The reaction mechanism and interfacial crystallization of Al nanoparticle-embedded Ni under shock loading
Yifn Xie , Jin-Li Sho ,b,*, Rui Liu , Pengwn Chen ,b,**
a State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing,100081, China
b Explosion Protection and Emergency Disposal Technology Engineering Research Center of the Ministry of Education, Beijing,100039, China
Keywords:Shock-induced reaction Molecular dynamics simulations Interfacial crystallization Reaction mechanism
ABSTRACT The shock-induced reaction mechanism and characteristics of Ni/Al system, considering an Al nanoparticle-embedded Ni single crystal, are investigated through molecular dynamics simulation.For the shock melting of Al nanoparticle, interfacial crystallization and dissolution are the main characteristics.The reaction degree of Al particle first increases linearly and then logarithmically with time driven by rapid mechanical mixing and following dissolution.The reaction rate increases with the decrease of particle diameter, however, the reaction is seriously hindered by interfacial crystallization when the diameter is lower than 9 nm in our simulations.Meanwhile, we found a negative exponential growth in the fraction of crystallized Al atoms, and the crystallinity of B2-NiAl (up to 20%) is positively correlated with the specific surface area of Al particle.This can be attributed to the formation mechanism of B2-NiAl by structural evolution of finite mixing layer near the collapsed interface.For shock melting of both Al particle and Ni matrix, the liquid-liquid phase inter-diffusion is the main reaction mechanism that can be enhanced by the formation of internal jet.In addition,the enhanced diffusion is manifested in the logarithmic growth law of mean square displacement, which results in an almost constant reaction rate similar to the mechanical mixing process.
1.Introduction
As a new type of energetic structural material,Ni/Al composites combine high energy density and excellent mechanical properties.The reaction initiation will be activated by a specific temperature environment [1-4] or mechanical shock condition [5-7] followed by the sustaining heat release.Based on the exothermic characteristics of no gas production,Ni/Al composites are widely used as heat sources in thermal batteries, welding, explosives, and other fields [8-11].
In recent years,a lot of shock experiments have been carried out to investigate the reaction properties of Ni/Al powder composites[5,6,12-15].The shock-induced reaction within the time scale of mechanical equilibration is enhanced by the structural changes and formation of defects following the shock compression [5].The pressure threshold for the ultrafast reaction initiation is usually determined indirectly from the measured shock velocity versus pressure.And, the critical value is related to the structure of the powder composites as well as the theoretical density [6,12].The combustion temperature(3600 K)of Ni/Al powder composites was measured at an impact velocity of 1.7 km/s [13].Furthermore, the reaction properties including energy release capacity are influenced by the manufacturing methods [14] and mechanical properties[15].Compared with the multi-layered Ni/Al prepared by cold rolling, the composites obtained by powder compact exhibit the advantages of small particle size and wide ignition temperature region under the shock loading [14].In addition to the reaction characteristics and properties, the understanding of the shockinduced reaction mechanism is crucial for the structural design and optimization of reactive composites.However, the limited in situ observation technology and the structural deformation of Ni/Al composites make it difficult to capture the interfacial structure details and reveal microscopic mechanisms in the experiments.
Nowadays, molecular dynamics (MD) simulation provides an effective method to deeply explore the relationship between microstructure and reactivity and reveal the intrinsic mechanism.Reviewing thermal ignition simulations of Ni/Al composites, the results show that the reaction path and mechanisms depend on the initial ignition temperature.When the ignition temperature is lower than the melting point of Al,the sluggish solid-state diffusion leads to heating of system followed by the formation of B2-NiAl phase [2,3].When the ignition temperature is higher than the melting point of Al but lower than the melting point of Ni, the reaction in Ni/Al composites is triggered by the exothermic dissolution of Ni into liquid Al, and the B2-NiAl grains will precipitate from the mixed liquid solution [16-19].In contrast, the shock compression of Ni/Al composites involves various complex processes[20-24]such as dislocation evolution,phase transition,void collapse,and interfacial instability.The initiation of shock-induced reaction is not directly affected by the dislocation evolution occurring inside the particle and near the shock wave front[21].It was indicated that the reaction of Ni/Al powder composites will be initiated at the collapsed void following the shock compaction[22,23].Previously, we explored the correlation mechanism between interface instability and shock-induced reaction from a fluid perspective[24].The results showed that the perturbation growth significantly promotes inter-diffusion and consequently the atomic mixing.Nonetheless, the investigation of reaction mechanism is always a challenging undertaking under shock loading.And, the solid-liquid reaction characteristics and mechanism of Ni/Al system under shock loading are not thoroughly understood.
In this study,we use MD simulation to investigate the dynamic response and reaction mechanism of Al nanoparticle embedded in Ni under long-time shock loading.Importantly,it is found that the B2-NiAl phase is formed on the particle interface at impact velocity up=2 km/s.The interfacial crystallization,exothermic dissolution,and the effect of particle size on crystallization are reported and analyzed in detail.Besides, we also focus on the diffusion mechanism within liquid-liquid phase reaction at up>2 km/s.It should be noted that the simulated shock wave is closer to the ideal state and may be different from the experiment or actual situation, such as narrower shock front and higher strain rate.The results related to reflection of shock wave may be more significant for the experiments with larger particle size.Besides, because the intense reaction driven by atomic diffusion behaves non-instantaneous, the reaction mechanism is also of important reference significance for more realistic case.This paper is organized as follows: the computational details are described in Section 2, the results are discussed in Section 3, followed by the conclusions in Section 4.
2.Computational details
All simulations are performed using the LAMMPS package[25].The interatomic interactions for Ni-Ni, Al-Al, and Ni-Al are described by an embedded atom method (EAM) potential developed by Purja Pun and Mishin [26], which provides an accurate description of melting,dissolution,and nucleation for pure metals and intermetallics such as NiAl,Ni3Al.This potential can predict and reproduce the chemical properties of Ni/Al composite under thermal [27] or shock [28] loading.In addition, by using this potential function, some researchers have investigated the solid-state reaction mechanism [3], reaction path [19], and the reaction process induced by laser irradiation [29] for the similar models, such as core/shell and embedded model.
