Synthesis of multifunctional additives for solid propellants: Structure,properties and mechanism
Pingn Zhng , Lin Sun , Jinmin Yun , Jinru Deng ,*
a College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, PR China
b College of Material Science and Engineering, Hunan University, Changsha 410082, PR China
c Hu Bei Sanjiang Aerospace Jianghe Chemical Technology Co., Ltd, Yichang 444200, PR China
Keywords:Hydroxyl-terminated polybutadiene (HTPB)HTPB propellant Chemical modification Bonding agent Mechanism
ABSTRACT To simplify the composite propellant formulation and address the current issue of the singlefunctionality present in existing additives, the multi-cyano, amine-based polybutadiene (AEHTPB-CN)was prepared based on AEHTPB by adopting appropriate synthesis strategies.By replacing 10% of HTPB binder in the propellant formulation,it can effectively enhance the interfacial bond strength between the propellant binder matrix and solid fillers (AP (ammonium perchlorate) and RDX (cyclotrimethylenetrinitramine)), the mechanical properties of the HTPB/AP/RDX/Al propellant were superior to blank control propellant with an improvement of 35.4%in tensile strength,62.0%enhancement in elongation at break, and reduce the propellant burn rate by 10.7% with any energy loss.The function mechanism of AEHTPB-CN was systematically elucidated through experiments and computer simulation techniques.The results show that the tertiary amine group in AEHTPB-CN can react with AP to form ammonium ionic bonds, and the hydroxyl and cyano groups can form hydrogen bonding interactions with AP, which enables AEHTPB-CN to be firmly adsorbed on the AP surface through chemical and physical interactions.For RDX, the interfacial bonding effect of AEHTPB-CN is attributed to their ability to form C-H···N≡C weak hydrogen bonding interaction between the cyano group and RDX methylene group.
1.Introduction
HTPB propellant is a type of composite propellant with hydroxyl-terminated polybutadiene (HTPB) as the polymeric binder,and it is one of the most widely used composite propellants[1-5].From the perspective of the overall composition, HTPB propellant is mainly composed of two parts:binder and solid fillers,in which the HTPB binder usually only accounts for 8%-10% of the mass of the propellant, while the content of solid fillers (ammonium perchlorate(AP),nitramine,aluminum powder)can be up to 88%-90%.Besides,to improve the performance of propellants,it is necessary to add various functional additives such as bonding agents [6,7], plasticizers [8,9], and burning-rate modifiers to the propellant formulation [10,11].
Among these additives, bonding agents are critical to the performance of the propellant.These bonding agents contain functional groups such as hydroxyl,amino,cyano,etc.which ameliorate the mechanical properties of propellant by forming physical or chemical interactions between the solid fillers and binder matrix of composite propellants[12,13].Usually,the bonding agents used in HTPB propellants are aziridine, polyamine, hydantoin, and their derivatives, but these existing bonding agents have a single function and poor adaptability to different fillers.Therefore,to achieve better performance, several bonding agents need to be used in combination, which makes the propellant composition more complicated and the propellant quality difficult to control.More importantly, the antagonism between components and thermodynamic incompatibility with the binder matrix will reduce the effect of various additives.
In recent years,the functional additives of composite propellant are developing in the direction of multi-functionality, and many researchers have conducted valuable explorations in this domain[14,15].Our research group reported a novel approach to the functionalization of HTPB to multi-amine,hydroxy polybutadienes(AEHTPB)by chemical modification[16].When added to the HTPB propellant formula, the hydroxyl groups in the AEHTPB molecule can increase the crosslink density of the binder matrix through the curing reaction, and the amine groups can react with AP filler to form strong chemical bonds, thus firmly binding to the particle surface.What is even more surprising is that the AEHTPB not only can effectively reduce the propellant burning rate but also has natural miscibility with HTPB.However, the application effect in the nitramine-based composite propellant is not significant.Studies have shown that cyano groups are effective bonding groups for nitramine fillers and cyano-containing bonding agents are often added to propellant systems to enhance the binder-nitramine filler interaction,but the mechanism of their interaction remains unclear[17,18].
On this basis, this study will adopt appropriate chemical methods to introduce cyano groups into the molecular structure of AEHTPB to prepare multi-cyano, amine-based polybutadiene(AEHTPB-CN) that has the bonding effect on AP and nitramine fillers, to further broaden the application of HTPB-based modified multifunctional additives.This paper has described the synthesis,characterization,and performance of AEHTPB-CN in the composite propellant.Also,the interaction mechanism of AEHTPB-CN with AP and RDX (cyclotrimethylene-trinitramine) was systematically elucidated through experiments and computer simulation techniques.
