were performed at room temperature at the Beijing Synchrotron Radiation Facility (BSRF).
3 Results and discussion
The XRD patterns of the samples of Bi1-xEuxFe0.95Co0.05O3are displayed in Fig.1.All the peaks of the patterns are indexed in different crystal systems and cell configurations with observed peak position.It can be seen that all the samples exhibit single-phase characteristics with no trace of other impurity phases (eg,Eu2O3,Bi2Fe4O9,and Bi25FeO39),which suggests that Eu and Co ions are incorporated into the BFO crystal structure[21].
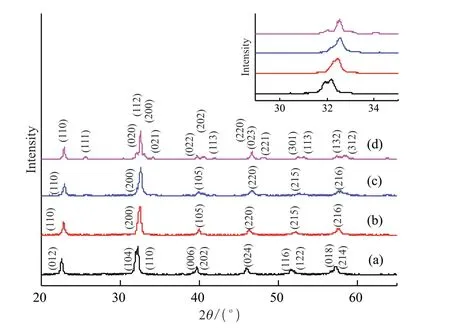
Fig.1 Room-temperature XRD patterns of the samples of Bi1-x EuxFe0.95Co0.05O3: (a) x=0.05;(b) x=0.10;(c) x=0.15;(d) x=0.20
A slight shift of the diffraction peak near 32 degrees to a higher angle can be observed with increasing doping concentration of Eu ions,as shown in the inset of Fig.1.This is because the radius of the Bi ion (0.117 nm) is larger than that of the Eu ion (0.107 nm).The lattice structure of the compound withx=0.05 is identical to that of BFO,which shows that (Eu,Co) co-doping in small quantity is ineffective in changing the lattice structure of the material.The diffraction peaks in the patterns of 0.10 ≤x≤ 0.15 samples characterize a polycrystalline rhombohedrally distorted perovskite structure with a space group R3c,and the doubly split peaks like (104) (110) partially merge to form a broadened peak as the Eu concentration increases,as shown in the inset of Fig.1.Furthermore,some other new peaks can be observed for thex=0.20 sample.It is obvious that the XRD patterns are close to that of orthorhombic EuFeO3,with Pn21a space group[22].All these results together suggest that a structural transformation with a continual change of crystal structure occurs in BFO when Eu substitution at the Bi site is increased.
Fig.2 shows the magnetic hysteresis loops for the Bi1-xEuxFe0.95Co0.05O3samples at 5 and 300 K.At room temperature,the saturation magnetization (Ms) and the remanent magnetization (Mr) decrease with increasing Eu doping rate.When the doping ratio is 0.05,the highest saturation magnetization is 1.13 emu/g,and the highest remanent magnetization is 0.30 emu/g.It is worth noting that when the doping ratio of Eu was 0.1,the coercivity (Hc) showed a rapid jump,but when the doping ratio further increased to 0.15,the coercivity dropped back to almost the original value.At 5 K,the magnetic changes are quite different.At this low temperature,the remanent magnetization and coercivity increase with an increase of doping proportion,while the saturation magnetization fluctuates up and down in a certain range,reaching a maximum value of 1.20 emu/g when the doping proportion is 0.10.Detailed data are shown in Table 1.
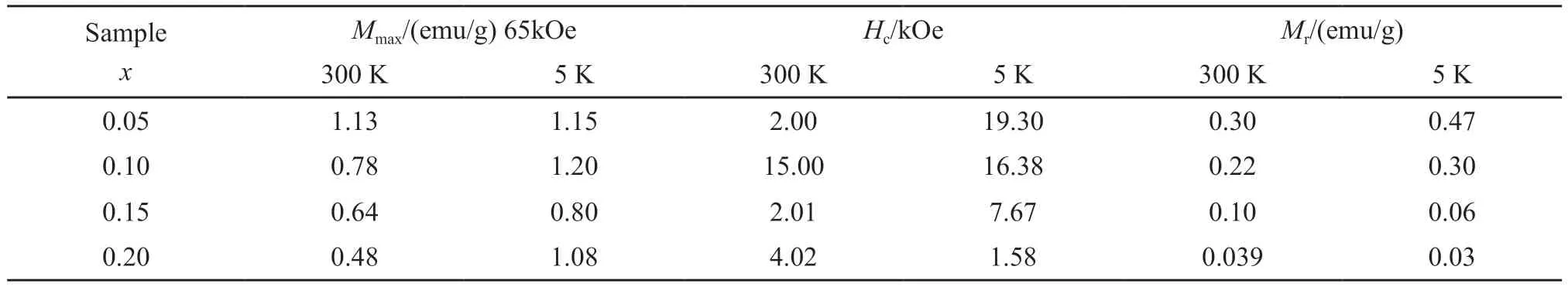
Table 1 Magnetization at high feild (Mmax),coercivity (Hc),and remanence (Mr) of the Bi1-xEuxFe0.95Co0.05O3 (x=0.05,0.10,0.15,and 0.20)samples
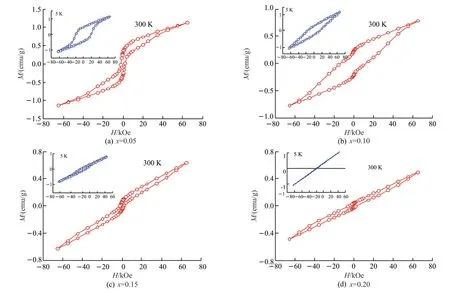
Fig.2 M-H curves for Bi1-xEuxFe0.95Co0.05O3 at room temperature and at 5 K
To understand the origin of the observed ferromagnetism in BiFeO3ceramic,zero-field-cooled (ZFC)and field-cooled (FC) temperature-dependent magnetization curves were measured at 5 000 Oe from 5 to 300 K,as shown in Fig.3.In these measurements,the sample is cooled to the desired temperature in the ZFC and then a magnetic field or FC is applied.In both cases,measurements are made while the sample is heated in a magnetic field[23,24].In previous studies,though the magnetization was small in BFO compared with the doped samples,there was a steep rise at lower temperatures.A notable feature is that the magnetic behavior of thex=0.2 sample is completely different from the others.It exhibits very low magnetism at high temperature and rapidly increases below 50 K,as shown in Fig.3(d).This may be due to a phase transition in the crystal structure that causes the Curie temperature of the sample to become lower and the crystal to exhibit paramagnetism when the temperature exceeds the Curie temperature,as shown by XRD results.