The initial configuration of the Ni/Al nanocomposite is shown in Fig.1.This model is simple and suitable for exploring the details of structural evolution and reaction mechanisms at particle interface,which is often used to study the effect of nanoparticle inclusion on the mechanical properties of metal composites, such as dynamic tensile strength[30],hardness[31],spalling strength[32].The x,y,and z axes are, respectively, along the [100], [010], and [001]crystallographic directions.The dimensions of the model are Lx=30 nm,Ly=30 nm,Lz=70 nm,and the lattice constants of Al and Ni are 4.05 Å and 3.52 Å, respectively.The initial model is prepared in the following way: a fcc single crystal Ni matrix is created firstly, then, a sphere of diameter dAlis cut out from the center of Ni matrix,and a spherical single crystal Al particle of the same diameter is put inside the pore.The system has ~6 × 106atoms, of which are ~7000 (dAl= 6 nm), 23,000 (dAl= 9 nm), and 55,000(dAl=12 nm)Al atoms.Before shock loading,the energy of system is minimized by conjugate gradient method followed by the thermodynamic relaxation.To reach the equilibrium state, the model relaxes in the isothermal isobaric(NPT)ensemble under the condition of zero pressure at 300 K over 150 ps.
Here,the generation of shock wave and the long-time evolution are achieved using momentum mirror technique and absorbing boundary condition [33,34].The shock wave is generated by a moving wall (at desired velocity up) which can reflect atoms.During the propagation of shock wave, the opposite side is set as free boundary condition.When the elastic wave first reaches the free surface,the Ni layer between plastic wave front and free surface is set to a rigid body moving with up.This approach can minimize the effects of reflection of elastic and plastic waves for the region behind the plastic wave front, which is equivalent to adopting an absorbing boundary condition.Then, long-time evolution of shocked state can be simulated.Except for shock direction, the other two directions always adopt periodic boundary conditions.Both the nonequilibrium shock and long-time shock loading processes are performed under the microcanonical (NVE) ensemble with a time step of 1 fs.
Atomic visualizations are performed using the open-access software OVITO [35].The interfacial crystallization is explored with the help of the adaptive common neighbor analysis (a-CNA)[36].The shock temperature is calculated by equipartition theoremwhere the center-of-mass velocity should be subtracted.And,the stress is calculated based on the virial theorem[37].The spatial distributions of physical quantities are obtained by 1D-binning analysis with a bin width of 3 Å along shock direction.The central atomic layer (140 Å <x <160 Å) is selected for 2Dbinning analysis with a bin size of 8 Å × 8 Å.
3.Results and discussion
3.1.Shock response of the Al nanoparticle in Ni
Firstly, we examine the propagation of shock wave in the Ni/Al nanocomposite with dAl=12 nm at up=2 km/s.Fig.2(a)shows the two-dimensional (2D) distribution of stress in the sample with dAl= 12 nm at various times.Initially, the plane shock wave generated in the Ni matrix propagates to the rear surface of the Al nanoparticle with a certain wave velocity.Due to the impedance mismatch between Ni and Al, the first reflection and transmission occurs at the rear surface of the Al nanoparticle at t=5.2 ps.At this time, the reflected rarefaction wave R propagates backward in a semi-elliptical shape in a 2D plane because the Ni/Al interface is spherical.The transmission wave TAlis generated in Al nanoparticle and propagates towards the front surface of the Al nanoparticle.At t=6.2 ps,the second reflection and transmission occur at the front surface of the Al nanoparticle when the TAlcrosses through the Ni/Al interface.At this time, the reflected compression wave C is formed in Al nanoparticle when the TAlpropagates from low density (Al) to high density (Ni) region.The transmission wave TNigenerated in the Ni propagates towards in an arc-shaped feature in a 2D plane.Then, the TNigradually evolves into a plane wave as shown at t = 8 ps.
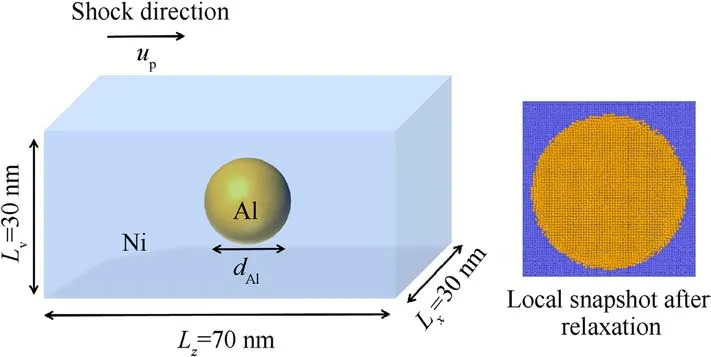
Fig.1.Schematic configuration of Ni/Al nanocomposite under shock compression.Al nanoparticle with diameter ranging from 6 nm to 12 nm is embedded in the center of Ni matrix.The shock direction is along z axis.Note that there are very few Ni and Al atoms at the interface diffuse each other after the relaxation.This can be ignored for the subsequent intermixing reaction process induced by mechanical shock.
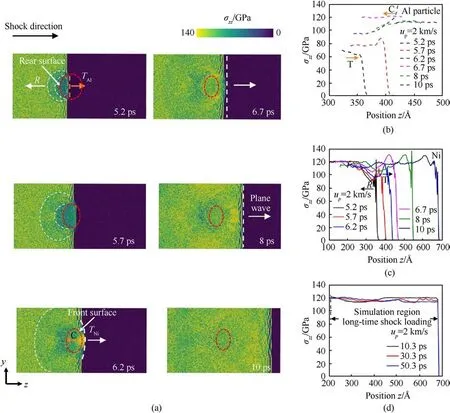
Fig.2.(a)The reflection and transmission of shock wave in the nanocomposite at up=2 km/s.The atoms are color-coded by the normal stress along shock direction.The location and region of Al particle are marked by the red dotted lines.Both reflection and transmission occur at the rear and front surfaces of the Al particle.Profiles of normal stress along the shock direction for (b) Al nanoparticle and (c) Ni at up = 2 km/s.The diameter of initial Al particle is 12 nm.(d) Profiles of normal stress along shock direction for the simulation region during long-time evolution.The onset of long-time shock loading is at t = 10.3 ps.
