Hypomethylation of glycine dehydrogenase promoter in peripheral blood mononuclear cells is a new diagnostic marker of hepatitis B virus-associated hepatocellular carcinoma
Li-Li Mio ,Jing-Wen Wng ,Hui-Hui Liu ,Shui Go ,Yu-Chen Fn ,c ,Ki Wng ,c,∗
a Department of Hepatology, Qilu Hospital of Shandong University, Jinan 250012, China
b Experimental Center, Shandong University of Traditional Chinese Medicine, Jinan 250355, China
c Institute of Hepatology, Shandong University, Jinan 250012, China
Keywords: Hepatocellular carcinoma Glycine dehydrogenase DNA methylation Peripheral blood mononuclear cells
ABSTRACT Background: Glycine dehydrogenase (GLDC) plays an important role in the initiation and proliferation of several human cancers.In this study,we aimed to detect the methylation status of GLDC promoter and its diagnostic value for hepatitis B virus-associated hepatocellular carcinoma (HBV-HCC).Methods: We enrolled 197 patients,111 with HBV-HCC,51 with chronic hepatitis B (CHB),and 35 healthy controls (HCs).The methylation status of GLDC promoter in peripheral mononuclear cells (PBMCs) was identified by methylation specific polymerase chain reaction (MSP).The mRNA expression was examined using real-time quantitative polymerase chain reaction (qPCR).Results: The methylation frequency of the GLDC promoter was significantly lower in HBV-HCC patients(27.0%) compared to that in CHB patients (68.6%) and HCs (74.3%) (P < 0.001).The methylated group had lower alanine aminotransferase level (P=0.035) and lower rates of tumor node metastasis (TNM) III/IV(P=0.043) and T3/T4 (P=0.026).TNM stage was identified to be an independent factor for GLDC promoter methylation.GLDC mRNA levels in CHB patients and HCs were significantly lower than those in HBV-HCC patients (P=0.022 and P < 0.001,respectively).GLDC mRNA levels were significantly higher in HBV-HCC patients with unmethylated GLDC promoters than those with methylated GLDC promoters(P=0.003).The diagnostic accuracy of alpha-fetoprotein (AFP) combined with GLDC promoter methylation for HBV-HCC was improved compared with that of AFP alone (AUC: 0.782 vs.0.630,P < 0.001).In addition,GLDC promoter methylation was an independent predictor for overall survival of HBV-HCC patients (P=0.038).Conclusions: The methylation frequency of GLDC promoter was lower in PBMCs from HBV-HCC patients than that from patients with CHB and HCs.The combination of AFP and GLDC promoter hypomethylation significantly improved the diagnostic accuracy of HBV-HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers all over the world [1].Approximately 50%-80% of HCC occurred in patients with chronic hepatitis B virus (HBV) [2].Early diagnosis of HCC is essential for improving the therapeutic outcomes.
At present,serum alpha-fetoprotein (AFP) and ultrasonography are widely used for the screening of HCC [3].However,the sensitivity and specificity of AFP were relatively low for HCC [4].The pathological analysis is the gold standard for diagnosing HCC.However,liver biopsy is invasive and has poor patient compliance.Therefore,there is an urgent need for exploring new biomarkers to detect HCC at its early stages.
Glycine dehydrogenase (GLDC) is an essential metabolic enzyme in glycine and serine metabolism.GLDCgene is an oncogene and plays an important role in the development and proliferation of several tumors [5–7].In non-small cell lung cancer,GLDC was able to promote tumorigenesis by upregulating pyrimidine biosynthesis [5].GLDC is highly expressed and contributes to the proliferation of human gioblastoma [6].Overexpression of GLDC facilitated the proliferation of triple-negative breast cancer (TNBC) cells,whereas GLDC knockdown had the opposite effects [7].Recent study suggested that GLDC might also play an important role in the development and progression of HCC [8].As a subunit of glycine cleavage system (GCS),GLDC was selected from the Cancer Genome Atlas (TCGA) and was found markedly highly expressed in HCC [8–10].In addition,GLDC was found to be the post-transcriptional target of miR-30d-5p in HCC [8].
DNA methylation is important for the regulation of gene expression [11],and is an important event in carcinogenesis [12].Multiple studies have demonstrated that DNA methylation of certain genes can be used as biomarkers for diagnosis,prognosis or therapeutic strategies [13,14].Hypermethylation ofGLDCgene promoter was discovered in gastric cancer tissues [15].However,to the best of our knowledge,the methylation status of GLDC in HBV-HCC has not yet been explored.
