催化邻羟基苯基取代对亚甲基醌与酮亚胺环加成反应合成二氢-1,3-苯并噁嗪化合物
孙一丹,李 鑫,2
(1.南开大学化学学院,天津 300071;2.物质绿色创造与制造海河实验室,天津 300192)
苯并噁嗪是一种优良的杂环化合物,具有特殊的分子结构,极具应用价值[1,2].含有二氢-1,3-苯并噁嗪骨架的化合物,如T615,T638[3](抗病毒性),CX-614[4](安帕金药)和Seclazone(抗菌性)[5]等(Scheme 1),展示了出色的生物和药用活性[6~8].
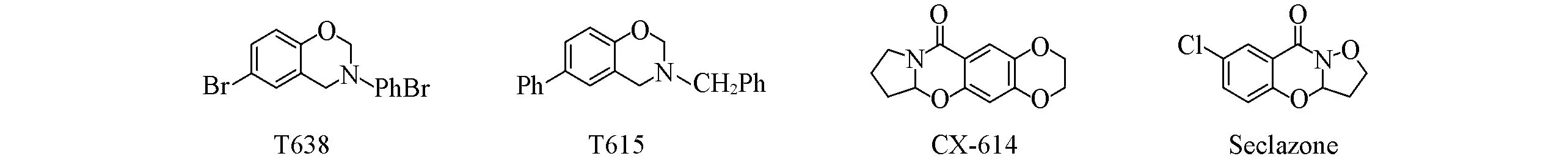
Scheme 1 Bioactive molecules containing dihydro-1,3-benzoxazine skeleton
此外,邻羟基苯基取代对亚甲基醌化合物(p-QMs)自发现以来即备受关注[9],其可以作为一种重要的杂环合成子[10~13].邻羟基苯基取代对亚甲基醌是醌的衍生物,其羰基被亚甲基取代引起分子偶极矩的变化,从而导致p-QMs的极化.羰基邻位的叔丁基体积大,能有效屏蔽亲核试剂对羰基和烯酮的攻击.同时,亚甲基不受大基团的影响,仍然是一个良好的亲电位点[14~17].当—OH 作为亲核取代基引入芳基取代基的2号位时,p-QMs 表现为具有亲电和亲核中心的双功能分子[18].基于其独特的两性离子芳香共振结构,Ender 等[9]利用p-QMs 与靛红衍生的烯酸酯反应得到了4-苯基取代的色满[Scheme 2(A)].在此基础上,多种杂环化合物应运而生.2019年,Zhao等[19]通过三氯化铁催化p-QMs和亚胺酯的环化,合成了2,4-二芳基苯并噁嗪化合物[Scheme 2(B)].基于此,本文提出了一种通过p-QMs和酮亚胺的[4+2]环加成反应制备二氢-1,3-苯并噁嗪化合物的方法.将磷酸催化剂用于邻羟基苯基取代对亚甲基醌与酮亚胺的[4+2]环加成反应,考察了不同溶剂和不同添加剂对反应非对映选择性和产率的影响;筛选出最优条件后,应用于不同取代的邻羟基苯基取代对亚甲基醌和酮亚胺的反应.实验还探索了将B(C6F5)3加入体系后反应的产率和非对映选择性的变化.

Scheme 2 The reactions of ortho-hydroxyphenyl substituted p-QMs and isatin olefin ester derivatives(A) and ortho-hydroxyphenyl substituted p-QMs and imidates(B)
1 实验部分
1.1 试剂与仪器
60-90石油醚(PE)和乙酸乙酯(EA),分析纯,天津渤化工程有限公司;反应过程中使用薄层层析色谱(TLC)监测反应;所有萃取的有机相均用无水硫酸钠干燥,并经旋转蒸发仪浓缩;柱层析分离使用200~300目硅胶,硅胶和薄层层析板均为烟台江友硅胶开发有限公司产品;所用溶剂和药品均为分析纯或化学纯试剂,使用前未经纯化处理.参照文献[9,20]方法合成邻羟基苯基取代对亚甲基醌.参照文献[21~23]方法合成酮亚胺.
Bruker Avance-500 型核磁共振波谱仪(NMR,以Acetone-d6和DMSO-d6为溶剂),德国Bruker 公司;Daicel Chiralpak IA 和IF 型手性色谱柱,日本大赛璐公司;LC-20A 型高效液相色谱仪(HPLC),日本岛津公司;Varian 7.0T FTMS型傅里叶变换高分辨质谱仪(ESI-HRMS),美国Varian公司.
1.2 实验过程
将0.12 mmol各种取代的邻羟基苯基取代对亚甲基醌(1)、0.1 mmol 各种取代的酮亚胺(2)和0.005 mmol磷酸依次加入干燥的5 mL反应管中,再加入1 mL超干二氯甲烷;室温下搅拌反应,用薄层层析色谱监测反应至完全,粗产品直接经柱层析(V石油醚∶V乙酸乙酯=30∶1)进行分离纯化,得到各种取代的产物12-(3,5-二叔丁基-4-羟基苯基)-5a-苯基-12H-苯并[5,6][1,3]噁嗪[3,2-a]吲哚-6(5aH)-酮(3)(Scheme 3).

Scheme 3 General procedure to synthesize 3
将0.12 mmol各种取代的邻羟基苯基取代对亚甲基醌(1)、0.1 mmol各种取代的酮亚胺(2)、0.005 mmol磷酸和0.005 mmol B(C6F5)3依次加入干燥的5 mL反应管中,再加入1 mL超干三氯甲烷;室温下搅拌反应,用薄层层析色谱监测反应至完全,粗产品直接经柱层析(V石油醚∶V乙酸乙酯=30∶1)进行分离纯化,得到各种取代的非对映异构体12-(3,5-二叔丁基-4-羟基苯基)-5a-苯基-12H-苯并[5,6][1,3]噁嗪[3,2-a]吲哚-6(5aH)-酮(4)(Scheme 4).

Scheme 4 General procedure to synthesize 4
1.3 产物的表征
所有产物的1H NMR,13C NMR及HPLC谱图见本文支持信息图S1~图S132.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3aa),黄色固体,产率85%,m.p.145~147 ℃.1H NMR(400 MHz,Acetone-d6),δ:7.63~7.60(m,2H),7.50(d,J=7.0 Hz,1H),7.33~7.22(m,6H),7.13~7.09(m,2H),7.01(d,J=7.7 Hz,1H),6.81~6.72(m,2H),6.13(s,1H),6.05(d,J=8.5 Hz,2H),1.35(s,18H);13C NMR(101 MHz,Acetone-d6),δ:194.2,158.6,153.6,150.7,138.2,137.0,135.6,131.7,128.8,128.1,127.9,127.7,127.0,125.0,124.9,122.6,91.9,59.1,34.3,29.7;ESI-HRMS(C35H36NO3+计算值),m/z:518.2684(518.2690)[M+H]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),主要非对映体:tR=5.2 min,tR=5.8 min;次要非对映体:tR=9.2 min,tR=10.1 min;96∶4d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-fluoro-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ba),黄色固体,产率99%,m.p.168~170 ℃.1H NMR(400 MHz,Acetoned6),δ:7.61(d,J=7.3 Hz,2H),7.51(d,J=7.7 Hz,1H),7.32~7.26(m,6H),7.18(dd,J=9.0,4.7 Hz,1H),6.94~6.88(m,1H),6.81~6.75(m,2H),6.17(s,1H),6.06(d,J=8.1 Hz,2H),1.35(s,18H);13C NMR(101 MHz,Acetone-d6),δ:195.4,159.9,159.2(d,J=239.3 Hz),155.3,148.4,139.8,138.5,136.8,132.6,130.8,130.4,130.3,128.5,127.1,126.5,126.3,122.0,121.9,120.1,119.8,116.3(d,J=24.0 Hz),115.5(d,J=24.9 Hz),113.3,93.5,60.5,35.8,31.1;19F NMR(376 MHz,DMSO-d6),δ:-119.65,-121.05;ESI-HRMS(C35H34FNNaO3+计算值),m/z:558.2416(558.2415)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),主要非对映体:tR=5.2 min,tR=5.6 min;次要非对映体:tR=6.9 min,tR=9.3 min;88∶12d.r.