Fig.2(b)and 2(c)shows the stress profiles at different times of Al particle and Ni during the propagation of shock wave,respectively.After the first reflection and transmission at rear surface (t = 5.2 ps), the TAlis formed in Al nanoparticle, and the stress is about 60 GPa at the wave front.Meanwhile, as shown in Fig.2(c), the stress(~90 GPa)becomes lower in Ni near the interface caused by the interaction between the R and the incident wave.The stress of loading side that is not influenced by R maintains 120 GPa.Then,the second reflection and transmission occur at front surface(t = 6.2 ps).Affected by the reflected C, the shape of the curve changes and the wave in Al particle becomes stronger.As shown in Fig.2(c), the strength of TNiis similar to that of incident wave.Finally, the curve of Al particle behaves plateau feature, and the stress is stable at 110 GPa at t = 10 ps.And, the drop,plateau, and raise obviously can be found in the curve of Ni, which can be explained by the propagation of reflected R and transmissive TNi.Fig.2(d) shows the stress profiles of simulation region during the long-time shock loading.Due to the continuous reflection of internal waves, the stress distribution gradually tends to a plateau characteristic after oscillation.It can reflect the long-time evolution process of the shocked state,which will be analyzed in detailed in subsection 3.2.
After multiple reflections and transmissions, both volume and morphology of Al particle change under the high-pressure condition.For different shock intensities, the volumetric and morphological responses of Al particle are shown in Fig.3(a).The Al nanoparticle-embedded Ni at different upfollows a multi-stage volumetric change involving the pre-shock stage, compression stage,and intermixing stage.In the pre-shock stage,the shock wave has not interacted with the Al particle, and the volume does not change with time.In the compression stage, the volume of the particle decreases linearly with time due to the shock loading,and the change rate increases with the impact velocity.In the intermixing stage,the volume continues to decrease at a certain change rate(smaller than the preceding stage)except for up=1.5 km/s.It can be seen from Fig.3(c)that the surface Al atoms of particle and Ni atoms form a disordered mixing layer at up>1.5 km/s.This indicates that the reaction initiation leads to the continuous intermixing and consequently the reduction of particle volume.At up= 1.5 km/s, only a few Al atoms on the rear surface are mixed with Ni atoms because of the deformation of particle.Meanwhile,the diffusion and reaction are weak when both the Ni and Al particle are in a solid state.So, the volume of particle is almost no longer reduced at up= 1.5 km/s shown in Fig.3(a).Besides, Al particle is compressed from spherical to ellipsoid at low shock intensity and collapsed from spherical to bowl-like shape at high intensity.
The atomic structural state of Al particle is very important for the reaction during the long-time shock loading.Fig.3(b)shows the Al-Al pair radial distribution function (RDF) of particle at the complete shocked state.After the shock compression, the position of the first peak is shifted to the left for all the up, which indicates that the Al-Al nearest-neighbor distance is reduced.At up= 1.5 km/s, all peaks of the pair RDF do not disappear substantially, indicating solid state of the Al particle.At up= 2 km/s, the peaks become weaker and some peaks at r >5 Å disappear.Combined with the microstructure shown in Fig.3(c), part of Al at the collapsed interface is amorphous, which can be regarded as the fluid-like atoms.At up>2 km/s, the curves of pair RDF behave a platform feature at r >5 Å, which indicates that Al atoms change from an ordered lattice arrangement to an amorphous arrangement, and Al particle is fully melted.
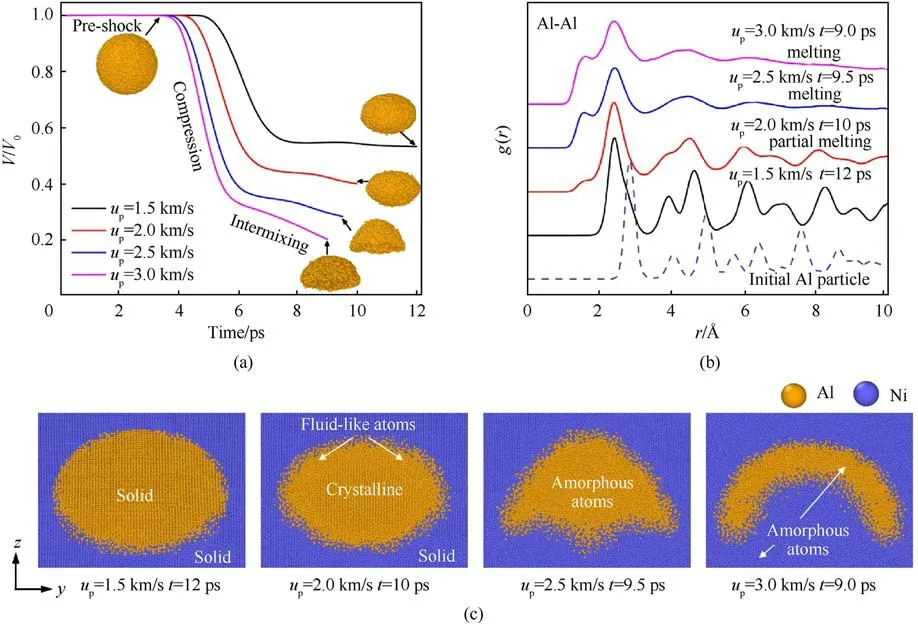
Fig.3.(a) The variation of Al particle volume at different up, where V0 is the initial volume and V is the volume of the shocked state.The end of the curves corresponds to the complete shocked state of the Ni/Al sample;(b)The pair RDF of Al-Al at different up.(dashed line,initial state;solid lines,complete shocked state);(c)Snapshots of the complete shocked configuration at different up.The diameter of initial Al particle is 12 nm.