2.Experimental and computational section
2.1.Materials
The multi-amine,hydroxy-based polybutadiene(AEHTPB)used has a molecular weight(Mn)~5685 g/mol and a hydroxyl value of 193.5 mg of KOH/g, was synthesized according to the method reported in our previous work.Acrylonitrile,Isophorone diisocyanate(IPDI), and t-BuOK were procured from Sinopharm Chemical Reagent Co.Ltd (China).Ammonium perchlorate (AP,100 mesh) and hexogen (RDX, 100 mesh) were kindly provided by Jianghe State Chemical Plant (China).All other chemicals were at the highest purity available and used without further purification.
2.2.Synthesis of AEHTPB-CN
The AEHTPB-CN was prepared through the AEHTPB reaction with acrylonitrile in the presence of t-BuOK (Scheme 1).For this,AEHTPB (30 g) was dissolved in a toluene solution (150 mL) and introduced into the reactor, which was submerged in a wellcontrolled-temperature water bath.Then, 0.3 g t-BuOK (dissolved in 15 mL t-BuOH solution) and an appropriate amount of acrylonitrile were added successively.The reaction was carried out at 45°C for 8 h.At the end of the reaction, the cooled solution was filtered and the filtrate was allowed to evaporate under a vacuum at 90°C.The product is a reddish-brown viscous liquid with a yield of 98%.
2.3.Structural characterization
Fourier transform infrared (FTIR) spectra were recorded on a Nicolet IS10 FTIR spectrometer in the range of 500-4000 cm-1,and the polymers were coated on the KBr disk to form the film.1H NMR spectra were recorded using a Bruker Avance-III 400 (400 MHz)spectrometer in CDCl3solvent.
2.4.Preparation of elastomers
The elastomer based on HTPB/AEHTPB-CN mixed binder was prepared with curing agent IPDI at a stoichiometry ratio(NCO/OH)of 1.0 without any catalyst.Firstly, HTPB and AEHTPB-CN were stirred evenly at 50°C and mixed with a calculated amount of the IPDI.Then, the above mixture was poured into a polytetrafluoroethylene mold and vacuumed for 30 min at 40°C to remove bubbles.After that,the mixture was cured at 60°C for 7 days.The cured elastomers were cut into appropriate-size specimens for mechanical performance testing.
2.5.Preparation of composite propellants
Table 1 provides the detailed composition of the HTPB-based propellant.Briefly, the procedure for the preparation of propellant is as follows: The AEHTPB-CN was first mixed with HTPB at 50°C in the mixer.Then, the mixture was moved to Vertical Kneader, and various fillers were added gradually with stirring.In the subsequent phase, the required amount of curing agents was added and mixed thoroughly, followed by degassing in a vacuum oven at 40°C and curing at 60°C for 5 d.At last, the cured propellant samples were tested for mechanical properties.
2.6.Mechanical property
The static mechanical properties of the cured elastomers and propellants were evaluated using Universal Testing Machine (INSTRON Model 5582).Dumbbell specimens were cut following ASTM D412 and GJB770B-2005 standard methods, and the tests were performed with a strain rate of 100 mm/min at room temperature.For each sample,at least three specimens were tested to check the reproducibility.The mechanical properties including maximum tensile strength (σ) and elongation under maximum tensile strength (ε) were recorded.
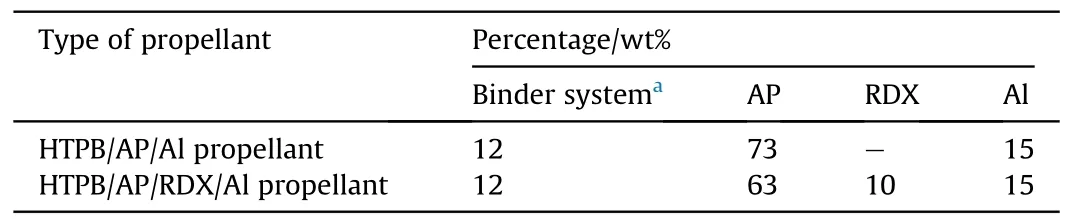
Table 1 The composition of the propellant.
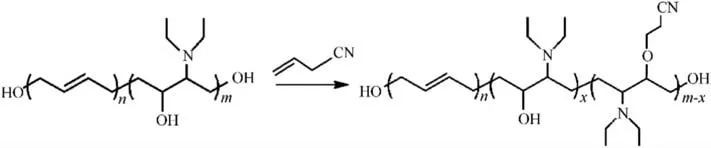
Scheme 1.Synthetic route for AEHTPB-CN.
2.7.Microscopic study
The fracture morphology of propellant specimens was examined by a scanning electronic microscope (Hitachi S-4800, Japan)instrument operated at 15 KV.