(5aR,12R)-2-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ca),黄色固体,产率91%,m.p.146~148 ℃.1H NMR(400 MHz,Acetoned6),δ:7.58(dd,J=8.1 Hz,2H),7.51(d,J=7.6 Hz,1H),7.34~7.24(m,5H),7.21~7.11(m,2H),7.04(d,J=2.4 Hz,1H),6.92(d,J=6.0 Hz,1H),6.76(t,J=7.4 Hz,1H),6.17(d,J=10.4 Hz,1H),6.08(s,1H),6.05~6.03(d,1H),1.35(s,14H),1.28(s,5H);13C NMR(101 MHz,Acetone-d6),δ:193.8,158.4,153.9,149.7,138.6,138.4,137.1,137.0,135.2,131.0,129.7,129.1,128.9,128.4,128.2,128.1,128.0,127.7,127.1,127.0,125.7,125.1,124.9,120.8,120.3,119.8,118.7,118.3,111.8,110.9,100.0,92.0,58.8,57.4,34.3,34.1,29.7,29.6;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2110(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),主要非对映体:tR=5.2 min,tR=5.6 min;次要非对映体:tR=6.6 min,tR=10.0 min;80∶20d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3da),黄色固体,产率75%,m.p.196 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.55~7.53(m,2H),7.49(d,J=7.1 Hz,1H),7.34~7.25(m,4H),7.16(s,2H),7.09(d,J=8.8 Hz,1H),6.96(s,1H),6.74~6.61(m,3H),6.06~6.02(m,2H),3.54(s,3H),1.26(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.6,158.5,154.3,153.1,143.6,139.6,137.4,135.2,131.7,128.9,128.5,126.7,125.0,123.9,119.8,118.6,117.6,113.2,112.6,112.0,91.8,58.3,55.0,34.5,30.3;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2605(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=6.0 min,tR=7.5 min;次要非对映体:tR=9.3 min,tR=14.5 min;99∶1d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-methyl-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ea),黄色固体,产率90%,m.p.162~163 ℃.1H NMR(400 MHz,acetoned6),δ:7.57~7.55(m,2H),7.44(d,J=6.4 Hz,1H),7.27~7.17(m,6H),6.97(d,J=8.2 Hz,1H),6.84~6.79(m,1H),6.67(t,J=7.4 Hz,1H),6.06~5.97(m,3H),1.99(s,3H),1.29(s,18H);13C NMR(101 MHz,Acetone-d6),δ:194.4,158.7,153.5,148.5,138.1,136.9,135.9,131.8,128.8,128.7,128.2,127.4,127.0,124.9,124.8,118.8,118.4,111.9,91.9,59.2,34.3,29.7,19.8;ESI-HRMS(C36H37NNaO3+计算值),m/z:554.2666(554.2666)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),主要非对映体:tR=5.0 min,tR=5.7 min;次要非对映体:tR=8.1 min,tR=10.6 min;92∶8d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3fa),黄色固体,产率87%,m.p.170 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.73(t,J=7.9 Hz,1H),7.52(td,J=21.6,20.2,7.6 Hz,2H),7.30(ddd,J=21.8,16.5,9.2 Hz,3H),7.12~6.82(m,7H),6.69(s,2H),6.10(d,J=61.9 Hz,1H),1.23(s,7H),1.16(s,11H);13C NMR(101 MHz,DMSO-d6),δ:194.2,193.9,161.6,158.3,153.3,153.0,152.0,150.9,139.8,139.1,138.4,137.6,134.5,134.5,132.5,131.8,131.1,131.0,130.2,129.5,129.2,129.1,128.4,128.1,126.7,126.6,125.7,125.1,125.0,124.1,123.2,122.0,121.9,120.3,118.9,118.8,117.7,117.3,116.7,111.7,110.8,91.6,89.6,57.8,56.1,34.5,34.2,30.2,30.0;ESIHRMS(C35H34ClNNaO3+计算值),m/z:574.2124(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.9 min,tR=5.5 min;>99.5∶0.5d.r.
(5aR,12R)-3-bromo-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ga),黄色固体,产率81%,m.p.163 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.54(tt,J=14.6,7.6 Hz,3H),7.44~7.27(m,3H),7.17~6.96(m,6H),6.86~6.82(m,1H),6.73(d,J=4.7 Hz,1H),6.11(d,J=57.3 Hz,1H),5.95(d,J=8.4 Hz,1H),1.26(s,12H),1.19(s,6H);13C NMR(101 MHz,DMSO-d6),δ:193.7,161.4,158.0,156.8,153.1,152.8,151.9,150.7,139.6,138.9,138.2,137.4,134.2,130.8,130.2,129.6,129.0,128.9,128.2,127.9,126.9,126.5,125.9,124.9,124.9,124.6,123.9,121.5,119.8,118.6,117.1,116.5,111.5,110.6,99.3,91.4,57.6,56.0,34.3,34.0,30.0,29.8;ESI-HRMS(C35H34BrNNaO3+计算值),m/z:618.1619(618.1614)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),主要非对映体:tR=4.9 min,tR=5.6 min;次要非对映体:tR=9.1 min,tR=13.0 min;60∶40d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-3-methyl-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ha),黄色固体,产率90%,m.p.178 ℃.1H NMR(400 MHz,Acetone-d6),δ:7.62~7.59(m,2H),7.51(dd,J=16.4,8.1 Hz,1H),7.33~7.20(m,5H),7.16~7.06(m,1H),6.98~6.86(m,2H),6.73(dd,J=14.4,7.0 Hz,1H),6.61(d,J=7.9 Hz,1H),6.13(s,1H),6.01(t,J=8.0 Hz,2H),2.25(d,J=46.8 Hz,3H),1.31(d,J=27.1 Hz,18H);13C NMR(101 MHz,Acetone-d6),δ:195.8,163.6,160.1,155.0,154.7,153.2,152.0,140.2,139.9,139.6,139.4,138.4,138.3,137.4,137.2,133.4,133.3,130.3,129.9,129.7,129.5,129.3,128.7,128.4,127.2,127.0,126.5,126.3,126.1,125.1,124.0,121.8,121.4,120.6,119.8,119.8,119.7,113.2,112.2,93.3,60.4,59.1,35.8,35.6,31.2,31.1,21.8,21.6;ESI-HRMS(C36H37NNaO3+计算值),m/z:554.2659(554.2666)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=5.1 min,tR=5.7 min;次要非对映体:tR=8.6 min,tR=17.6 min;78∶22d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-3-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ia),黄色油状液体,产率74%.1H NMR(400 MHz,Acetone-d6),δ:7.58~7.55(m,2H),7.46(d,J=6.9 Hz,1H),7.30~7.17(m,6H),6.82(d,J=8.6 Hz,1H),6.71~6.67(m,2H),6.35(dd,J=8.6,2.5 Hz,1H),6.08(s,1H),5.97~5.92(m,2H),3.69(s,3H),1.31(s,18H);13C NMR(101 MHz,Acetone-d6),δ:195.6,161.0,160.1,155.0,153.1,139.6,138.5,137.1,133.3,130.3,130.2,128.3,126.5,126.3,121.1,119.8,113.1,110.7,105.1,101.4,93.3,60.2,56.2,31.2;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2616(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),主要非对映体:tR=5.8 min,tR=6.4 min;>99.5∶0.5d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-4-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ja),黄色固体,产率91%,m.p.162 ℃.1H NMR(400 MHz,Acetone-d6),δ:7.60(d,J=6.5 Hz,2H),7.46(d,J=9.2 Hz,1H),7.27~7.18(m,6H),6.72~6.67(m,3H),6.56(dd,J=6.9,2.4 Hz,1H),6.06(s,1H),6.01(d,J=8.4 Hz,1H),5.97(s,1H),3.85(s,3H),1.29(s,18H);13C NMR(101 MHz,Acetone-d6),δ:194.2,158.7,153.5,150.1,140.3,138.1,136.9,136.0,131.6,128.9,128.8,126.5,125.0,124.8,122.6,119.3,118.4(d,J=5.5 Hz),111.9,110.3,92.3,59.1,55.5,34.3,29.7;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2606(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),主要非对映体:tR=6.0 min,tR=7.0 min;>99.5∶0.5d.r.
(5aR,12R)-4-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ka),黄色固体,产率85%,m.p.171 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.56~7.54(m,1H),7.48(d,J=6.4 Hz,1H),7.34~7.24(m,4H),7.20(dd,J=8.0,1.4 Hz,1H),7.11(s,2H),6.99~6.96(m,2H),6.81(t,J=7.9 Hz,1H),6.72(t,J=7.4 Hz,1H),6.07(s,1H),5.95(d,J=8.4 Hz,1H),1.23(s,18H);13C NMR(101 MHz,DMSO-d6),δ:193.7,158.2,153.4,145,139.8,137.6,134.4,130.9,129.8,129.4,129.1,128.2,126.8,126.2,125.2,124.2,123.7,122.5,118.9,117.3,111.8,92.1,58.1,34.5,30.2,30.0;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2122(574.2119)[M+Na]+;HPLC(Chiralpak IF,Vhexane∶Vi-PrOH=25∶1,1.0 mL/min,254 nm),主要非对映体:tR=10.0 min,tR=11.1 min;次要非对映体:tR=14.6 min,tR=15.7 min;99∶1d.r.