3.2.Interfacial crystallization and exothermic dissolution
As we know,the melting of Al accelerates the reaction initiation,and the situation is adaptive for us to reveal the intrinsic mechanism of the reaction.Interestingly, for the shock loading with up= 2 km/s, the B2-NiAl phase crystallized at Ni/Al interface is observed, which has not been thoroughly discussed in other studies.
During the long-time shock loading, the reaction degree, interfacial crystallization,and accompanying thermodynamic effect are analyzed.It should be noted that the simulation of long-time shock loading continues to conduct within the adiabatic condition, so there is no energy exchange between the system and the external.And, this simulation process can be regarded as a long-time evolution of shocked state.Fig.4 shows the evolutions of thermodynamic states and the chemical properties of the Ni/Al embedded region at up= 2 km/s.Besides, the effect of Al particle size on the shock-induced chemical reaction is considered.As shown in Fig.4(a), the temperature decreases rapidly followed by a slow increase at a certain rate,which indicates that the Ni/Al embedded region successively experiences a short-term endothermic and a long-term exothermic process.In the beginning, the Al particle is not completely melted, and its interior is still in a crystal state, as shown in Fig.3(c).The fluid-like Al and Ni atoms at the collapsed interface react with each other through short-range migration,providing a part heat for the complete melting of Al particle.The drop of temperature is consistent with results of competition between melting and reaction obtained by Zhao et al.[38].In the following stage, the solid Ni dissolves into molten Al and releases heat continuously, which causes the system temperature to rise under adiabatic conditions.It can be clearly seen that the temperature is proportional to the Al particle diameter.In addition, the evolution of pressure is shown in Fig.4(b).The overall pressure oscillation in the Ni/Al embedded region does not exceed 2 GPa,and the pressure for the samples with various particle diameters is basically the same.Because the low volume fraction of Al nanoparticle, the change of Al particle state has little effect on the pressure of Ni/Al embedded region.
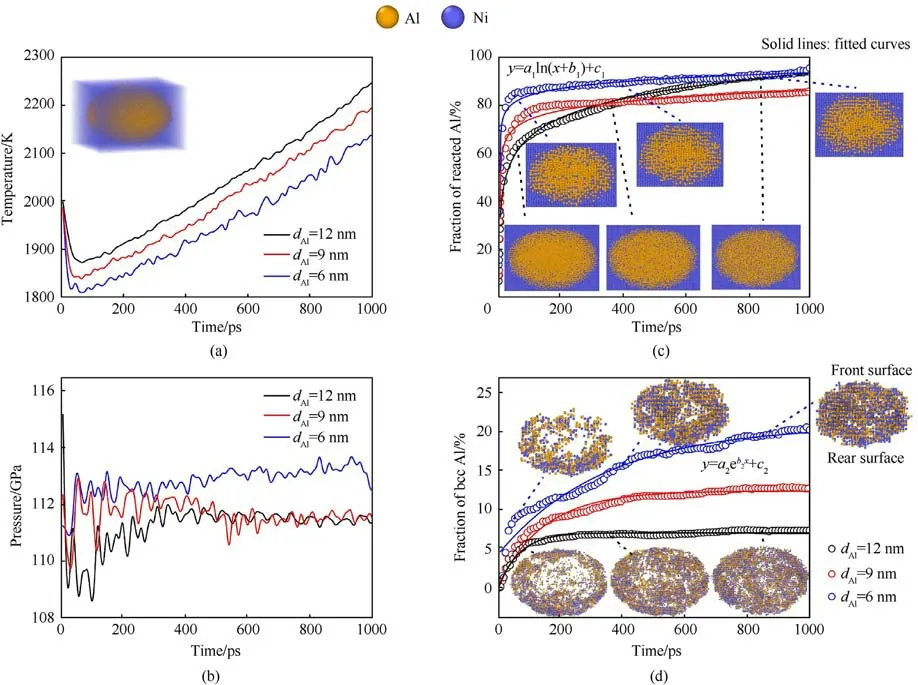
Fig.4.Evolutions of(a)temperature and(b)pressure for the embedded region at up=2 km/s.The effect of initial Al particle size on the thermodynamic state of reactive region is shown.The fraction of(c)reacted Al and(d)Al atoms crystallized at the interface as a function of time at up=2 km/s.The results of Al particles with different diameters are shown.Here, the initial states are shocked state and signify the onset of long-time shock loading.
Generally, in the shock simulations of Ni/Al system [39], the reaction degree is usually characterized by the reacted fraction of Al atoms.The central Al atom is regarded as a reacted atom if at least one Ni atom appears within the cutoff radius (0.24 nm) sphere.Based on the interatomic distance,this technique can simply judge the reaction state of Al and effectively reflect the reaction or mixing degree of the system.Initially,it should be noted that the Al atoms at Ni/Al interface are inevitably identified as the reacted atoms,but this does not affect the analysis of the exothermic reaction in the subsequent shock compression.As shown in Fig.4(c), the reaction fraction of Al increases significantly in two stages with time.In the first stage, the reacted Al atoms increase approximately linearly with time, which can be attributed to the thermomechanical mixing at the Ni/Al interface.The fluid-like atoms retained at the collapsed interface possess higher mobility and mix rapidly with each other.In the second stage, the curve of reaction fraction exhibits a slow growth trend with a decreasing change rate, and the growth of reaction satisfies a logarithmic law.Unlike the first stage,the mixing reaction is mainly driven the dissolution of solid Ni into molten Al, and the lower atomic mobility leads to a sluggish reaction rate.In addition, for the samples with various particle diameters,the reaction rate decreases with the increase of diameter.However,in the second stage,the reaction rate is obviously higher when the particle diameter bigger than 9 nm.By comparing the microstructural evolutions, it is found that the degree of atomic mixing in the inner region of the large-sized particle increases significantly with time,while the inside of the small-sized particle contains a small amount of diffused Ni atoms.Importantly, for all samples, the intermetallics B2-NiAl is observed at the Ni/Al interface after ~50 ps.The size effect of the reaction inside the particle may be directly related to the interfacial crystallization process.
