不对称二亚胺席夫碱的合成、表征和抗菌活性
Nartop Dilek Gürkan Perihan
(1Department of Chemistry,Faculty of Arts and Science,Nevs¸ehir University,Nevs¸ehir 50300,Turkey(土耳其))
(2Department of Chemistry,Faculty of Science,Gazi University,Ankara 06500,Turkey(土耳其))
0 Introduction
Schiff bases,named after Schiff[1],are one of the most widely used compounds in Coordination Chemistry.Some Schiff bases and their metal complexes were previously reported as effective corrosion inhibitors for various metals[2-3],efficient catalysts[4-6],and pH sensing florescent molecules[7-8].Schiff bases have also been shown to exhibit a broad range of biological activities such as antibacterial[9-10], antimalarial[11],antifungal[12]and antiviral[13].
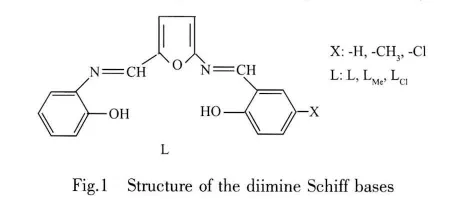
Symmetrical diimines, including two imine groups,bounded an aromatic ring (-N=HC-Ar-CH=N)or(-CH=N-Ar-N=CH)type,can be synthesized with the reactions of dialdehydes with amines[14]or diamines with aldehydes[15-16].Since a reaction occurs between-NH2and-CHO groups in the same aromatic ring,(-N=HC-Ar-N=CH)type unsymmetric diimines cannot be synthesized directly.Recently we reported synthesis and characterizations of{-N=HC-Ar-N=CH(Ar:phenylene)}type unsymmetric diimines and some metal complexes[17-18].
In this work,we report synthesis and characterizations of three novel {-N=HC-Ar-N=CH (Ar:furylene)}type unsymmetric dimine Schiff bases(L,LMeand LCl)and their Feバ and Niギ complexes.Thesecompoundsarenew unsymmetricdiimines because the furylene rings are in the middle of the imine groups.The antimicrobial activity of the ligands and the complexes against Pseudomonas aeruginosa ATCC 29212,Staphylococcus aureus ATCC 25973,Escherichia coliATCC 11230,Bacillus subtilis ATCC6633 and Yersinia enterecolitica ATCC1501 are also studied.
1 Experimental
1.1 Instrumentation
All the chemicals were purchased from Sigma-Aldrich or Merck and used without further purification.IR spectra in the 4 000~400 cm-1were recorded on a Mattson-1000 FTIR and UV-Vis spectra were taken on a Shimadzu UV-160A spectrophotometer.1H and13C-NMR spectra were recorded with a Bruker AvanceDPX-400 usingTMS asinternal standard in DMSO-d6.Carbon,hydrogen and nitrogen analyses were obtained using a LECO CHNS-932 analyzer.Metal ions were determined with a Perkin Elmer Analyst 800 model AAS.Mass spectra were recorded at 70 eV on an Agilent 1100 MSD mass spectrometer.The TGA curves were obtained using a Du Pont Instrument 951 between 35~800 ℃ at a heating rate of 10 ℃·min-1in nitrogen.Molar conductivities in DMSO (1 mmol·L-1)at 20 ℃ were measured using a Siemens WPA CM 35 apparatus.Magnetic susceptibilities ofthe complexes were determined using a Sherwood Scientific MKI model Evans magnetic balance.
1.2 Synthesis of the unsymmetric diimines:General procedure
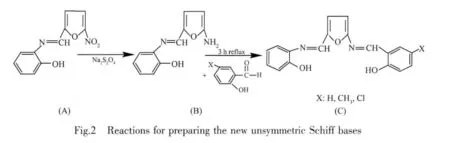
At first,the starting Schiff base 2-hydroxy-N-(5-nitrofurylidene)aniline(SB-NO2)was obtained using 5-nitro-furfuraland 2-hydroxyaniline,as described previously[19-20].Unsymmetric diimines(L,LMeand LCl)were prepared with our two-step method,as shown in Fig.2.2 mmol(0.46 g)of SB-NO2was dissolved in 1∶1(V/V)ethanol:water solution(60 mL)at 70 ℃.5~8 mmol (0.86 ~1.38 g)of solid sodium dithionite was slowly added to the solution in small portions through 1 h and then stirred for 1 h at 50oC for completion of reducing process.Consequently,amino derivative of the starting Schiff base (SB-NH2)was formed in the solution,as shown in Fig.2(B).2 mmol of 2-hydroxy-5-X benzaldehyde(X=H,CH3and Cl)in 10 mL ethanol was added to this solution with stirring and was refluxed 3~4 h at 60 ℃.The resulting solution was evaporated at room temperature for approximately 3 d,until a yellow-white precipitate was formed.The crude product was treated with warm water,filtered twice and then recrystallized from ethanol.