2.8.Quantum chemical calculations
Density functional theory(DFT)calculations were performed to investigate the electrostatic potentials of RDX and AEHTPB-CN molecules using the Gaussian 09 program.According to the calculation results of electrostatic potential,we analyze the binding site of AEHTPB-CN and RDX molecule and construct binary complex structure models.The complex structures were then optimized using the B3LYP functionals and 6-311++G(d,p)basis set with the DFT-D correction method [19-23].Single-point calculations were performed on all optimized geometries and the interaction energies of all complexes were calculated to quantify the adsorption affinity between AEHTPB-CN and RDX molecular.
2.9.Molecular dynamics simulation
Molecular dynamics simulations were performed to study the interaction between the AEHTPB-CN and RDX crystal using Material Studio.In this paper,the crystal parameters of RDX used in the simulations were derived from the X-ray diffraction experiment and the RDX(1 0 0)surface was selected as the adsorption surface[24].The COMPASS force field was chosen to describe the adsorption system, which has been applied successfully in nitramine explosives and propellant systems[25-28].All MD simulations were subsequently performed in the NVT ensemble with a time step of 1 fs.The temperature and the pressure were maintained by the Nosˊe-Hoover thermostat and Andersen barostat, respectively.Each simulation ran for a total of 10 ns and the last 1 ns of which was sampled every 100 fs for the calculation of ensemble averages.
3.Results and discussions
3.1.Structure characterization
The FTIR spectra of AEHTPB and AEHTPB-CN are enumerated in Fig.1.Comparing the spectra, it can be found that the absorption peak due to-CN stretching appeared at 2245 cm-1in AEHTPB-CN,and the sharp peak observed at around 1110 cm-1attributed to the-C-O-C stretching vibrations.Meanwhile, the absorption peak centered at 1070 cm-1,which corresponds to the secondary alcohol(C-O, stretching vibration), as well as the hydroxyl group broad signal at 3400-3600 cm-1of AEHTPB are decreased, indicating AEHTPB was cyanoethylated by reaction with acrylonitrile.1H NMR analysis further confirmed the structure of AEHTPB and AEHTPBCN, the corresponding assignments of chemical shifts (δ ppm)from1H NMR are presented in Fig.2.
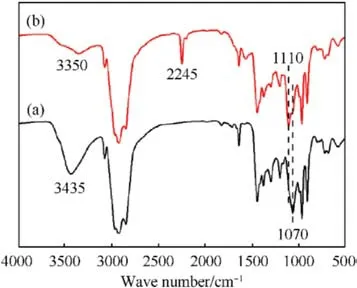
Fig.1.FTIR spectra: (a) AEHTPB; (b)AEHTPB-CN.
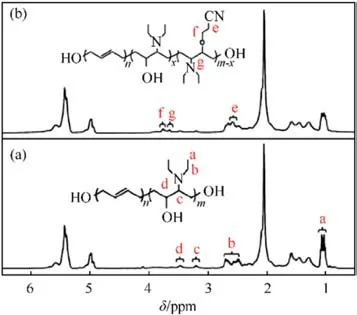
Fig.2.1H NMR spectra: (a) AEHTPB; (b)AEHTPB-CN.
The physicochemical properties,such as density,viscosity,glass transition temperature(Tg),hydroxyl value,and amine value of the product were measured.The results are displayed in Table 2.
It can be seen from Table 2 that the viscosity of AEHTPB-CN is lower than that of AEHTPB.The reason is that the hydroxyl group content in the AEHTPB-CN decreases,which reduces the hydrogen bond and the interaction between molecules.Besides,the increase in molecular weight makes the amine value of the AEHTPB-CN lower than AEHTPB.
3.2.Mechanical properties
The stress-strain curves of elastomers with different mass ratios of AEHTPB-CN/HTPB were exhibited in Fig.3.It can be observed that the tensile strength of the elastomer increased with the AEHTPB-CN content, while the elongation at break gradually decreases, indicating that the addition of AEHTPB-CN has a strengthening effect on the elastomer.The mechanical properties of AEHTPB-CN/HTPB elastomers were compared with those of AEHTPB/HTPB and pure HTPB elastomers, and the results are shown in Table 3 (The percentages in Table 3 are the additions of AEHTPB and AEHTPB-CN).We found that the tensile strength of AEHTPB-CN/HTPB elastomers is even higher than that of AEHTPB/HTPB elastomers when the addition amount is the same.
However, the data in Table 2 shows that the hydroxyl value of AEHTPB-CN is much lower than that of AEHTPB,which means that the chemical cross-linking density of AEHTPB-CN/HTPB elastomers is lower than that of AEHTPB/HTPB elastomers,so what causes the strength increase of AEHTPB-CN/HTPB elastomer? To answer this question, we used molecular dynamics simulation to analyze the cross-linking curing system of AEHTPB-CN/HTPB elastomer.The results show that the cyano groups in AEHTPB-CN and the H atoms in carbamate groups can form a large number of N-H…N≡C hydrogen bonds (Fig.4), which increase the physical cross-linking density of AEHTPB-CN/HTPB elastomers, so the strength of the elastomer is improved.