The effect of particle diameter on the interfacial crystallization is reflected in Fig.4(d).Unlike thermal ignition,the arrangement of Ni and Al atoms is compressed from the FCC structure to the BCC structure at this shock intensity[40].Therefore,only a-CNA cannot accurately distinguish the intermetallics B2-NiAl (BCC phase)formed at the interface.Combined with the approach of reaction identification,we utilize the number of reacted Al atoms in the BCC phase to quantify the crystallinity of B2-NiAl.Using this method,B2-NiAl can be indirectly captured and quantified in the complex structure after compression,and the influence of transformed BCC phase pure metal is minimized.As shown in Fig.4(d),the fraction of crystallized Al atoms increases negatively exponentially with time.It can be seen from the microstructure that the nucleation and growth of B2-NiAl occur near the Ni/Al interface.A large number of Ni and Al atoms firstly form the BCC structure on the rear surface of the particle,and then the crystallized region gradually evolves into a complete ellipsoid.Besides, the interfacial crystallinity increases with the decrease of the initial Al particle diameter, which can be traceable to specific surface area(6/dAl).The particles with a small diameter have a large specific surface area,and a large proportion of atoms located on the surface.So, after shock compression, the fraction of mixed atoms at interface is higher for the smallerdiameter sample due to the deformation of particle.Similarly, in the second growth stage,the fraction(~7%)of crystallized Al atoms grows extremely slowly for the sample with particle diameter bigger than 9 nm.It can be concluded that the exothermic reaction is dominated by dissolution for the sample with a large particle diameter.And, for the sample with a small particle diameter, the continuous interfacial crystallization hinders the dissolution of Ni to a certain extent, further slowing down the reaction inside the particle.
Fig.5 comprehensively reveals the mechanism of crystallization and dissolution as well as the accompanying mass and thermal diffusion.The spatial distributions of temperature in the local region can better reflect the formation of hot spot and the reaction kinetics process.As shown in Fig.5(a), at t = 10 ps, the Al nanoparticle can be divided into three distinct regions from the outside to the inside: mixed with Ni atoms, molten or amorphous, and crystalline.Consequently, the potential energy distribution for Al atoms at this time is non-uniform showing in Fig.5(d).The potential energy Epof Al atoms in the outermost mixed region is comparatively lower than that in the crystallization region at the center, owing to the exothermic reaction.In contrast, the Epof Al atoms in the melting region is higher due to the absorption of heat.Along with thermal diffusion,the local hot spots on the rear surface of the Al particle disappear, and the interior of particle gradually melts.During the long-time shock loading, the finite mixing layer transforms from amorphous phase to typical B2-NiAl phase, and solid Ni continues to dissolve into molten Al.The crystallization behavior can be traced to disordered atoms trapped at the Ni/Al interface.The retained atoms migrate short-range to form an intermetallic compound with a thermodynamically stable state.At t=1000 ps,the interfacial crystalline region and the inner mixing region reform into local hot spots, as shown in Fig.5(c).Subsequently, with exothermic dissolution and thermal diffusion, the temperature of the entire embedded region is uniformly distributed.At this time(t=1500 ps),the atomic composition ratio of the crystalline region is close to 1:1, and the proportion of diffused Ni atoms inside the Al particle accounts for ~30%,which is consistent with the saturation concentration (25%-30%) [16].Besides, the potential energy of the mixed Al atoms becomes lower, and the potential energy forms a stepped distribution similar to the atomic composition ratio.
3.3.Atomic diffusion mechanism within the reaction
In this section, we explore the reaction under the condition where both Al particle and Ni are in a liquid state.For these higher particle velocities, the temperature evolution of the embedded region is mainly controlled by the combination of thermal diffusion and exothermic reaction during long-time shock loading.Fig.6 shows the reaction fraction of Al and temperature as a function of time at up=2.5 km/s and up=3 km/s.Firstly,it can be seen that the Ni atoms near particle changed from FCC to amorphous structure when the shock wave crosses through Al particle.By comparing Figs.6(a) and Fig.4(c), it is found that the time for the complete reaction of the Al particle is significantly shorter than that in the case of up= 2 km/s, indicating the enhancement of reaction by Ni melting.Although Ni near the interface is in liquid state, the evolution characteristics of reaction fraction are similar for up= 2.5 km/s and up= 2 km/s.The linear growth stage (t0-t1) of reacted Al atoms is caused by the shock-induced mechanical mixing, and the second slow growth stage (t1-t3) is due to the interdiffusion as shown in Fig.6(b).Meanwhile, the temperature shows a growth trend similar to the reaction degree.It should be noted that the temperature rises to the platform in advance compared to the reaction degree at 40 ps.This is the result of slow reaction rate and thermal diffusion from embedded region to Ni matrix.Instead, for up= 3 km/s, the reaction fraction always increases linearly with time until the Al particle is fully reacted,without evident stage characteristics.Due to the rapid and continuous mixing reaction, the rise of temperature is consistent with the reaction degree at up= 3 km/s.Besides, the time for the complete reaction of the Al particle at up=2.5 km/s is almost three times that of the case of up= 3 km/s.As shown in Fig.6(b), the morphological change and the formation of jet increase the contact area and the degree of inter-diffusion at up= 3 km/s.