(5aR,12R)-8-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ab),黄色固体,产率89%,m.p.163~164 ℃.1H NMR(400 MHz,DMSOd6),δ:7.52~7.49(m,3H),7.33~7.24(m,4H),7.13~7.03(m,4H),6.96(d,J=4.3 Hz,2H),6.80(t,J=7.5 Hz,1H),6.03~5.98(m,2H),1.23(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.0,157.6,153.8,150.2,140.3,137.5,134.9,131.8,129.7,129.6,128.6,128.4,127.8,127.2,124.6,124.6,123.6,123.1,119.5,119.1,113.9,92.4,58.7,35.0,30.7;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2112(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.6 min,tR=5.1 min;>99.5∶0.5d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-9-methyl-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ac),黄色固体,产率89%,m.p.200~201 ℃.1H NMR(400 MHz,DMSOd6),δ:7.52(d,J=7.2 Hz,2H),7.37~7.26(m,4H),7.12(dq,J=15.4,8.1 Hz,4H),7.02(s,1H),6.97(d,J=7.7 Hz,1H),6.83(t,J=7.4 Hz,1H),6.55(d,J=7.8 Hz,1H),5.97(s,1H),5.57(s,1H),2.01(s,3H),1.28(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.0,159.2,153.9,150.6,148.9,140.3,135.5,131.8,129.4,129.4,128.6,128.5,127.6,127.2,125.3,123.2,120.3,119.3,115.6,112.5,100.0,91.9,58.9,35.0,30.7,22.6;ESI-HRMS(C36H37NNaO3+计算值),m/z:554.2654(554.2666)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.9 min,tR=6.6 min;>99.5∶0.5d.r.
(5aR,12R)-4-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ad),黄色固 体,产 率79%,m.p.175~176 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.60~7.56(m,3H),7.36~7.10(m,8H),7.00~6.93(m,2H),6.87~6.83(m,1H),6.49(s,1H),6.11(d,J=24.2 Hz,1H),3.72(s,3H),1.26(s,16H),1.23(s,3H);13C NMR(101 MHz,DMSO-d6),δ:194.5,165.4,158.2,153.5,149.9,139.8,136.9,134.3,130.6,129.3,129.2,128.2,127.0,126.8,125.2,124.7,123.0,120.4,118.9,118.8,112.7,99.6,91.6,58.4,52.4,34.5,30.2;ESI-HRMS(C37H37NNaO5+计算值),m/z:598.2565(598.2564)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=5.2 min;次要非对映体:tR=8.2 min,tR=11.3 min;97∶3d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-9-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ae),黄色固 体,产率88%,m.p.126~128 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.53~7.48(m,2H),7.38~7.26(m,4H),7.17~7.07(m,5H),6.96(d,J=6.4 Hz,1H),6.84~6.80(m,1H),6.28~6.21(m,1H),5.99(s,1H),5.31(d,J=2.1 Hz,1H),3.40(s,2H),1.24(d,J=30.2 Hz,18H);13C NMR(101 MHz,DMSO-d6),δ:191.5,168.5,166.8,164.1,160.7,153.3,152.9,151.2,150.0,139.9,138.4,135.2,131.4,128.9,128.8,128.1,128.0,127.2,127.0,126.7,126.6,126.4,125.3,124.6,122.7,121.6,118.8,117.9,110.1,109.7,109.0,108.5,99.5,94.5,94.3,91.7,58.4,56.5,56.0,55.0,34.5,34.3,30.2,30.1;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2610(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=6.2 min,tR=7.4 min;次要非对映体:tR=20.4 min,tR=30.7 min;89∶11d.r.
(5aR,12R)-9-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3af),黄色固体,产率93%,m.p.187~188 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.48(dd,J=30.2,7.8 Hz,3H),7.28(dt,J=11.6,6.9 Hz,3H),7.15~7.03(m,5H),6.92(d,J=7.6 Hz,1H),6.79(t,J=7.4 Hz,1H),6.70(d,J=8.1 Hz,1H),5.99(s,1H),5.77(s,1H),1.25(s,18H);13C NMR(101 MHz,DMSO-d6),δ:193.5,159.1,154.1,150.3,142.9,140.4,134.8,131.0,129.7,129.5,128.6,128.6,127.2,127.1,126.9,125.3,123.4,119.3,119.1,116.5,112.2,92.0,58.9,35.0,30.7;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2121(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.7 min,tR=5.3 min;次要非对映体:tR=8.4 min,tR=11.6 min;>99.5∶0.5d.r.
(5aR,12R)-9-bromo-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ag),黄色固体,产率89%,m.p.185 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.52(d,J=7.4 Hz,2H),7.36(d,J=8.2 Hz,1H),7.32~7.23(m,3H),7.14~7.03(m,5H),6.91(d,J=7.8 Hz,1H),6.81(dd,J=19.5,7.7 Hz,2H),5.96(d,J=20.4 Hz,2H),1.25(s,18H);13C NMR(101 MHz,DMSO-d6),δ:193.3,158.6,153.6,149.9,139.9,134.2,132.0,130.5,129.2,129.1,128.1,128.1,126.7,126.6,126.4,124.8,122.9,121.4,118.8,116.2,114.8,91.3,58.4,34.5,30.2;ESI-HRMS(C35H34BrNNa计算值),m/z:618.1604(618.1614)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.7 min,tR=5.3 min;>99.5∶0.5d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-(4-fluorophenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ah),黄色固体,产率93%,m.p.145~147 ℃.1H NMR(400 MHz,DMSOd6),δ:7.60~7.53(m,2H),7.46(d,J=7.7 Hz,1H),7.26~6.93(m,8H),6.80(q,J=8.8,8.0 Hz,2H),6.68(dd,J=13.8,6.3 Hz,1H),6.03(s,1H),5.97(d,J=8.4 Hz,1H),1.18(d,J=26.7 Hz,18H);13C NMR(101 MHz,DMSO-d6),δ:194.9,163.0(d,J=245.6 Hz),159.0,153.7,153.4,151.5,150.2,140.2,139.6,138.8,138.0,132.0,131.9,129.6,129.5,128.5,128.4,128.1,126.2,125.6,125.5,124.6,123.6,122.7,122.1,120.6,119.4(d,J=25.3 Hz),118.1,117.8,116.5,116.3,115.3(d,J=22.1 Hz),112.5,111.3,91.5,58.7,56.3,34.9,34.7,30.7,30.5;19F NMR(376 MHz,DMSO-d6),δ:-112.63,-113.74;ESI-HRMS(C35H34FNNaO3+计算值),m/z:558.2403(558.2415)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=5.2 min,tR=6.3 min;次要非对映体:tR=9.3 min,tR=11.4 min;80∶20d.r.
(5aR,12R)-5a-(4-chlorophenyl)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ai),黄色油状液体,产率96%.1H NMR(400 MHz,Acetone-d6),δ:7.64(d,J=8.6 Hz,2H),7.52(d,J=7.6 Hz,1H),7.34~6.95(m,8H),6.88~6.73(m,2H),6.20~6.01(m,3H),1.31(d,J=22.5 Hz,18H);13C NMR(101 MHz,Acetone-d6),δ:195.6,195.4,163.3,160.1,155.1,154.8,153.1,151.9,140.0,139.6,138.6,138.3,136.2,135.9,135.2,133.0,130.4,130.3,130.3,129.6,129.5,129.2,127.1,126.5,126.3,124.3,124.1,123.1,121.6,120.4,120.1,119.6,119.3,119.2,113.5,112.2,92.8,90.9,60.6,58.4,35.7,35.5,31.1,31.0;ESIHRMS(C35H34ClNNaO3+计算值),m/z:574.2120(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=5.4 min,tR=8.3 min;次要非对映体:tR=9.2 min,tR=11.6 min;82∶18d.r.
(5aR,12R)-5a-(4-chlorophenyl)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3aj),黄色固体,产率90%,m.p.155~156 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.44(d,J=7.6 Hz,1H),7.31~6.93(m,11H),6.80(t,J=7.3 Hz,1H),6.68(t,J=7.4 Hz,1H),6.01(s,1H),5.95(d,J=8.4 Hz,1H),2.19(s,3H),1.23(s,18H);13C NMR(101 MHz,DMSO-d6),δ:195.0,158.9,153.6,150.5,140.2,138.7,137.9,135.4,132.1,130.2,129.4,128.4,128.1,127.5,125.5,124.6,124.4,123.4,119.5,119.0,117.9,112.3,100.0,92.0,58.7,35.0,34.7,30.7,30.5,21.5;ESI-HRMS(C36H37NNaO3+计算值),m/z:554.2660(554.2666)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.8 min;次要非对映体:tR=9.3 min,tR=10.2 min;98∶2d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-(3-methoxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3ak),黄色固体,产率95%,m.p.138~139 ℃.1H NMR(400 MHz,Acetone-d6),δ:7.50(d,J=7.6 Hz,1H),7.29(s,2H),7.25~7.07(m,6H),7.01(d,J=7.7 Hz,1H),6.82~6.77(m,2H),6.72(t,J=7.4 Hz,1H),6.11(s,1H),6.04(d,J=7.7 Hz,2H),3.71(s,3H),1.33(s,18H);13C NMR(101 MHz,Acetone-d6),δ:194.8,161.0,159.3,154.4,151.5,138.9,138.0,137.8,132.5,130.6,128.9,128.7,128.5,125.8,125.6,123.4,119.7,119.2,114.7,113.8,112.5,92.5,59.8,55.4,35.1,30.5;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2617(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=5.7 min,tR=7.4 min;>99.5∶0.5d.r.