3.3.Performance of AEHTPB-CN in propellant
To examine the practical effect of AEHTPB-CN,some propellantlevel studies were carried out using the mixture of AEHTPB-CN and HTPB(The mass ratio of AEHTPB-CN/HTPB is 1/9)as the binder.Forcomparison,the mechanical performances of the propellant with T-313 (Amine bonding agent), BAG-5 (Borate ester bonding agent),and AEHTPB as bonding agents were also evaluated.
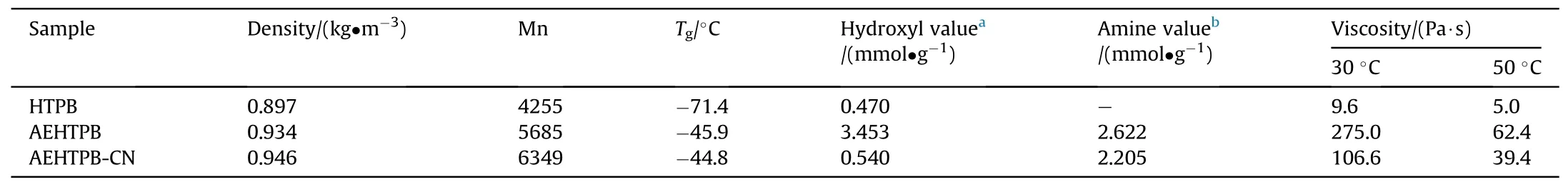
Table 2 Physicochemical properties of AEHTPB and AEHTPB-CN.
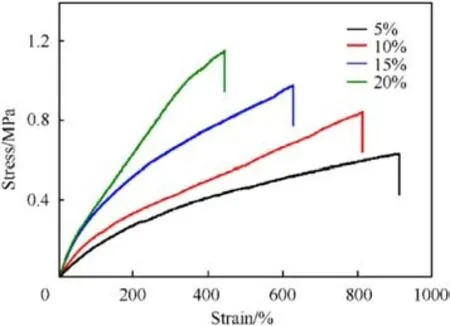
Fig.3.Stress-strain curve of AEHTPB-CN/HTPB elastomer.
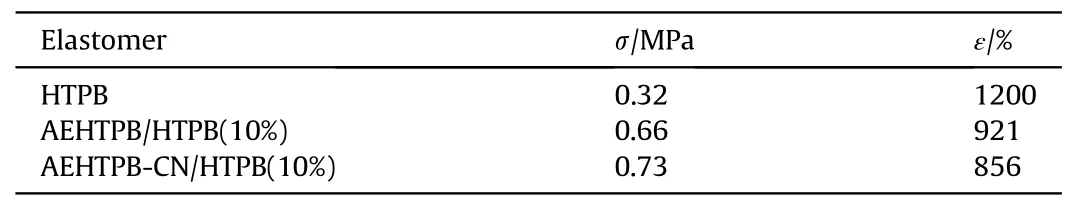
Table 3 Mechanical properties of different elastomers.
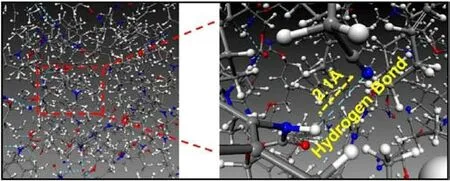
Fig.4.The molecular model of AEHTPB-CN/HTPB elastomer and intermolecular hydrogen bond (blue dotted line).
Fig.5 displays the maximum tensile strength (σ) and the elongation at break (ε) of propellant.As shown, the mechanical properties of the HTPB/AP/Al propellant added with AEHTPB-CN are further improved,and its tensile strength and elongation are higher than those of the propellant added with AEHTPB and T-313 bonding agent.For HTPB/AP/RDX/Al propellant, the tensile strength of the propellant grain can reach 0.84 MPa, which is slightly lower than that of the BAG-5 bonding agent (0.87 MPa), but the elongation increased to 62.9%, indicating that AEHTPB-CN can effectively improve the mechanical properties of the RDX-based propellant.In addition,AEHTPB-CN can also reduce the burning rate of HTPB/AP/RDX/Al propellant by 10.7% (Table 4).It is worth noting that the addition of AEHTPB-CN does not affect the overall energy level of the propellant since it can also be burned as a fuel.