1.3 Synthesis of the complexes
The metal complexes were synthesized by the reaction of unsymmetric Schiff base ligands(1 mmol)with Niギ and Feバ chlorides(1 mmol)in ethanol(25 mL).The mixture was refluxed for ca 3~5 h at about 70 ℃.Over a waiting period of 3~4 d,the solid complex obtained was filtered,washed with hot water,ethanol and ether,respectively,and dried in a vacuum desiccator over anhydrous CaCl2.
1.4 Antibacterial activities
In vitro antibacterial activities of 1 000 and 500 μg·L-1of the unsymmetric Schiff bases and their complexes againstPseudomonasaeruginosaATCC 29212, Staphylococcus aureus ATCC 25973,Escherichia coliATCC 11230 Bacillus subtilis ATCC6633 and Yersinia enterecolitica ATCC1501 were studied using well-diffusion method according to the procedure reported previously[17].
2 Results and discussion
The newly synthesized (-N=HC-Ar-N=CH)type unsymmetrical diimine Schiff bases L,LMeand LClare N2O3type pentadentate ligands with two imine nitrogens,two phenolic oxygens and the furan ring oxygen.By reaction of these ligands with Feバ and Niギchlorides,dimeric[ML]2X2(X=OH-,Cl-or H2O)type complexes are formed.Diimines show N2O3type pentadentate behaviour for FeL,FeLMe,FeLCland NiLClcomplexes,but N2O2type tetradentate behaviour is observed for NiL and NiLMecomplexes with exclusion of furan oxygen atom.
All the synthesized compounds are very stable in air.The diimines are soluble in common organic solvents.Furthermore,metal complexes are slightly soluble in methanol,ethanol and acetone but more soluble in DMF and DMSO.The molar conductance values of the complexes in 1 mmol·L-1DMSO solution are found in the range of 2.9~3.5 and 11.3~23.3 Ω-1·cm2·mol-1for Niギ and Feバ complexes,respectively,indicating their nonelectrolytic nature.Some analytical and physical properties of the compounds are given in Table 1.
2.1 IR spectra
Selected IR and electronic spectral bands of the diimines and their metal complexes are presented in Table 1.The IR spectra of the free ligands display two strong bands in 1600 cm-1and 1619 cm-1for L,1 571 cm-1and 1 597 cm-1for LMeand 1 560 cm-1and 1 581 cm-1for LCldue to stretching vibrations of the two imine groups in the molecules.Each of these two vibrations is shifted to higher wave number ca 10~30 cm-1in the spectra of the complexes,suggesting the coordination from the azomethine nitrogen atoms.The broad bands between 3 307~3 333 cm-1in the spectra of the diimines and the complexes are attributed to νOH of the phenol and water molecules.The vibrations νCO and δCO of furan ring occur at 1 509 ~1 516 cm-1and 829~835 cm-1in the spectra of diimine Schiff bases.The shifts to higher frequency by ca 20 cm-1for νCO and ca 13 cm-1for δCO in the spectra of the complexes(FeL,FeLMe,FeLCland NiLCl)indicate the coordination of furan oxygens to metal ions[21-23].This observation shows N2O3type pentadentate behaviour of the diimines.However, νCO and δCO frequencies of the NiL and NiLClcomplexes remains almost the same positions,comparing with those for the ligands L and LCl,suggesting that the furan oxygens are not involved in chelation and diimines act as N2O2type tetradentate ligands.In the spectra ofthe complexes,new additionalbands,which arenot present in the spectra of the ligands,are observed in the 479~505 cm-1and 455~483 cm-1regions.Thesebands can be assigned to νM-O and νM-Ν vibrations,respectively.

Table 1 Analytical,physical,electronic and selected IR spectral data(cm-1)of the diimine Schiff bases and their metal complexes
2.2 UV-Vis spectra
The electronic spectra of the ligands and the complexes were recorded in DMSO and are illustrated in Table 1.Diimines show three high intensity bands in the range of 220~225 nm,252~283 nm and 327~353 nm.The first and second peaks are assigned to π-π*transitions of the aromatic rings and n-π*transitions of the imines,respectively.The last bands,which appear at the lowest energy region,are ascribed to charge transfer transitions in the molecules.