Fig.3 FC and ZFC M-T curves for a field of 5 000 Oe for Bi1-x EuxFe0.95Co0.05O3 samples
The SXAS spectra of Fe 2p and Co 2p core levels of the prepared samples are shown in Fig.4.The 2p3/2binding energy values of Fe3+and Fe2+oxidation states are 711 and 709 eV[25,26],respectively.The binding energy of 2p3/2peaks is above 712 eV,indicating the existence of mixed valence states of iron (Fe2+and Fe3+).Notably,the peak binding energy (713.4 eV) of 2p3/2atx=0.10 moved toward the side with higher photon energy compared with the sample atx=0.05 (712.8 eV),indicating an increase in the overall mixed valence state of Fe,and then returning to the original position,which is in line with the magnetic change results and explains the increase in saturation magnetization atx=0.10.In the Co 2p core level,there are two peaks for each sample,which are caused by spin-orbit coupling.In thex=0.05 sample,a satellite peak at 790.2 eV was observed,but it disappeared when the Eu doping ratio was higher.In general,the excitation peaks of the oxidation state are more obvious than those of the metal state,which may indicate that more Co is reduced as the Eu doping ratio increases.
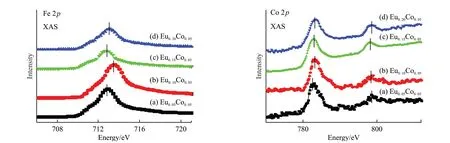
Fig.4 The soft X-ray absorption spectra of Fe and Co 2p core levels in samples of Bi1-xEuxFe0.95Co0.05O3 (x=0.05,0.10,0.15,and 0.20)
Fig.5 shows the polarization electric field hysteresis loops of the samples tested at room temperature.It can be clearly seen from the figure that P-E loops of(Eu,Co) co-doped samples reach saturation,indicating the existence of a ferroelectric effect[27].At the same time,the hysteresis loops of the samples do not have obvious downward bending,which may be due to a small leakage current.According to previous studies,fewer Fe2+ions indicate fewer oxygen vacancies,resulting in lower leakage current[28,29].
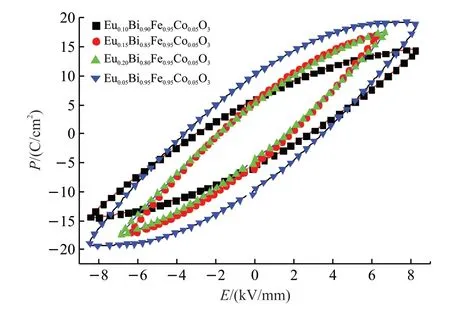
Fig.5 Polarization-electric field (P-E) hysteresis loops of samples of Bi1-xEuxFe0.95Co0.05O3 (x=0.05,0.10,0.15,and 0.20)
4 Conclusions
In summary,we prepared Bi1-xEuxFe0.95Co0.05O3(x=0.05,0.10,0.15,and 0.20) nanoparticles by a solgel method,then studied the structure,local electronic structure,and the magnetic and electrical properties of the nanoparticles in detail.Hysteresis loops and FC/ZFC show the change of magnetization with magnetic field intensity and temperature.Based on analysis of XRD and XAS and hysteresis loop results,we believe that the magnetic change of the sample can be attributed to the following,with an increase of Eu doping concentration,a structural phase transition occurs,and the presence of mixed valence states of Fe3+and Fe2+and the constant change of their proportion imply a change of leakage current and oxygen vacancy.
Acknowledgements
All the soft X-ray absorption spectroscopy measurements of the samples Bi1-xEuxFe0.95Co0.05O3were carried out at BSRF.This work is financially supported by the research project of Nanjing University of Posts and Telecommunications under contracts Nos.NY217096 and NY213124.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Fabrication of YAG: Ce3+ and YAG: Ce3+,Sc3+ Phosphors by Spark Plasma Sintering Technique
- Preparation of Modified UiO-66 Catalyst and Its Catalytic Performance for NH3-SCR Denitration
- Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
- Ultraviolet Photodetector based on Sr2Nb3O10 Perovskite Nanosheets
- Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
- Bio-inspired Hydroxyapatite/Gelatin Transparent Nanocomposites