To understand the bonding effect of AEHTPB-CN with different fillers,we prepared propellant samples containing a single filler(AP or RDX) and studied them through the scanning electron microscope(SEM)and mechanical testing.From Table 5,we find that the mechanical properties of propellants containing AP or RDX fillers are greatly improved after adding AEHTPB-CN, especially for RDX-based propellants, the elongation at room temperature has doubled.Further, SEM analysis was carried out on the tensile fracture surface morphology of samples, and the detection results were shown in Fig.6.
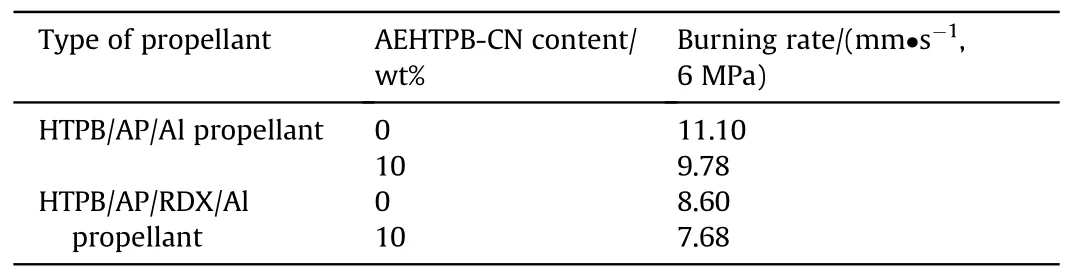
Table 4 Effect of AEHTPB-CN on the propellant burning rate.
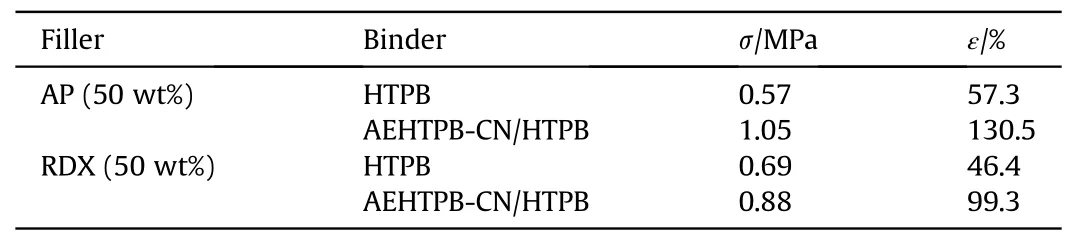
Table 5 Effect of AEHTPB-CN on mechanical properties of single filler propellant.

Fig.5.Effects of different agents on the mechanical properties of HTPB/AP/Al and HTPB/AP/RDX/Al propellants.
As depicted in Fig.6, the fracture surfaces of AP-based propellants and RDX-based propellants become smooth after adding AEHTPN-CN, and the surface of both AP and RDX fillers is covered with a coating layer.Moreover,the damage of the fracture surface is mainly manifested as internal tearing of the binder matrix, which indicates that AEHTPN-CN has a good bonding effect on AP and RDX particles.
3.4.Mechanism of interaction between AEHTPB-CN and AP
3.4.1.Chemisorption
The AEHTPB -CN molecule contains many tertiary amine groups,theoretically,these tertiary amine groups can react with the surface of AP particles to form ammonium salt ion bonds and release ammonia [31,32].The reaction formula is as follows:
Therefore,the AEHTPB molecules are firmly adsorbed on the AP surface by the bonding effect of ionic bonds, forming a dense coating layer.To verify that they can react to form ionic bonds, a small number of AP particles was put into excess AEHTPB-CN solvent(completely dissolved in ethyl acetate).Bubbles were observed in the solution,accompanied by a pungent ammonia smell,and the pH test result was alkaline (Fig.7(a)).With the progress of the reaction,the AP particles gradually became smaller until disappeared.After that,the reaction solution was filtered and measured by FTIR spectrometer,and the spectra are shown in Fig.7(c).Comparing the spectrum of pure AP,it is seen that there are asymmetric stretching and bending vibrations of perchlorate (Cl-O) (at 1070 and 625 cm-1) [33] in the filtrate.But, the characteristic peaks of ammonium(N-H,at 3298 cm-1and 1424 cm-1)[34]are not found in the solution, and the adsorption peak at 2245 cm-1informs about the-CN group present in the reaction solution.These results have given strong evidence that AEHTPB-CN and AP can react and form new ionic compounds.
3.4.2.Physisorption
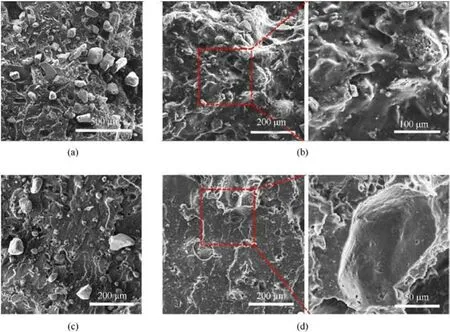
Fig.6.SEM image of propellant tensile fracture surface: (a) HTPB/AP; (b) AEHTPB-CN/HTPB/AP; (c) HTPB/RDX; (d) AEHTPB-CN/HTPB/RDX.