(5aR,12R)-5a-(4-chlorophenyl)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3al),黄色固体,产率76%,m.p.178~180 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.64(d,J=1.8 Hz,1H),7.54(d,J=7.9 Hz,1H),7.47(t,J=7.8 Hz,2H),7.27(t,J=7.8 Hz,2H),7.15~7.04(m,5H),6.92(s,1H),6.83(t,J=7.4 Hz,1H),6.73~6.67(m,1H),6.09(s,1H),6.03(d,J=8.4 Hz,1H),1.22(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.5,159.2,153.6,150.1,140.2,138.4,138.1,132.6,132.1,131.7,129.7,128.6,128.5,128.2,126.4,125.6,124.6,123.8,122.7,119.5,119.4,117.7,112.8,91.3,58.7,34.9,30.7;ESI-HRMS(C35H34BrNNaO3+计算值),m/z:618.1616(618.1614)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.9 min,tR=5.2 min;次要非对映体:tR=10.0 min;92∶8d.r.
(5aR,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-(3-fluorophenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3am),黄色固体,产率83%,m.p.161~162 ℃.1H NMR(400 MHz,DMSOd6),δ:7.47(d,J=7.6 Hz,1H),7.37~7.24(m,4H),7.16~7.01(m,6H),6.93(s,1H),6.82(t,J=7.4 Hz,1H),6.71(t,J=7.4 Hz,1H),6.08(s,1H),6.01(d,J=8.4 Hz,1H),1.22(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.0,162.4(d,J=244.8 Hz),158.6,153.1,149.7,139.7,138.2,137.6,131.5,131.1,131.0,128.1,128.0,127.7,125.1,124.1,123.2,122.8,119.0,118.8,117.2,116.1(d,J=21.1 Hz),113.6(d,J=22.8 Hz),112.2,90.9,58.2,34.4,30.2;19F NMR(376 MHz,DMSO-d6),δ:-111.87,-113.13;ESI-HRMS(C35H34FNNaO3+计算值),m/z:558.2416(558.2415)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.8 min,tR=5.2 min;次要非对映体:tR=9.9 min;98∶2d.r.
(5aR,12R)-5a-(3-chlorophenyl)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(3an),黄色固体,产率96%,m.p.169~171 ℃.1H NMR(400 MHz,DMSOd6),δ:7.50~7.46(m,3H),7.33~7.24(m,3H),7.15~7.03(m,5H),6.92(s,1H),6.84~6.77(m,1H),6.73~6.66(m,1H),6.08(s,1H),6.02(d,J=8.4 Hz,1H),1.21(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.5,159.2,153.6,150.1,140.2,138.2,138.1,134.1,132.0,131.4,129.7,128.6,128.5,128.2,126.9,126.0,125.6,124.6,123.8,119.5,119.4,117.7,112.8,91.4,58.7,34.9,34.7,30.7,30.5;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2122(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=4.8 min;次要非对映体:tR=10.1 min;96∶4d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4aa),黄色固体,产率85%,m.p.141~142 ℃.1H NMR(400 MHz,Acetone-d6),δ:7.74~7.70(m,1H),7.60(d,J=7.6 Hz,1H),7.55(d,J=8.3 Hz,1H),7.29~7.09(m,7H),6.97(t,J=7.6 Hz,1H),6.93~6.89(m,J=7.3 Hz,4H),6.09(s,1H),5.99(s,1H),1.27(s,18H);13C NMR(101 MHz,Acetone-d6),δ:195.9,163.5,154.7,153.4,139.9,138.3,137.3,133.2,130.2,129.7,129.5,128.6,128.4,127.2,127.0,126.3,124.8,123.0,121.4,119.5,112.2,91.4,59.1,35.6,31.1;ESI-HRMS(C35H35NNaO3+计算值),m/z:540.2510(540.2509)[M+H]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),次要非对映体:tR=5.1 min,tR=5.6 min;主要非对映体:tR=8.9 min,t=9.4 min;4∶96d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-fluoro-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ba),黄色固体,产率88%,m.p.206 ℃.1H NMR(400 MHz,Acetone-d6),δ:7.73(t,J=7.1 Hz,1H),7.61(d,J=8.8 Hz,1H),7.53(d,J=8.2 Hz,1H),7.32~7.22(m,3H),7.18~7.06(m,4H),6.99(t,J=7.4 Hz,1H),6.94(s,2H),6.63(dd,J=9.4,3.5 Hz,1H),6.14(d,J=24.9 Hz,1H),6.04(s,1H),1.35(s,2H),1.29(s,16H);13C NMR(101 MHz,Acetone-d6),δ:195.7,163.4,158.7(d,J=239.3 Hz),154.9,149.5,140.0,139.8,138.5,137.1,132.6,130.4,130.3,129.8,129.6,128.6,128.5,127.1,127.0,126.7,126.7,126.5,126.3,121.7,121.0,121.0,120.1,119.4,117.1(d,J=23.7 Hz),115.9(d,J=24.0 Hz),113.3,112.4,91.7,60.5,59.3,35.8,35.6,31.1,31.1;19F NMR(376 MHz,DMSO-d6),δ:-119.64,-121.04;ESI-HRMS(C35H34FNNaO3+计算值),m/z:558.2413(558.2415)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),次要非对映体:tR=5.3 min,tR=5.7 min;主要非对映体:tR=7.0 min,tR=9.6 min;10∶90d.r.
(5aS,12R)-2-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ca),黄色固体,产率80%,m.p.222 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.75(t,J=7.9 Hz,1H),7.58(d,J=7.6 Hz,1H),7.51(d,J=8.3 Hz,1H),7.33~7.26(m,2H),7.14~6.95(m,6H),6.86(d,J=15.3 Hz,2H),6.69(s,2H),6.23(s,1H),1.16(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.3,161.6,153.1,150.1,139.1,138.5,134.6,130.7,128.7,128.3,128.0,127.9,126.6,125.7,125.2,125.0,120.4,119.7,116.9,110.9,99.5,89.7,56.1,34.3,30.0;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2120(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=5.2 min,tR=5.5 min;主要非对映体:tR=6.6 min,tR=9.7 min;<0.5∶99.5d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4da),黄色固体,产率75%,m.p.219 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.77~7.72(m,1H),7.58(dd,J=7.6,1.3 Hz,1H),7.49(d,J=8.4 Hz,1H),7.18(d,J=8.9 Hz,1H),7.15~7.12(m,1H),7.07(d,J=4.3 Hz,4H),6.96(t,J=7.4 Hz,1H),6.88(dd,J=8.9,3.0 Hz,1H),6.84(s,1H),6.79(s,2H),6.36(d,J=2.3 Hz,1H),6.11(s,1H),3.58(s,3H),1.20(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.9,161.9,153.6,152.9,144.9,139.0,138.5,131.3,128.1,127.9,126.7,125.6,125.0,123.9,120.1,118.7,117.0,114.8,112.9,110.9,89.6,57.0,55.4,34.3,30.1;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2606(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=6.0 min,tR=7.4 min;主要非对映体:tR=9.2 min,tR=14.2 min;4∶96d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-methyl-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ea),黄色固体,产率83%,m.p.190 ℃.1H NMR(400 MHz,Acetone-d6),δ:7.68~7.64(m,1H),7.51(dd,J=19.5,7.9 Hz,2H),7.16~7.03(m,7H),6.89(d,J=14.8 Hz,3H),6.66(s,1H),5.99(s,1H),5.93(s,1H),2.10(s,3H),1.23(s,18H);13C NMR(101 MHz,Acetone-d6),δ:194.6,162.1,153.2,149.8,138.4,136.8,135.9,131.8,130.8,129.4,128.8,128.2,128.0,127.2,125.7,125.5,122.9,119.9,117.9,117.8,110.8,89.9,57.7,34.1,29.6,19.7;ESI-HRMS(C36H37NNaO3+计算值),m/z:554.2658(554.2666)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=8.4 min,tR=10.8 min;<0.5∶99.5d.r.