The difference in reaction rate extremely depends on the atomic diffusion, for which we calculate the mean square displacement(MSD) of two distinct components at different shock intensities.Here, we only consider the atomic lateral displacement, and the lateral MSD is defined as follows:
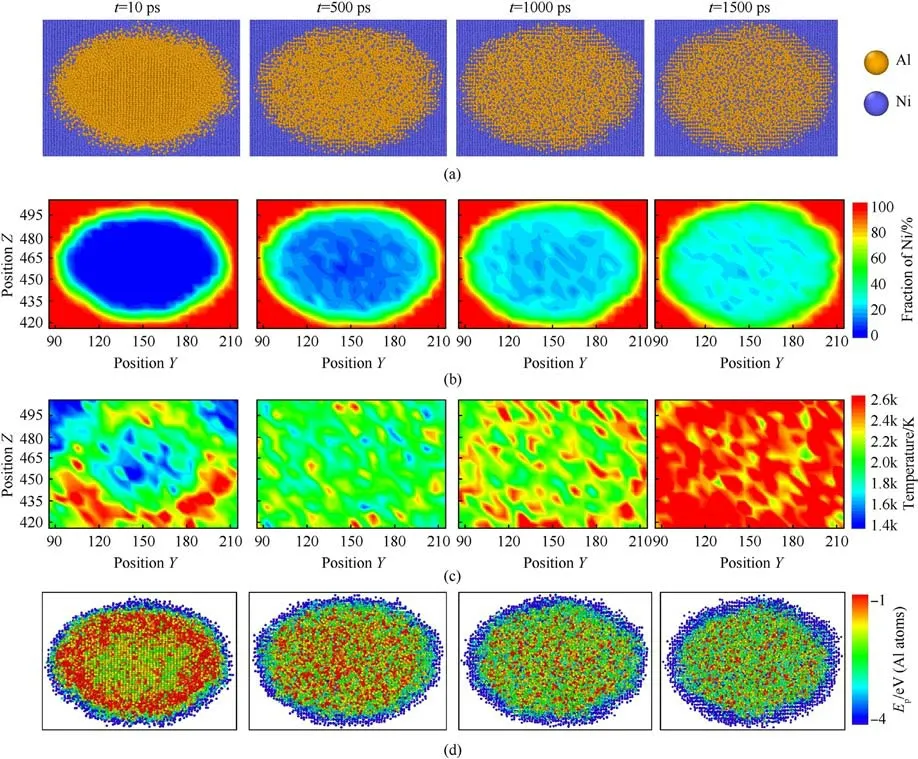
Fig.5.(a)Snapshots of the configuration at different times for up=2 km/s.The diameter of initial Al particle is 12 nm.The spatial distributions of(b)fraction of Ni atoms and(c)temperature in the reactive region.(d)Potential energy evolution at the interface and interior of Al nanoparticle during exothermic dissolution.Only Al atoms are shown and colorcoded by potential energy Ep.
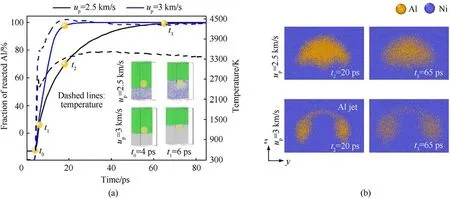
Fig.6.(a)Fraction of reacted Al atoms and temperature of embedded region as a function of time at up=2.5 km/s and up=3 km/s.The cut surfaces of Al particles are colored by the color yellow, and Ni atoms are color coded by a-CNA in the inset figures.(green: FCC, blue: BCC, grey: disorder) The results include the shock propagation and long-time shock processes, and the initial states are the states before shock; (b) Snapshots of the configuration at t2 and t3 for up = 2.5 km/s and up = 3 km/s.The diameter of initial Al particle is 12 nm.
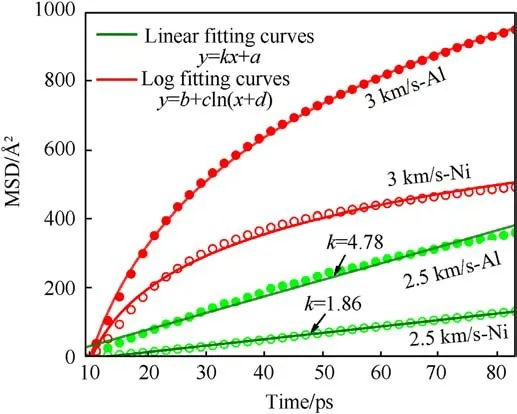
Fig.7.Lateral MSDs of Al(solid circles)and Ni(hollow circles)atoms at up=2.5 km/s and up=3 km/s.Scattered points and solid lines are simulated data and fitting curves respectively.The effects of shock intensity on the lateral atomic diffusion and the difference between components are shown.
where N represents the atomic number of Al particle or Ni,t is the time after the generation of waves,t0(9 ps)is the start time of longtime shock loading.Previously, the lateral MSD is also used to quantify the atomic mutual motion and characterize the deformation behavior in the shock simulations of Ni/Al composites[41].The effects of shock intensity on the lateral diffusion law are clearly shown in Fig.7.Firstly, it is easy to understand that the atomic lateral displacement increases with the shock intensity because the atomic motion and inter-diffusion can be intensified by the higher temperature in the stronger shock loading.However,the evolution characteristics of the MSD curves are distinctly different for up= 2.5 km/s and up= 3 km/s when both the Al particle and its nearby Ni are melting.At up=2.5 km/s,the lateral MSDs of both Ni and Al increase linearly with time.Instead, it can be seen that the lateral MSDs of both Ni and Al increase logarithmically with time at up= 3 km/s.It is worth noting that the difference in the lateral diffusion law is closely related to the dynamic process of Ni/Al.At up= 2.5 km/s, the linear growth of MSDs indicates that the fluidlike Ni and Al are mixed only by inter-diffusion at interface(Fig.6(b)).At up= 3 km/s, the atomic lateral MSDs grow rapidly during the formation of jet, and then the growth rate of MSDs decreases with time due to the atomic motion dominated by interdiffusion.In addition,the difference between components is mainly reflected in the quantity and growth rate of MSD.At the same shock intensity,the lateral displacement and growth rate of Al atoms are bigger than that of Ni atoms,which is consistent with the previous results [39,41].The softer Al is more easily deformed compared with Ni,so the atomic movement is more violent during the mixing process.