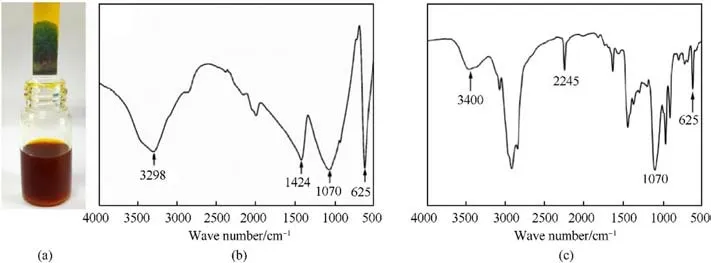
Fig.7.(a) pH test for the gas produced by the reaction; (b) FTIR spectra of AP; (c) FTIR spectra of the reaction solution.
In addition to the chemical adsorption described above,there is also a strong physisorption interaction between AEHTPB-CN and AP.In this study, molecular dynamics simulations will be used to investigate the physisorption interaction between them at the molecular level.Firstly, the interfacial adsorption model shown in Fig.8 was constructed, where Fig.8(a) shows the initial configuration of the model and Fig.8(b) is the adsorption equilibrium configuration after dynamics simulation.From Fig.8(b), it can be seen that the AEHTPB-CN chain can be well adsorbed on the AP surface.
To understand the nature of the interaction between AEHTPBCN and AP, the radial distribution function (RDF) analysis, which can describe how the specific atomic density varies as a function of the distance from one reference atom, was performed to characterize equilibrium configurations [35,36].Here, we calculated the RDF of the hydroxyl and cyano groups in the AEHTPB-CN to AP specific atoms defined in Fig.8(c).As shown, the first peaks in the curve of OH-H/AP-O appear near 1.8 Å,which denotes the average distance from the hydrogen atoms on the hydroxyl groups to the oxygen atoms on AP.Generally, the interaction distance range of the hydrogen bond is 1.1-3.0 Å, so we can confirm that these hydroxyl groups can form strong hydrogen bonds (O-H···O) with the AP.Similarly,a sharp peak is observed at approximately 1.8 Å in the curve of CN-N/AP-H, implying that the cyano groups in AEHTPB-CN can also form hydrogen-bonding interaction(N-H···C≡N) with AP [37], which highly corresponds to the AEHTPB-CN molecular adsorption situations on the AP surface in Fig.8(d).Apparently, since the cyano group can form hydrogen bonds with AP,the bonding effect of AEHTPB-CN to AP particles will be further improved compared with AEHTPB.
3.5.Mechanism of interaction between AEHTPB-CN and RDX
It has been often assumed that polar groups(such as the cyano group) of the bonding agents can form a strong intermolecular interaction with the nitroamine filler thus enhancing the interfacial bond strength [38,39].However, there are no clear conclusions on the key issues of how the cyano group interacts with the surface of the nitroamine and where the sites of action are.Here, the interaction mechanism between the cyano group and RDX molecules is discussed in terms of electrostatic potential,interaction energy,and interfacial adsorption simulation, using density functional theory and molecular dynamics methods.
3.5.1.Electrostatic potential analysis
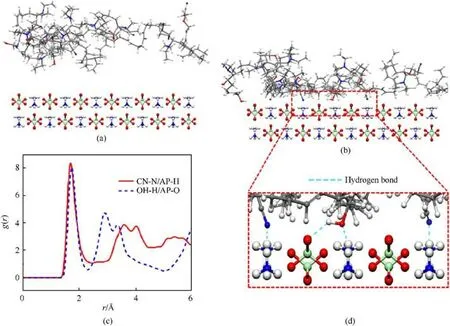
Fig.8.(a) The initial configuration and (b) equilibrium configuration of AEHTPB-CN adsorption on the AP surface; (c) The radial distribution function analysis for different atom pairs; (d) The AETPB-CN molecular adsorbs on the surface of AP and forms hydrogen bonds (blue dashed line).
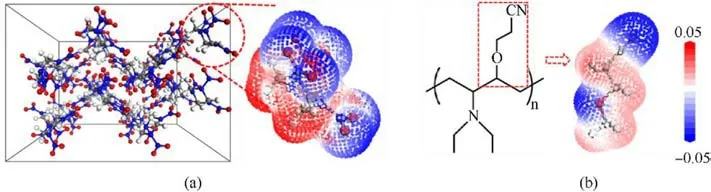
Fig.9.Electrostatic potential surface of (a) RDX molecule and (b) cyano unit in AEHTPB-CN.