The observed magnetic moment values of the the Feバ complexes are between 5.23~5.41 B.M.range.These values are slightly lower than the spin only value of high spin (S=5/2)ironバion,and they are also less than the spin only value expected for a system containing two independent ironバ centers(8.37μB/Fe2).This results show the presence of significant antiferromagnetic coupling between Feバions in the dimer,in Ohgeometry[24].For the Niギcomplexes, μeffvalues are between 2.09 ~2.50 B.M.These results exhibit more reduced magnetic moments than that of the spin only values,which may result from spin density exchange between two metal centers in Ohgeometry[25].
Electronic spectra of Feバcomplexes shows the new band between 503~507 nm may be due to the L→M charge transfer,which obscuresthe low intensity,spin forbidden d-d bands[24].Two d-d transition bands are observed in the regions 416~425 nm and 631~669 nm in the spectra of the Niギ complexes.They are attributed to3A2g(F)→3T1g(F)and3A2g(F)→3T1g(P)transitions for octahedral d8ions;respectively[26].
2.3 NMR spectra
1H and13C-NMR spectral data of the ligands are listed in Table 2.In the1H-NMR spectra of the ligands appear two different signals in the range 8.88~8.94 and 9.58~9.60 which are attributed to unsymmetric imine groups due to their different chemical environments.Two singlet peaks in the 10.18~10.51 and 13.40~13.70 range are attributed to the phenolic protons.OH signalsaredefined alsowith D2O exchange.The multisignals at 6.68~7.76 are assignedto the aromatic protons.Methyl protons of LMeare observed at 2.28 ppm as a triplet.

Table 2 1H and13C-N.M.R.chemical shift(ppm)of the Shiff bases
In the13C-NMR spectra of the diimines,the resonances of the unsymmetric imine carbon atoms are observed in the range 159.5~163.3 and 159.9~163.5.As shown in Table 2,the signals of the carbon atoms,bounded to hydroxy groups (3,4)and furan oxygen atom (6,7),appeared at the range between 151~161.3 and 151.7 ~134.5,respectively.The signals of the other aromatic carbon atoms are shown at 106.4 ~135.6 range as expected.
2.4 LC-Mass spectra
The importantmass spectralresults ofthe compounds are given in Table 3.L and LClgive the molecular ion peaks as[M-H]+and[M+8H]+,whereas molecular ion peak could not be observed for the diimine LMe.
The loss of the fragment C6H5O is observed as the base peaks for L and LMeand a 28%intensity peak for LCl.The base peak of LCl,occurred at m/z 228.0,may be attributed to the elimination of the fragment C6H5Cl.

Table 3 Molecular ion peaks of the compounds.
As shown in Table 3,the molecular ion peaks of the complexes occur at the desired positions,which confirm the suggested dimeric structures.The fragmentations of the complexes consisted of the different patterns.
2.5 TGA analysis
Thermal analyses data of the complexes are presented in Table 4.There is no weight loss up to ca 320℃,indicating the absence of crystal or coordinated water molecules in the thermograms of the Feバcomplexes and NiLCl.The TG curves show continuous weight loss in the range between 320~650 ℃ and complete as horizontal line until 800℃.The residues are expected to be Fe2O3and NiO for NiLCl.
Niギcomplexes show two decomposition steps between ca 55~285 ℃ and ca 268~800 ℃.First weight loss range is attributed to the removal of water molecules.The second one is due to the pyrolysis of the ligand molecules.The final residues are NiO for both of the complexes.First decomposition step of the NiL and NiLMecomplexes consists the sum of two parts:removal of the crystal and coordinated water molecules.These two step can be seperated for NiLMecomplex,but proceeded together for NiL as shown in Fig.3.
On the basis of spectral,analytical data andmagnetic measurements,a dimeric structure could be suggested for the complexes.For NiLCland all of the Feバcomplexes,diimine Schiff bases act as N2O3type pentadentate ligands involving two imine nitrogens,two phenol oxygens and furan ring oxygen,as shown in Fig.4(a).Furthermore,diimine Schiff bases act as N2O2type tetradentate ligands for NiL and NiLMecomplexes from the unemployed furan oxygen,as shown in Fig.4(b).

Table 4 Thermal data of the complexes


The other coordination sites are occupied by OH-or Cl-for Feバ complexes and H2O for the Niギcomplexes.
2.6 Antimicrobial activity
The antibacterial activities of the diimines and the complexes are given in Table 5.The inhibition zones of the compounds against P.aeruginosa show that the diimines and the Niギcomplexes(except NiLMe)are inactive.However,the biggest inhibition zones are measured for the Feバcomplexes as similar to our previous work[17].Although it may result from the Feバion in the molecules,it is difficult to have an exact explanation without more extensive research.The activities of the compounds against S.aureus are found as analogous but considerably small in respect to P.aeruginosa.The diimine ligands and Niバcomplexes(except NiLCl)show small activities against E.coli,while Feバcomplexes are inactive.All compounds show moderate activities towards B.subtilis and Y.enterecolitica.Reports have shown that FeCl3·6H2O and NiCl2·6H2O have nobiologicalactivityonbacteria and fungi species[27-28].