In this study,we evaluated the methylation status and the mRNA levels of GLDC in HBV-HCC,and aimed to investigate the diagnostic value of GLDC methylation in HBV-HCC.
Methods
Subjects
Totally 197 patients were enrolled including 111 HBV-HCC cases,51 chronic hepatitis B (CHB) cases and 35 healthy individuals at the Department of Hepatology,Qilu Hospital of Shandong University from May 2019 to February 2021.A clinical follow-up for the HBV-HCC patients was performed from the date of diagnosis to May 2022.
This study was complied with theDeclarationofHelsinkiand authorized by the Regional Research Ethics Committee of Qilu Hospital,Shandong University.All the participants signed the informed consent.
HBV-associated HCC was diagnosed based on the 2018 practice guidance for Management of HCC by the American Association for the Study of Liver Diseases (AASLD) [16].Explicit history of chronic HBV infection was required in the HBV-HCC subjects.In the diagnostic criteria for chronic hepatitis B,the patients must have positive result on hepatitis B surface antigen (HBsAg)for at least six months.Patients with CHB were collected according to the latest information for prevention,diagnosis and treatment of chronic hepatitis B in the 2018 hepatitis B guidance from AASLD [17].Healthy controls (HCs) were serologically negative for hepatitis virus,and there was no medical or surgical history.
Patients with history of other tumors,pregnancy,fatty liver disease,autoimmune hepatitis (AIH) or co-infection with hepatitis virus except for HBV,and other chronic liver diseases were excluded.
Isolation of mononuclear cells from peripheral blood
Five mL of peripheral venous blood was sampled from all participants,and citric acid was used as an anticoagulant.Peripheral blood mononuclear cells (PBMC) was isolated from fresh citrate anticoagulant blood by Ficoll density gradient centrifugation (GE healthcare,Uppsala,Sweden),and stored at-20◦C.
Extracting of DNA and sodium sulfite modification
The genomic DNA was obtained from PBMCs using QIAamp DNA Mini Kit (Qiagen,Valencia,CA,USA).The DNA modification was then performed with EZ DNA methylated gold kit (Zymo Research,Orange,CA,USA).Finally,we obtained the bisulfiteconverted DNA and reserved it at-20◦C.
Methylation specific polymerase chain reaction (MSP)
MSP was developed to confirm the methylation status of GLDC promoter.Meth-Primer software (Applied Biosystems,Carlsbad,CA,USA) was used to design the specific primers for methylated (M)and unmethylated (U) GLDC promoter.The primer was as follows:methylated GLDC primer,forward 5′-GTT TTG GGT GGA GTT ATA ATT TTG C-3′ and reverse 5′-CCG ACC TAA AAC CCC TTT CG-3′ ;unmethylated GLDC primer,forward 5′-TGT TTT GGG TGG AGT TAT AAT TTT GT-3′ and reverse 5′-CCC AAC CTA AAA CCC CTT TCA C-3′.
The estimated PCR product size was 396 bp.The reaction system was designed according to the instructions,consisting of 2μL bisulfate modified DNA,10.5μL nuclease-free water,0.5 μL of each primer and 12.5μL of Premix Taq (Zymo Research,Orange,CA,USA).The final volume was 26 μL.MSP started at 95 °C (10 min),35 cycles included degeneration at 94 °C (30 s),annealing at 59.5◦C (30 s),and extension at 72◦C (30 s),then cooled to 4 °C.PCR product was electrophoresed on 2% agarose gel,marked by gel red(Biotium,Hayward,CA,USA),and displayed under UV light.All the tests were done in triple.
RNA separation from PBMCs and real-time quantitative polymerase chain reaction (qPCR)
The RNA was separated from PBMCs using phenol chloroform isopropanol method.With the aid of the first strand cDNA synthesis Kit (Fermentas,Vilnius,Lithuania),1μg RNA was reverse transcribed into complementary DNA (cDNA).Expression of GLDC mRNA in each group was detected using real-time qPCR.The GLDC primers were forward 5′-AAC CAG GGA GCA ACA CAT TC-3′ and reverse 5′-GCA ACC AGT TCT GCA GAT GA-3′.β-actin was the internal control.Forward primer ofβ-actin: 5′-ACA CTG TGC CCA TCT ACG AGG-3′.Reverse primer ofβ-actin: 5′-AGG GGC CGG ACT CGT CAT ACT-3′.The reaction system consisted of 4.1 μL nucleasefree water,0.2 μL forward primer,0.2 μL reverse primer,5 μL SYBR Green (Toyobo,Osaka,Japan) and 0.5 μL of cDNA.real-time qPCR was performed as follows: denaturation at 95◦C for 30 s,then 45 cycles at 95◦C for 5 s,60◦C for 30 s and 72◦C for 30 s.The mRNA levels were calculated by the comparative (2-ΔΔCT) means.