(5aS,12R)-3-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4fa),黄色固体,产率78%,m.p.211 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.73(t,J=7.9 Hz,1H),7.55(dd,J=17.8,8.0 Hz,2H),7.37(s,1H),7.14~6.93(m,7H),6.87~6.82(m,2H),6.69(s,2H),6.17(s,1H),1.16(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.7,162.1,153.5,152.5,139.6,138.9,135.0,133.0,131.6,130.7,128.9,128.6,127.1,126.2,125.5,122.4,120.8,118.2,117.2,111.3,100.0,90.1,56.6,34.7,30.5;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2114(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=4.9 min,tR=5.4 min;主要非对映体:tR=8.3 min,tR=11.8 min;4∶96d.r.
(5aS,12R)-3-bromo-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ga),黄色固体,产率77%,m.p.209 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.73(t,J=7.1 Hz,1H),7.58~7.49(m,3H),7.14~7.02(m,6H),6.95(t,J=7.4 Hz,1H),6.83~6.78(m,2H),6.69(s,2H),6.15(s,1H),1.23(s,1H),1.15(s,17H);13C NMR(101 MHz,DMSO-d6),δ:194.2,161.6,153.1,152.1,139.1,138.4,134.5,131.0,130.4,128.4,128.1,126.7,125.7,125.1,124.8,122.5,120.7,120.5,120.3,116.7,110.9,89.6,56.2,34.3,30.1;ESI-HRMS(C35H34BrNNaO3+计算值),m/z:618.1614(618.1614)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),次要非对映体:tR=5.0 min,tR=5.6 min;主要非对映体:tR=9.3 min,tR=13.3 min;6∶94d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-3-methyl-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ha),黄色固体,产率84%,m.p.144~146 ℃.1H NMR(400 MHz,DMSOd6),δ:7.70(t,J=7.8 Hz,1H),7.54(d,J=7.6 Hz,1H),7.48(d,J=8.3 Hz,1H),7.10~7.02(m,6H),6.91(t,J=7.4 Hz,1H),6.79(s,1H),6.73~6.67(m,4H),6.05(s,1H),2.24(s,3H),1.15(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.8,161.8,152.9,151.0,139.0,138.3,135.1,131.6,128.3,128.2,128.0,126.7,125.6,125.1,122.6,120.0,119.9,117.9,116.8,110.8,89.3,56.6,34.2,30.1,20.7;ESI-HRMS(C36H37NNaO3+计算值),m/z:554.2657(554.2666)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=8.6 min,tR=17.3 min;<0.5∶99.5d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-3-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ia),黄色固体,产率49%,m.p.202 ℃.1H NMR(400 MHz,Acetone-d6),δ:7.74(t,J=7.8 Hz,1H),7.62(d,J=6.3 Hz,1H),7.55(d,J=8.4 Hz,1H),7.23~7.11(m,5H),7.01~6.94(m,3H),6.83(d,J=2.5 Hz,1H),6.79(d,J=8.6 Hz,1H),6.55(dd,J=8.6,2.5 Hz,1H),6.00(d,J=11.3 Hz,2H),3.84(s,3H),1.29(s,18H);13C NMR(101 MHz,Acetone-d6),δ:194.5,162.1,160.2,153.2,152.8,138.4,136.8,135.8,132.0,129.3,128.2,128.0,127.2,125.6,125.5,119.9,117.7,115.1,110.7,108.7,102.6,90.0,57.4,54.9,34.1,29.6;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2615(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=10.2 min,tR=12.2 min;<0.5∶99.5d.r.
(5aS,12R)-2-(3,5-di-tert-butyl-4-hydroxyphenyl)-4-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ja),黄色固体,产率85%,m.p.194~196 ℃.1H NMR(400 MHz,Acetoned6),δ:7.69~7.65(m,1H),7.56(d,J=7.7 Hz,1H),7.45(d,J=8.3 Hz,1H),7.25(d,2H),7.16~7.08(m,3H),6.96~6.89(m,4H),6.80(t,J=8.0 Hz,1H),6.35(d,J=7.8 Hz,1H),5.95(d,J=14.1 Hz,2H),3.90(s,3H),1.25(s,18H);13C NMR(101 MHz,Acetone-d6),δ:194.4,162.3,153.3,149.5,141.5,138.4,137.0,135.9,132.0,128.4,128.1,127.0,125.8,125.5,121.3,120.1,120.0,118.0,111.0,110.8,90.3,58.2,55.5,34.2,29.7;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2607(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,210 nm),主要非对映体:tR=7.1 min,tR=10.2 min;<0.5∶99.5d.r.
(5aS,12R)-4-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ka),黄色固体,产率97%,m.p.185 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.74(t,J=7.8 Hz,1H),7.56(dd,J=14.0,8.0 Hz,2H),7.40(d,J=6.5 Hz,1H),7.15~7.11(m,1H),7.09~7.02(m,4H),6.94(q,J=7.8 Hz,2H),6.82(d,J=8.1 Hz,2H),6.70(s,2H),6.22(s,1H),1.16(s,18H);13C NMR(101 MHz,DMSO-d6),δ:193.9,161.5,153.1,146.8,139.1,138.4,134.2,131.1,129.0,128.6,128.2,127.5,126.3,125.7,125.1,122.2,121.6,120.4,116.7,110.9,90.1,56.5,34.2,30.0;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2109(574.2119)[M+Na]+;HPLC(Chiralpak IF,Vhexane∶Vi-PrOH=25∶1,1.0 mL/min,254 nm),次要非对映体:tR=10.1 min,tR=11.3 min;主要非对映体:tR=14.4 min,tR=15.6 min;2∶98d.r.
(5aS,12R)-8-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ab),黄色固体,产率66%,m.p.140~141 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.74(d,J=8.7 Hz,1H),7.55(d,J=9.1 Hz,2H),7.25~7.18(m,2H),7.08~7.01(m,5H),6.91(t,J=7.4 Hz,1H),6.83~6.78(m,2H),6.68(s,2H),6.16(s,1H),1.13(s,18H);13C NMR(101 MHz,DMSO-d6),δ:193.7,160.3,153.0,151.0,138.5,138.3,134.5,131.2,128.9,128.6,128.4,128.0,126.6,125.1,124.7,123.9,122.7,121.8,118.1,117.8,112.6,89.7,56.5,34.2,30.0;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2123(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=6.6 min,tR=11.9 min;<0.5∶99.5d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-9-methyl-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ac),黄色固体,产率70%,m.p.186~188 ℃.1H NMR(400 MHz,DMSOd6),δ:7.47(d,J=7.8 Hz,1H),7.39(s,1H),7.25(dt,J=15.1,7.9 Hz,2H),7.13~7.04(m,5H),6.94(t,J=7.5 Hz,1H),6.87~6.76(m,5H),6.12(s,1H),2.42(s,3H),1.19(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.4,162.6,153.4,151.7,150.9,138.8,135.8,132.0,129.2,129.1,128.6,128.5,127.1,125.9,125.8,123.6,122.2,121.9,118.3,115.1,111.6,90.3,57.1,34.8,30.6,22.9;ESI-HRMS(C36H37NNaO3+计算值),m/z:554.2623(554.2666)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=11.5 min,tR=22.4 min;<0.5∶99.5d.r.
(5aS,12R)-methyl12-(3,5-di-tert-butyl-4-hydroxyphenyl)-6-oxo-5a-phenyl-5a,6-dihydro-12H-benzo[5,6][1,3]oxazino[3,2-a]indole-9-carboxylate(4ad),黄色固体,产率79%,m.p.180~181 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.60~7.56(m,3H),7.36~7.10(m,8H),7.00~6.93(m,2H),6.87~6.83(m,1H),6.49(s,1H),6.11(d,J=24.2 Hz,1H),3.72(s,3H),1.26(s,16H),1.23(s,3H);13C NMR(101 MHz,DMSO-d6),δ:194.5,165.4,158.2,153.5,149.9,139.8,136.9,134.3,130.6,129.3,129.2,128.2,127.0,126.8,125.2,124.7,123.0,120.4,118.9,118.8,112.7,99.6,91.6,58.4,52.4,34.5,30.2;ESI-HRMS(C37H37NNaO5+计算值),m/z:598.2566(598.2564)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=5.2 min;主要非对映体:tR=8.3 min,tR=11.4min;2∶98d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-9-methoxy-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ae),黄色固体,产率73%,m.p.135~137 ℃.1H NMR(400 MHz,DMSOd6),δ:7.50(dd,J=10.9,7.8 Hz,1H),7.38~7.23(m,2H),7.17~7.05(m,6H),6.99~6.92(m,1H),6.84~6.80(m,3H),6.51(dd,J=8.6,2.0 Hz,1H),6.20(s,1H),3.95(s,2H),1.23(d,J=30.4 Hz,18H);13C NMR(101 MHz,DMSO-d6),δ:191.9,168.3,163.9,152.8,151.1,139.8,138.3,135.3,131.6,128.8,128.6,128.4,128.0,127.9,127.0,126.6,125.1,123.0,121.5,117.8,109.6,108.9,94.1,89.8,56.4,55.8,54.8,34.3,34.1,30.1,29.9;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2617(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=6.3 min,tR=7.6 min;主要非对映体:tR=21.1 min,tR=31.9 min;24∶76d.r.