The electrostatic potential is a measurable and fundamentally significant physical property of compounds, which can provide insight into the charge density distribution and reactivity of entire molecules [40,41].Fig.9 shows the electrostatic potential distribution of RDX and AEHTPB-CN molecules.To reduce computation cost, we simplified the computational model of AEHTPN-CN and focused on the electrostatic potential of the structural units containing the cyano group.It can be seen that the negative electrostatic potentials (blue region) of the RDX molecule are mainly distributed on nitro groups(-NO2)with strong electron absorption capacity, while the positive potentials (red region) are located around the nonpolar methylene groups (-CH2) of RDX molecule[42].In the cyano unit of AEHTPB-CN, the negative potentials are focused on the strong polar cyano group(-CN).From the viewpoint of electrostatic attractions,molecules always tend to approach each other in a complementary manner.Based on this,we can speculate that there may be a strong electrostatic interaction between the cyano group of the AEHTPB-CN molecule and the methylene group in the RDX molecule.
Fig.10 shows the surface structures and electrostatic potential distribution of RDX habit crystal faces.It is observed that the molecular arrangements of all crystal faces present a "valley" shape,where the nitro groups are exposed at the top and the valley bottom areas are dominated by the methylene groups [43].However,due to the different surface chemistry of each crystal face, the orientation, position, and density of specific groups on different crystal faces will also change.Furthermore, the electrostatic potential distribution of the different crystal faces shows that the nitro groups exposed at the top have higher negative potentials,while the valley bottom regions have more methylene groups and show a stronger positive potential.From this,we can infer that the interaction sites of the cyano groups with RDX are located in the cavities of the RDX crystal face, which can maximize their contact with RDX molecules.
3.5.2.Interaction energy calculation
To quantify the strength of the interaction between the cyano unit in AEHTPB-CN and the RDX crystal face, the interaction energies of the two-component models are calculated.The initial structures of the model composed of cyano unit and RDX molecular are depicted in Fig.11.Each model is optimized and characterized to the relative energy minimum of the system at the B3LYP/6-311++G(d,p) level and their interaction energies are presented in Table 6.The interaction energies(ΔE)between the cyano unit in AEHTPB-CN and RDX molecular is obtained using the following formulae [44,45]:
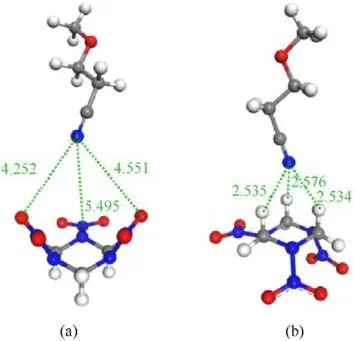
Fig.11.Optimized structures of the two-component models formed between cyano unit and RDX molecular.
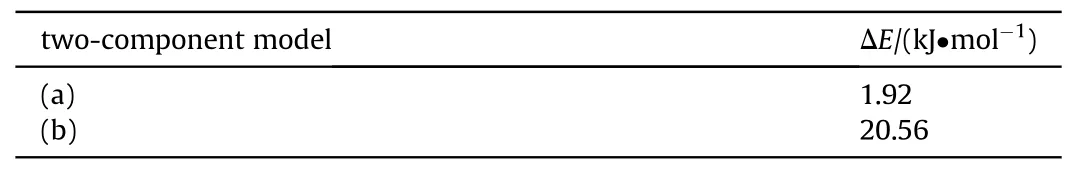
Table 6 Interaction energies of cyano unit and RDX molecular.
where EABis the total energy of the complex (AB).EAand EBrepresent the energies of the RDX molecular(A),and the cyano unit(B)respectively.EBSSEis the basis set superposition error correction.
Several interaction geometries were investigated for cyano structure unit adsorbed at RDX molecular and the corresponding results are presented in Fig.11.As we can see, the adsorption affinity of cyano structure unit to RDX molecule with different orientations varies in magnitude.In Fig.11(a),the nitro group of RDX is facing the cyano group of AEHTPB-CN,the optimized model shows that the distance between the nitrogen atom of the cyano group and the oxygen and nitrogen atoms on the nitro group of RDX in the range 4.2-5.5 Å, while the distance between the nitrogen atom of the cyano group and the hydrogen atoms of the methylene groups in Fig.11(b) are around 2.6 Å.On the other hand, the adsorption affinity of the cyano group and methylene group is relatively stronger.The calculated ΔE value in Fig.11(a) is 1.92 kJ/mol,whereas, it is 20.56 kJ/mol in the case of Fig.11(b), which further confirms that the cyano group can form strong intermolecular interactions with the methylene group in the RDX molecule.Judging by the interaction distance and the strength of the interaction(The bond energy of weak hydrogen bonds is less than 25 kJ/mol)[46-48], the cyano group and RDX molecular can form C-H···N≡C weak hydrogen bonding interaction.