Table 5 Antibacterial activities of all compounds(diameter of inhibition zone in mm)
3 Conclusions
Three novel unsymmetric diimine Schiff bases(L,LMeand LCl)were prepared with two-step method,and their dimeric,binuclear[ML]2X2(X=OH-,Cl-or H2O)type Feバ and Niギ complexes were synthesized.The compounds were characterized by physicaland spectroscopic techniques.Diimine Schiff bases are N2O3type pentadentate ligands for all of the Feバcomplexes and NiLCl,involving two imine nitrogens,two phenol oxygens and furan ring oxygen.But for NiL and NiLMecomplexes Diimine Schiff bases act as N2O2type tetradentate ligands,exceptthe furan oxygen.The other coordination sites are filled by OH-or Cl-for Feバ complexes and H2O for the Niギcomplexes.
The antibacterial activities of all compounds are determined against P.aeruginosa,S.Aureus,E.coli,B.subtilis and Y.enterecolitica.The highest activity values are measured for the Feバcomplexes against P.aeruginosa while ligands and Niギcomplexes are inactive.
Acknowledgements:This work is supported by the Research Foundation of Gazi University (FEF 05/2003-64)and(FEF 05/2005-37).We are thankful to Dr.Servet Çete for antimicrobial activities of the compounds.
[1]Schiff H.Justus Liebigs Ann.Chem.,1864,131:118-119
[2]Behpour M,Ghoreishi S M,Salavati-Niasari M,et al.Corros.Sci.,2009,51(5):1073-1082
[3]Stanly Jacob K,Parameswaran G.Corros.Sci.,2010,52:224-228
[4]Kılıç A,Kayan C,Baysal A,et al.Appl.Organomet.Chem.,2011,25(5):390-394
[5]Zhou M D,Zhao J,Kühn F E,et al.Chem.Eur.J.,2007,13(1):158-166
[6]Yue S,Li J,Gu X P,et al.Russ.J.Coord.Chem.,2010,36:547-551
[7]Derinkuyu S,Ertekin K,Çetinkaya E,et al.Dyes and Pigm.,2008,76:133-141
[8]Aksuner N,Henden E,Cukurovali A,et al.Dyes and Pigm.,2009,83(2):211-217
[9]Shi L,Ge H M,Tan S H,et al.Eur.J.Med.Chem.,2007,42(4):558-564
[10]Pandeya S N,Sriram D,De Clercq,E.et al.Il Farmaco,1999,54(9):624-628
[11]Rathelot P,Vanelle P,Maldonado J,et al.Eur.J.Med.Chem.,1995,30:503-508
[12]Martins C V B,da Silva D L,de Resende M A,et al.J.Antimicrob.Chemother.,2009,63(2):337-339
[13]Sriram D,Yogeeswari P,Saraswat V,et al.Bioorg.Med.Chem.Lett.,2006,16:2127-2129
[14]Humphrey L C F,Rodolphe C,Sally B.Polyhedron,2010,29(4):1353-1357
[15]Potgieter K,Mayer P,Booysen I N,et al.Polyhedron,2009,28(13):2808-2812
[16]Shadrick I M P,Ünige A L,Shengwen L.Inorg.Chim.Acta,2010,363(13):3390-3398
[17]Nartop D,Gürkan P,Çete S,et al.J.Coord.Chem.,2008,61(21):3516-3524
[18]Güngör O,Gürkan P,Spectrochim.Acta Part A:Mol.Biomol.Spect.,2010,77(1):304-311
[19]Csaszar J.Acta Chim.Hung.,1987,124:245-257
[20]Zhelyazkov L,Zikolova S,Farmatsia(Sofia,Bulgaria),1964,10(25):16-21
[21]Kandil S S,Ali G Y,El-Dissouky A.Trans.Met.Chem.,2002,27:398-406
[22]Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds.New York:Wiley,1986.
[23]Sar N,Gürkan P.Trans.Met.Chem.,2003,28:687-693
[24]Lever A B P.Inorganic Electronic Spectroscopy.New York:Elsevier,1984.
[25]Dubey R K,Dubey U K,Mishra C M.Trans.Met.Chem.,2006,31(7):849-855
[26]Nag J K,Pal S,Sinha C.Trans.Met.Chem.,2005,30:523-526
[27]Adediji J F,Obaleye J A,Akinremi C A,et al.J.Chem.Pharm.Res.,2012,4(8):4073-4078
[28]Joshua A O,Johnson F A,Mattehew A A.Molecules,2011,16:5861-5874