(5aS,12R)-9-chloro-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4af),黄色固体,产率88%,m.p.193~195 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.67(d,J=9.8 Hz,1H),7.57(d,J=8.1 Hz,1H),7.27~7.21(m,2H),7.13~7.01(m,5H),6.94(t,J=8.5 Hz,2H),6.83(d,J=10.9 Hz,2H),6.72(s,2H),6.23(s,1H),1.38(s,1H),1.16(s,17H);13C NMR(101 MHz,DMSO-d6),δ:193.9,192.4,162.5,153.5,151.5,144.4,139.1,138.8,135.1,131.5,129.3,129.1,128.8,128.5,127.7,127.3,127.1,125.8,123.3,122.3,120.7,118.4,116.2,111.4,90.2,57.0,34.7,30.5;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2111(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=4.7 min,tR=5.3 min;主要非对映体:tR=8.4 min,tR=11.6 min;1∶99d.r.
(5aS,12R)-9-bromo-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-phenyl-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ag),黄色固体,产率76%,m.p.168~170 ℃.1H NMR(400 MHz,DMSOd6),δ:7.82(s,1H),7.48(d,J=8.2 Hz,1H),7.38~7.20(m,2H),7.13~7.03(m,6H),6.92(t,J=7.5 Hz,1H),6.81(d,J=11.3 Hz,2H),6.72(s,2H),6.21(s,1H),1.26(s,1H),1.16(s,17H);13C NMR(101 MHz,DMSO-d6),δ:194.2,162.4,153.6,151.5,138.9,135.1,133.9,131.5,129.3,129.1,128.9,128.6,127.7,127.1,125.8,123.5,123.4,122.4,118.4,116.5,114.4,90.1,57.1,34.8,30.6;ESI-HRMS(C35H34BrNNaO3+计算值),m/z:618.1606(618.1614)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=4.1 min,tR=4.5 min;主要非对映体:tR=6.7 min,tR=10.9 min;2∶98d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-(4-fluorophenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ah),黄色固体,产率83%,m.p.154~156 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.72(t,J=7.7 Hz,1H),7.57(dd,J=10.8,8.0 Hz,2H),7.27~7.17(m,2H),7.01~6.91(m,5H),6.81~6.77(m,3H),6.66(s,2H),6.23(s,1H),1.15(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.5,161.8(d,J=245.2 Hz),161.5,152.9,151.0,139.1,138.3,131.4,131.1,128.9,128.8,128.8,128.7,125.7,124.9,122.2,121.6,120.1,117.6,116.8,114.8(d,J=21.8 Hz),110.7,88.9,55.8,34.2,30.0;19F NMR(376 MHz,DMSO-d6),δ:-100.01,-113.74;ESIHRMS(C35H34FNNaO3+计算值),m/z:558.2404(558.2415)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=5.2 min,tR=6.3 min;主要非对映体:tR=9.3 min,tR=11.4 min;2∶98d.r.
(5aS,12R)-5a-(4-chlorophenyl)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ai),黄色固体,产率78%,m.p.166~168 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.74~7.70(m,1H),7.57(dd,J=13.8,8.0 Hz,2H),7.24~7.16(m,2H),7.04(d,J=8.7 Hz,2H),6.98~6.91(m,5H),6.80(s,1H),6.66(s,2H),6.23(s,1H),1.15(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.3,161.5,152.9,150.9,139.1,138.2,134.1,133.1,131.3,128.9,128.8,128.5,127.9,125.7,125.0,122.3,121.7,120.2,117.6,116.7,110.8,88.8,55.8,34.2,30.0;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2120(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=9.2 min,tR=11.5 min;<0.5∶99.5d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-(m-tolyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4aj),黄色固体,产率83%,m.p.145~146 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.71(t,J=7.8 Hz,1H),7.56(dd,J=14.6,7.9 Hz,2H),7.26~7.17(m,2H),7.00~6.86(m,6H),6.77(s,1H),6.67(s,2H),6.51(s,1H),6.21(s,1H),1.90(s,3H),1.14(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.6,161.4,152.8,151.2,139.0,138.0,136.9,134.7,131.8,129.0,128.8,127.9,127.2,125.7,125.0,123.8,122.2,121.4,119.8,117.7,116.7,110.5,89.1,55.8,34.2,30.0,20.8;ESI-HRMS(C36H37NNaO3+计算值),m/z:554.2668(554.2666)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=4.8 min;主要非对映体:tR=9.3 min,tR=10.1 min;1∶99d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-(3-methoxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4ak),黄色固体,产率85%,m.p.154~155 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.72(t,J=7.9 Hz,1H),7.56(t,J=7.5 Hz,2H),7.25~7.18(m,2H),7.03(t,J=8.0 Hz,1H),6.92(t,J=7.7 Hz,2H),6.85(d,J=7.8 Hz,1H),6.80~6.76(m,2H),6.71(s,2H),6.66(d,J=8.3 Hz,1H),6.31(s,1H),6.17(s,1H),1.14(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.9,162.0,159.2,153.4,151.7,139.5,138.7,136.8,132.3,129.7,129.3,126.2,125.7,123.0,122.1,120.4,119.5,118.3,117.2,114.9,112.4,111.2,89.6,56.6,54.9,34.7,30.5;ESI-HRMS(C36H37NNaO4+计算值),m/z:570.2614(570.2615)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),主要非对映体:tR=10.2 min,tR=11.6 min;<0.5∶99.5d.r.
(5aS,12R)-5a-(3-bromophenyl)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4al),黄色固体,产率60%,m.p.163~165 ℃.1H NMR(400 MHz,DMSO-d6),δ:7.74(t,J=7.7 Hz,1H),7.60(dd,J=24.9,8.0 Hz,2H),7.28~7.19(m,3H),7.06~6.93(m,6H),6.77(s,1H),6.66(s,2H),6.27(s,1H),1.16(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.7,162.1,153.5,151.4,139.7,138.6,138.2,131.7,130.6,129.6,129.5,129.3,126.3,126.1,125.3,122.6,122.2,121.8,120.8,118.0,117.2,111.4,89.0,56.2,34.7,30.5;ESI-HRMS(C35H34BrNNaO3+计算值),m/z:618.1614(618.1614)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=4.9 min,tR=5.3 min;主要非对映体:tR=10.0 min;1∶99d.r.
(5aS,12R)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-5a-(3-fluorophenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4am),黄色固体,产率90%,m.p.155~156 ℃.1H NMR(400 MHz,DMSOd
6),δ:7.72(t,J=7.7 Hz,1H),7.58(dd,J=11.5,8.0 Hz,2H),7.27~7.18(m,2H),7.08(q,J=7.0,6.2 Hz,1H),6.91(dd,J=12.2,7.1 Hz,5H),6.78(s,1H),6.68(d,J=11.7 Hz,3H),6.23(s,1H),1.15(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.1,161.7(d,J=243.5 Hz),161.7,152.9,150.9,139.1,138.2,138.1,138.0,131.2,130.0,129.9,128.9,128.7,125.7,124.9,122.7,122.4,121.7,120.3,117.6,116.8,115.2(d,J=20.9 Hz),113.4(d,J=23.5 Hz),110.9,88.8,56.0,34.2,30.0;19F NMR(376 MHz,DMSO-d6),δ:-111.86,-113.13;ESI-HRMS(C35H34FNNaO3+计算 值),m/z:558.2404(558.2415)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=4.8 min,tR=5.2 min;主要非对映体:tR=9.9 min;4∶96d.r.