Fig.10.Surface structures and the electrostatic potential surface of RDX crystal faces: (a) 200; (b) 020; (c) 002; (d) 210.
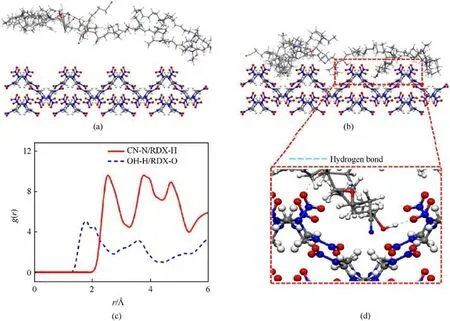
Fig.12.The initial configuration(a)and equilibrium configuration(b)of AEHTPB-CN adsorption on the RDX surface;(c)The radial distribution function analysis for different atom pairs; (d) The AETPB-CN molecular adsorbs on the surface of RDX and forms hydrogen bonds (blue dashed line).
3.5.3.Molecular dynamics simulation
Molecular dynamics simulations were adopted to give deep insights into the adsorption of the AEHTPB-CN molecules on the RDX surface.For this, the adsorption model of AHTPB-CN on the RDX surface was established, where Fig.12(a) is the initial model and the optimized model after molecular dynamics equilibrium is shown in Fig.12(b).Fig.12(c)depicts the RDF between AEHTPB-CN and RDX atoms,it is seen that the first peak of OH-H and RDX-O is located at 2.0 Å, implying the existence of hydrogen bonding interactions among the hydroxyl groups of AEHTPB-CN and nitro groups of RDX.For CN-N and RDX-H atom pair, there is a distinct peak at 2.6 Å,which means the cyano group in AEHTPB-CN is about 2.6 Å above the methylene group of RDX, and this observation highly corresponds to the previous DFT calculation.
The detailed view of the adsorption situations on the RDX surface is demonstrated in Fig.12(d).It is observed that hydrogen bonds are formed between the hydroxyl groups and the nitro groups (blue dashed line).On the other hand, the small molecular size of the cyano group(one-eighth of the methyl group) allows it to penetrate deep inside the RDX surface and interact with the hydrogen atoms of the methylene groups.Considering that the hydroxyl groups are consumed during the curing process,the weak hydrogen bonding between the cyano group and RDX will be the main driving force for AEHTPB-CN to achieve effective adsorption.
4.Conclusions
In this study, a multi-cyano, amine-based polybutadiene(AEHTPB-CN) was prepared based on AEHTPB through cyanoethylation of the hydroxyl group.The prepared AEHTPB-CN has good compatibility with the HTPB binder.Adding it to the propellant formula(replacing 10%of the HTPB binder)can not only effectively enhance the strength of the binder matrix, but also improve the interfacial bonding effect between the matrix and solid fillers (AP and RDX).At room temperature, the mechanical properties of the HTPB/AP/RDX/Al propellant are superior to the control propellant with an improvement of 35.4% in tensile strength, and 62.0%enhancement in elongation at break.Meanwhile, the burning rate of propellant containing AEHTPB-CN is found to be reduced by 10.7% compared to HTPB-based propellants without any energy loss, showing good application potential in composite propellants.In addition,the interaction mechanisms of AEHTPB-CN with AP and RDX fillers were systematically studied by combining experiment and computational simulation methods.The results show that the tertiary amine group in AEHTPB-CN can react with AP to form ammonium salt ionic bonds, and the hydroxyl and cyano groups can form strong O-H···O, N-H···C≡N hydrogen bonding interaction with AP,which enables AEHTPB-CN to be firmly adsorbed on the AP surface through chemical and physical interactions.Finally,our findings affirm that the interfacial bonding performance of AEHTPB-CN to RDX fillers is attributed to their ability to form C-H···N≡C weak hydrogen bonds and the adsorption sites mainly originate from the cavities formed at the basin area of RDX crystal face.The results reported here will provide a fundamental comprehension of AEHTPB-CN and solid fillers interactions, especially providing some experimental and theoretical support for the design of nitroamine filler bonding agent.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Defence Technology的其它文章
- Explosion resistance performance of reinforced concrete box girder coated with polyurea: Model test and numerical simulation
- An improved initial rotor position estimation method using highfrequency pulsating voltage injection for PMSM
- Target acquisition performance in the presence of JPEG image compression
- Study of relationship between motion of mechanisms in gas operated weapon and its shock absorber
- Data-driven modeling on anisotropic mechanical behavior of brain tissue with internal pressure
- The effect of reactive plasticizer on viscoelastic and mechanical properties of solid rocket propellants based on different types of HTPB resin