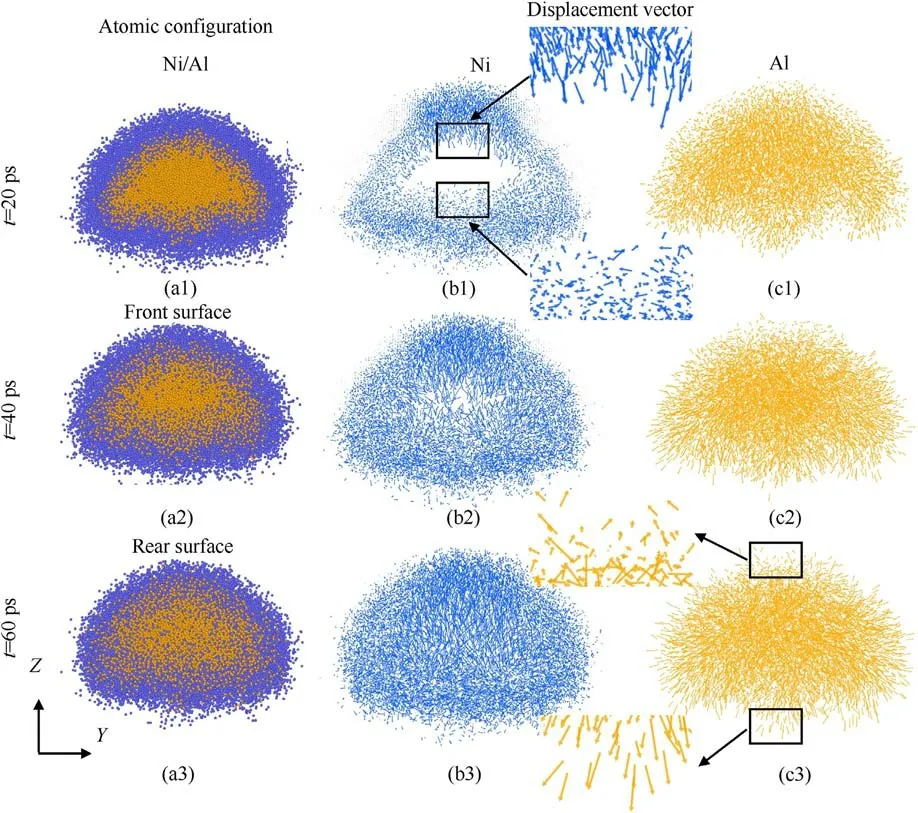
Fig.8.(a) Snapshots of the configuration at different times for up = 2.5 km/s.Atomic migration vector (Y-Z plane) diagram of (b) Ni atoms and (c) Al atoms at up = 2.5 km/s.
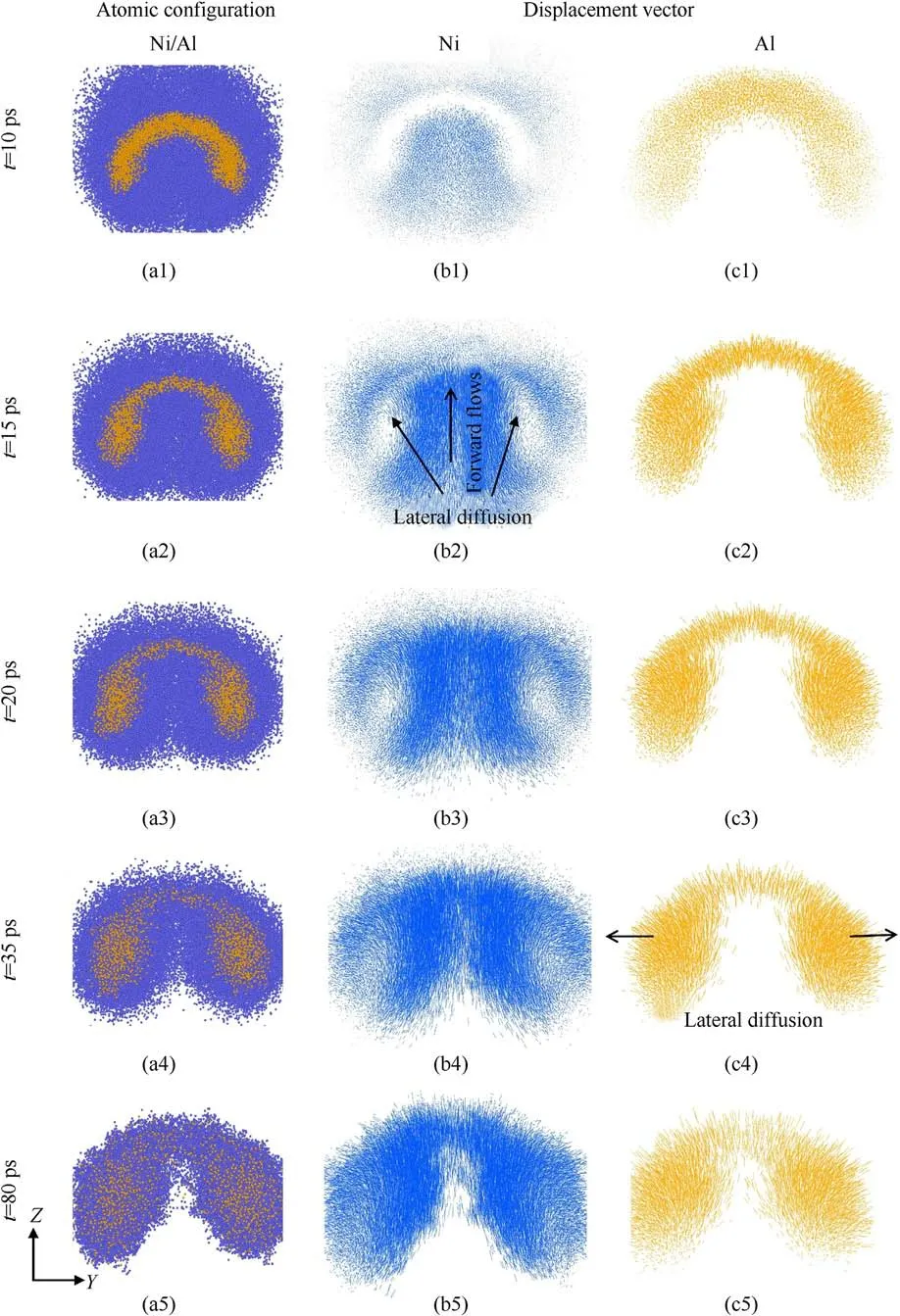
Fig.9.(a) Snapshots of the configuration at different times for up = 3 km/s.Atomic migration vector (Y-Z plane) diagram of (b) Ni atoms and (c) Al atoms at up = 3 km/s.