(5aR,12R)-5a-(3-chlorophenyl)-12-(3,5-di-tert-butyl-4-hydroxyphenyl)-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-6(5aH)-one(4an),黄色固体,产率96%,m.p.151~152 ℃.1H NMR(400 MHz,DMSOd6),δ:7.71(t,J=7.8 Hz,1H),7.61(d,J=8.4 Hz,1H),7.55(d,J=7.7,1H),7.23~6.99(m,5H),6.93~6.90(m,3H),6.82(s,1H),6.75(s,1H),6.65(s,2H),6.25(s,1H),1.13(s,18H);13C NMR(101 MHz,DMSO-d6),δ:194.1,161.6,153.0,150.9,139.2,138.1,137.5,132.8,131.2,129.8,129.0,128.8,128.3,126.3,125.8,125.2,124.8,122.1,121.7,120.3,117.6,116.7,110.9,88.7,55.7,34.2,30.0;ESI-HRMS(C35H34ClNNaO3+计算值),m/z:574.2110(574.2119)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=90∶10,1.0 mL/min,254 nm),次要非对映体:tR=4.8 min,tR=5.2 min;主要非对映体:tR=10.1 min;1∶99d.r.
tert-butyl(2,6-di-tert-butyl-4-((5aR,12R)-6-oxo-5a-phenyl-5a,6-dihydro-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-12-yl)phenyl)carbonate(5),黄色油状 液体,产率92%.1H NMR(400 MHz,Acetone-d6),δ:7.76~7.72(m,1H),7.61(t,J=8.2 Hz,2H),7.31(td,J=7.5,6.6,2.0 Hz,1H),7.25~7.11(m,7H),7.05(d,J=2.2 Hz,1H),7.02~6.92(m,3H),6.21(s,1H),1.52(s,9H),1.19(d,J=7.8 Hz,18H);13C NMR(101 MHz,Acetone-d6),δ:194.3,161.9,152.4,151.9,147.7,142.5,142.2,138.5,137.8,135.5,129.0,128.8,128.4,128.2,127.2,127.1,126.7,125.6,122.4,121.7,120.2,118.2,117.8,110.7,89.9,82.5,57.1,35.1,35.0,30.7,27.1;ESI-HRMS(C40H43NNaO5+计算值),m/z:640.3020(640.3033)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=19∶1,1.0 mL/min,254 nm),主要非对映体:tR=4.5 min,tR=5.5 min;次要非对映体:tR=11.5 min,tR=12.3 min;80∶20d.r.
tert-butyl(2,6-di-tert-butyl-4-((5aS,12R)-6-oxo-5a-phenyl-5a,6-dihydro-12H-benzo[5,6][1,3]oxazino[3,2-a]indol-12-yl)phenyl)carbonate(6),黄色油状液体,产率98%.1H NMR(400 MHz,Acetoned6),δ:7.64~7.62(m,2H),7.53~7.51(m,1H),7.44(s,1H),7.33~7.06(m,7H),7.00~6.95(m,1H),6.84~6.80(m,1H),6.78~6.75(m,1H),6.19(s,1H),6.07(d,J=8.4 Hz,1H),1.52(d,J=7.2 Hz,9H),1.30(s,8H),1.23~1.19(m,10H);13C NMR(101 MHz,Acetone-d6),δ:195.2,195.0,159.2,152.7,151.5,139.4,138.5,138.1,136.4,129.9,129.8,129.7,129.2,129.1,128.9,128.0,127.9,127.8,127.5,127.1,126.8,126.4,125.9,123.7,122.5,121.1,120.0,119.5,119.2,119.0,118.7,112.7,92.7,83.5,59.7,36.1,31.6,31.5,31.5,27.9;ESI-HRMS(C40H43NNaO5+计算值),m/z:640.3018(640.3033)[M+Na]+;HPLC(Chiralpak IA,Vhexane∶Vi-PrOH=19∶1,1.0 mL/min,254 nm),主要非对映体:tR=11.3 min,tR=12.1 min;<0.5∶99.5d.r.
2 结果与讨论
2.1 反应条件的优化
以无取代的邻羟基苯基取代对亚甲基醌(1a)与无取代的酮亚胺(2a)的反应作为模型,以消旋磷酸(PA)为催化剂,在二氯甲烷中反应进行反应条件的筛选.反应30 min后,以99%的产率得到预期产物3aa,反应的非对映选择性为96∶4(表1 中Entry 1).随后,考察了溶剂和添加物对反应的影响,由表1可见,当溶剂为二氯甲烷时为最优反应条件.将摩尔分数为5%的B(C6F5)3加入到反应体系中反应12 h后,以54%的产率和2∶98的非对映选择性得到产物4aa(表2中Entry 1).对反应溶剂和添加物进行筛选发现,当以三氯甲烷为溶剂时反应的收率达到90%,非对映选择性达到4∶96(表2中Entry 4).

Table 1 Optimization of the phosphoric catalytic reaction conditionsa
2.2 邻羟基苯基取代对亚甲基醌的底物拓展
将PA催化体系的最优反应条件应用于不同取代的邻羟基苯基取代对亚甲基醌(1)与酮亚胺(2a)的环加成反应,考察了反应体系的普适性,结果见Scheme 5.实验还考察了p-QMs 苯环上的取代基对产物产率和非对映选择性的影响.结果表明,无论是吸电子基团还是给电子基团取代的邻羟基苯基取代对亚甲基醌均能很好地适应反应条件,产物3ba~3ka均获得了良好的收率(74%~99%)和优异的非对构选择性(>99.5∶0.5d.r.).对于PA和B(C6F5)3协同催化的反应体系,同样进行了p-QMs苯环上一系列取代基团的拓展,结果表明,无论基团电子性质如何,各取代基团都具有良好的普适性(化合物4ba~4ka的产率高达97%,<0.5∶99.5d.r.)(Scheme 6).

Table 2 Optimization of the phosphoric-B(C6F5)3 catalytic reaction conditionsa

Scheme 6 Scope of p-QMs for phosphoric-B(C6F5)3 catalytic system
2.3 酮亚胺的底物拓展
对于PA催化体系,考察了不同取代基的化合物2对产物非对映选择性和产率的影响.由Scheme 7可见,在最佳条件下,当骨架苯环4,5 号位为—Me,—OMe 等给电子基团和卤素等吸电子基团时,所得产物3ab~3ag具有较高的产率(79%~93%)和高的非对映选择性(>99.5∶0.5d.r.).此外,无论取代苯环上的间位和对位基团是吸电子基还是给电子基,都获得了优异的非对映选择性(高达98∶2d.r.),其产率可达76%~96%.随着化合物3的高非对映选择性合成范围的扩大,化合物4的合成范围引起了关注(Scheme 8).在最优反应条件下,无论骨架苯环上取代基性质如何,产物4ab~4ag都具有较高的产率(70%~88%)和非对映选择性(高达<0.5∶99.5d.r.).采用相同条件测试了一系列间位取代和对位取代的化合物2,结果表明,反应物在此条件下均可很好地反应.化合物3ka(CCDC:2252433)和4ka(CCDC:2252430)的绝对构型通过X射线衍射分析确定,其它产物的绝对构型通过类比分配.

Scheme 7 Scope of 2-aryl-3H-indol-3-ones for phosphoric catalytic system
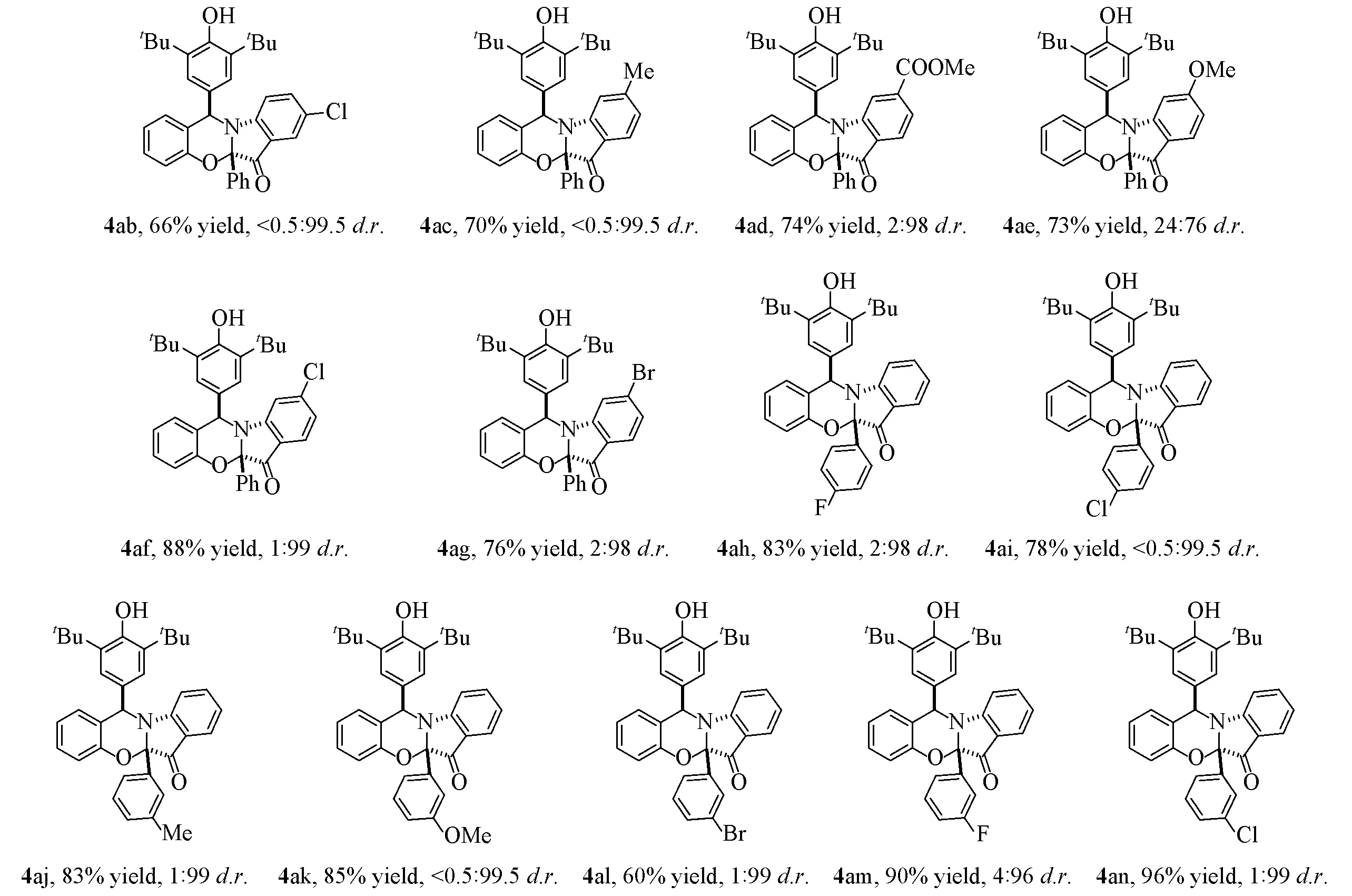
Scheme 8 Scope of 2-aryl-3H-indol-3-ones for phosphoric-B(C6F5)3 catalytic system
2.4 扩大化和衍生化反应
为了验证该方法的应用性,对化合物1a和2a在最优反应条件下进行了克级反应.结果表明,反应进行顺利,化合物3aa的产率为85%,非对映选择性为96∶4d.r.,化合物4aa的产率为87%,非对映选择性<0.5∶99.5d.r.[Scheme 9(A)].产物可进一步转化,在4-二甲氨基吡啶(DMAP)条件下,化合物3aa与Boc2O反应生成化合物5[24],得到90%的产率和80∶20d.r.,化合物4aa反应生成化合物6,具有较高的产率,且产物的非对映选择性得以保持[Scheme 9(B)].

Scheme 9 Large-scale reaction and synthetic transformation
2.5 可能的反应机理
根据单晶表征结果,提出了一个可能的过渡态.如Scheme 10所示,在磷酸催化时,反应以分子内的方式进行,磷酸通过氢键的方式活化p-QMs,酮亚胺从内部进攻,生成化合物3aa.当加入B(C6F5)3时,其与磷酸形成络合物,B(C6F5)3具有大位阻,占据了内部空间,并迫使酮亚胺从外部进攻,从而产生非对映选择性不同的产物.
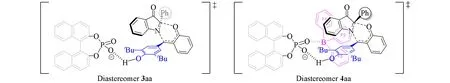
Scheme 10 Proposed reaction transition states
2.6 不对称反应
对化合物1a和2a之间的[4+2]不对称环加成反应进行了研究.由表3可见,尝试采用一系列手性磷酸和方酰胺催化该反应(催化剂结构见本文支持信息图S133).遗憾的是,在所有条件下,产物3aa的对映选择性都很低(0~16%e.e.),这意味着控制该环加成反应的对映选择性极具挑战.
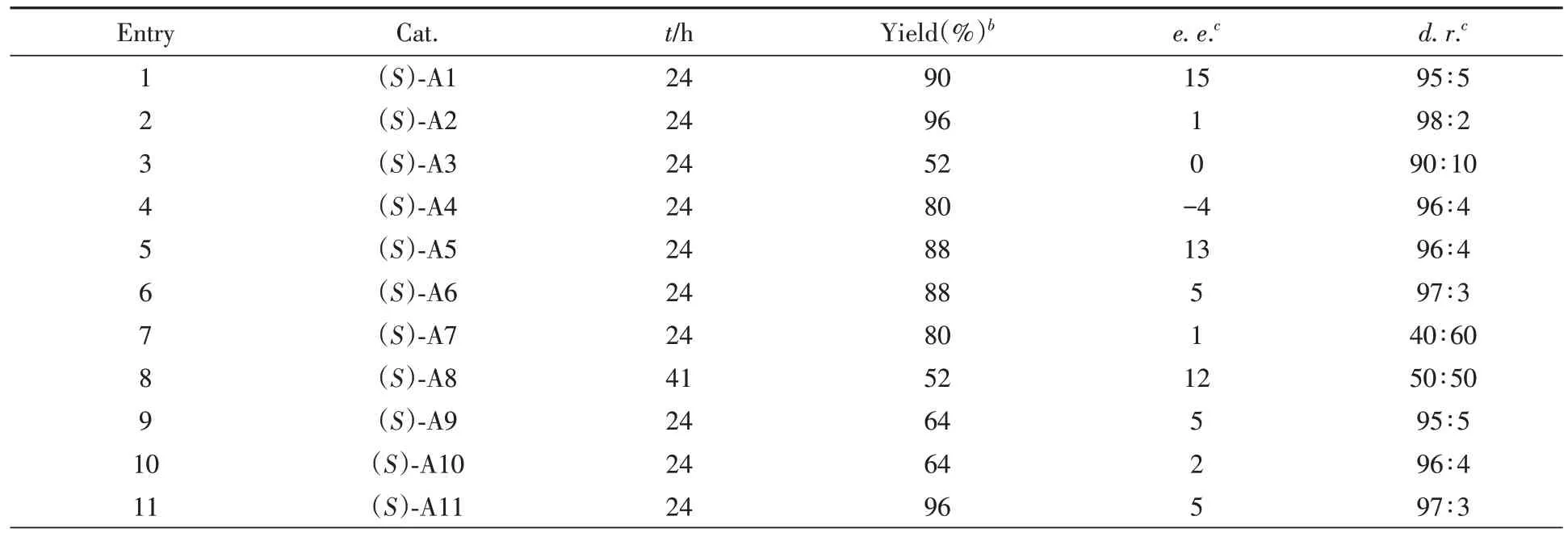
Table 3 Preliminary investigation on the catalytic asymmetric versiona
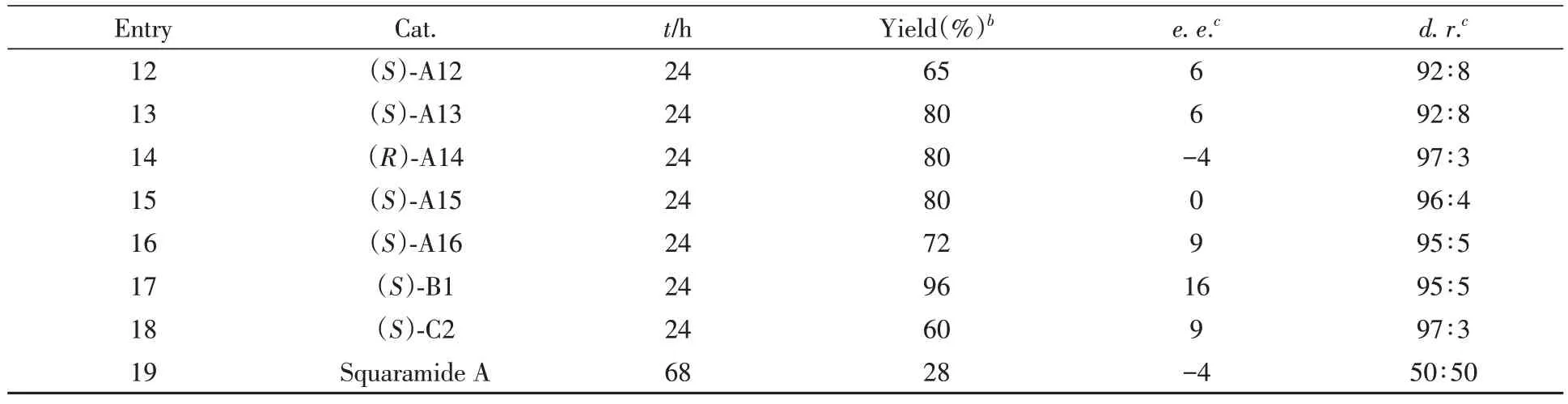
Continued
3 结论
开发了一种磷酸催化p-QMs和酮亚胺的[4+2]环加成反应,并通过简单地加入B(C6F5)3获得了具有相反非对映选择性的二氢-1,3-苯并噁嗪化合物.利用该方法,以高收率和优异的非对映选择性合成了多种二氢-1,3-苯并噁嗪化合物.产物的克级合成和转化反应结果表明该方法具有合成效用.此外,该反应还为合成二氢-1,3-苯并噁嗪类化合物提供了一种新方法.
支持信息见http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20230473.