At the same time, the difference in atomic diffusion law also reveals the different microscopic mechanisms of reaction.To further explore the diffusion mechanism,Figs.8 and 9 compare the atomic migration process for the two shock intensities(up=2.5 and 3 km/s).The atomic movements of different components at up=2.5 km/s are shown in Fig.8.The end of the arrow represents the initial position of the atom at the reference moment(t=9 ps)when the long-time shock loading starts.Overall, it can be seen from Fig.8(a1) and 8(a2) that the ring-shaped mixing layer gradually expands from the interface to the center of deformed Al particle due to the inter-diffusion.In Fig.8(a3), the ring-shaped mixing layer eventually develops into an almost uniform ellipsoidal mixing region at t = 60 ps.From the perspective of the atomic movements, as shown in Fig.8(b1), the motion patterns of Ni atoms located on the front and rear surfaces are significantly different.At this time, the Ni atoms on the front surface move toward the center of the Al particle, while the Ni atoms on the rear surface are disordered.And, the magnitude of displacement is larger for the front surface Ni atoms.This indicates that the reaction of the front surface is controlled by the atomic diffusion,while the reaction mechanism of the rear surface is the short-range migration occurring in the residual atoms.As time increases, it can be seen from Fig.8(b2) and 8(b3) that Ni atoms on the front surface also begin to diffuse into the Al particle.The displacement of Ni atoms further increases until the Al particle are fully reacted.Meanwhile,Al atoms diffuse into Ni from the interface during the reaction.As shown in Fig.8(c3), the average length of the arrows on the front surface is significantly larger than that on the rear surface.The reactions of the front and rear surfaces are certainly initiated by the diffusion of Al and Ni atoms, respectively.
Fig.9 shows the atomic movements of different components at up=3 km/s.At this strong shock intensity,the dynamics of molten particle is similar to the evolution of bubble subjected to shock wave, which is a classic compressible viscous flow problem[42-44].As shown in Fig.9(a1) and 9(a5), the Al particle changes from the original spherical shape to a bowl-like shape followed by the formation of jet, which agrees well with other simulations[45,46].The Al jet is not further elongated due to the melting of Ni and intense reaction, and then an internal jet of Ni/Al mixture is gradually formed.Furthermore, we interpret the diffusion mechanism accompanying the internal jet development through the migration process of the two components.At t = 10 ps, the displacement of the atoms is very small.Then,Ni atoms move along the shock direction while migrating to both sides of the Al jet,indicating forward flows and lateral diffusion.Meanwhile,it can be found from Fig.9(c4) that the motion mode of Al atoms on both sides is dominated by lateral diffusion.
4.Summary
In summary, MD simulations are performed to investigate the reaction characteristics and mechanism of the Al nanoparticle embedded in Ni under long-time shock loading.Two typical combinations of Ni and Al with solid or liquid phases are considered by adjusting the initial impact velocity up.The one is solid Ni/liquid Al(up=2 km/s),the other one is liquid Ni/liquid Al(up=2.5 km/s and up= 3 km/s).Within simulation scale, the intermetallics B2-NiAl appear in the case of solid Ni/liquid Al at the lower up.At the higher up, the violent reaction is driven by the inter-mixing between molten Ni and Al,and the completely molten NiAl compound forms the final product.At up= 2 km/s, the interfacial crystallization,exothermic dissolution,and the effect of particle size are discussed in detail.At up≥2.5 km/s, the reaction mechanism and liquidliquid phase diffusion are explored.
At up= 2 km/s, the increase of reaction degree with time is divided into two stages.The first stage behaves as a linear growth driven by the fast mechanical mixing,and the second stage behaves as a logarithmic growth driven by slow dissolving mixing.It is found that the reaction rate increases with the decrease of particle diameter.However, in the second stage, exothermic dissolution is obviously hindered by interfacial crystallization for the sample with a diameter lower than 9 nm in our simulation scale.Importantly, the structural evolution of the interface shows that the B2-NiAl phase nucleates at the rear surface of the particle and grows into a complete ellipsoid.The B2 phase is formed by the short-range migration of retained atoms around the compressive interface.A negative exponential growth in the fraction of crystallized Al atoms is found,and the interfacial crystallinity(7%~20%)increases with the decrease of the initial Al particle diameter.This is because the smaller the particle size,the larger the specific surface area and the larger the ratio of retained atoms.
At up>2 km/s, the liquid-liquid phase diffusion is the main reaction mechanism that provides a high mixing rate.When the internal atomic jet is formed, the evolution of the reaction is accelerated to approximate linearity.And, the growth of lateral MSD approximately satisfies the logarithmic law with time.In addition, the microscopic mechanism is explored by the evolution of atomic migration vector.It is found that the reaction initiation mechanism around the front and rear surfaces of Al particle are not the same.The reaction initiation of the rear surface is driven by the diffusion of Ni atoms,and the reaction of the front surface is mainly initiated by the diffusion of Al atoms.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by the State Key Program of National Natural Science Foundation of China (Grant No.12132003), and State Key Laboratory of Explosion Science and Technology (Grant No.QNKT20-07).
- Defence Technology的其它文章
- Explosion resistance performance of reinforced concrete box girder coated with polyurea: Model test and numerical simulation
- An improved initial rotor position estimation method using highfrequency pulsating voltage injection for PMSM
- Target acquisition performance in the presence of JPEG image compression
- Study of relationship between motion of mechanisms in gas operated weapon and its shock absorber
- Data-driven modeling on anisotropic mechanical behavior of brain tissue with internal pressure
- The effect of reactive plasticizer on viscoelastic and mechanical properties of solid rocket propellants based on different types of HTPB resin