Clinical and laboratory parameter collections
The necessary clinical and laboratory parameters of the subjects were tested at the Department of Laboratory Medicine,Qilu Hospital of Shandong University,including virological indices: [HBsAg,hepatitis B e antigen (HBeAg),hepatitis B virus DNA (HBV-DNA)],hepatic biochemical indices [alanine aminotransferase (ALT),aspartate aminotransferase (AST),total bilirubin (TBIL),albumin (ALB),AFP]and blood coagulation indices [prothrombin time activity(PTA),international normalized ratio (INR)].
The value of AFP above 20 ng/mL are currently recommended for the investigations of HCC.The values 200 ng/mL and 400 ng/mL are frequently used as confirmatory tests for HCC diagnosis [18–21].And the 400 ng/mL remains to be the diagnostic cut-off value for HCC in the newest guidelines of primary liver cancer in China [18,22].So the 20 ng/mL,200 ng/mL and 400 ng/mL were regarded as the cut-off values for AFP in this study.
The HBV DNA cut-off levels of 2000 IU/mL was proposed by the National Institutes of Health (NIH) workshop to characterize inactive carriers [23].
Statistical analysis
The SPSS statistics 26.0 software (SPSS Inc.,Chicago,IL,USA)was utilized to analyze all the data.Quantitative data were expressed as median (interquartile range) and contrasted through Kruskal-Wallis test and Mann-WhitneyUtest.The classification data were compared with Chi-square test.The association between variables was evaluated by Spearman correlation test.Multivariate logistic regression was used to determine the relationship between GLDC promoter methylation and clinicopathological parameters.The diagnostic performance of AFP and GLDC promoter methylation in HCC was assessed by the area under the curve (AUC) of the receiver operating characteristic (ROC).Cox regression analyses were applied to screen independent predictors of overall survival(OS) in HCC patients.
Results
General characteristics
This study recruited 197 subjects including 111 HBV-HCC patients,51 CHB patients and 35 HCs.The clinical and virological characteristics are shown in Table 1.There were significant differences between the HBV-HCC and CHB groups with the respects to positive HBeAg (23.4% vs.66.7%,P<0.001),percentage of HBV DNA ≥2000 IU/mL (25.2% vs.70.6%,P<0.001),ALT (40.00 vs.218.00 U/L,P<0.001),AST (51.00 vs.158.00 U/L,P<0.001) and AFP (88.67 vs.34.00 ng/mL,P=0.008).

Table 1Clinical features of subjects.
Methylation status of GLDC promoter in HBV-HCC, CHB and HCs
Fig.1 shows the methylation status of GLDC promoter of all the participants.The methylation frequency was 27.0% (30/111) in HBVHCC patients,68.6% (35/51) in CHB patients and 74.3% (26/35) in HCs.The methylation frequency of GLDC promoter in the HBV-HCC group was significantly lower than that in the CHB and HCs groups(P<0.001).There was no significant difference between CHB patients and HCs (P=0.570).Fig.2 shows the representative results of MSP in methylation status of GLDC promoter.

Fig.1. Comparison of GLDC methylation frequencies in different groups.HCC: hepatocellular carcinoma;CHB: chronic hepatitis B;HCs: healthy controls.
Relevance between clinicopathological characteristics and GLDC promoter methylation in HBV-HCC patients
Table 2 shows the relationship between clinical characteristics and GLDC promoter methylation of HBV-HCC patients.The methylated group had lower ALT level (27.50 vs.42.00 U/L,P=0.035)and lower rates of TNM stage III/IV (23.3% vs.44.4%,P=0.043)and T3/T4 (6.7% vs.25.9%,P=0.026).However,other clinical characteristics involved in this study were not significantly different between the methylated group and unmethylated group.In addition,our multivariate logistic regression showed that TNM stage III/IV was less likely to be methylated than TNM stage Ⅰ/ Ⅱ,suggesting that TNM stage was an independent factor for GLDC promoter methylation (OR=0.161,95% CI: 0.031-0.839,P=0.030;Table 3).
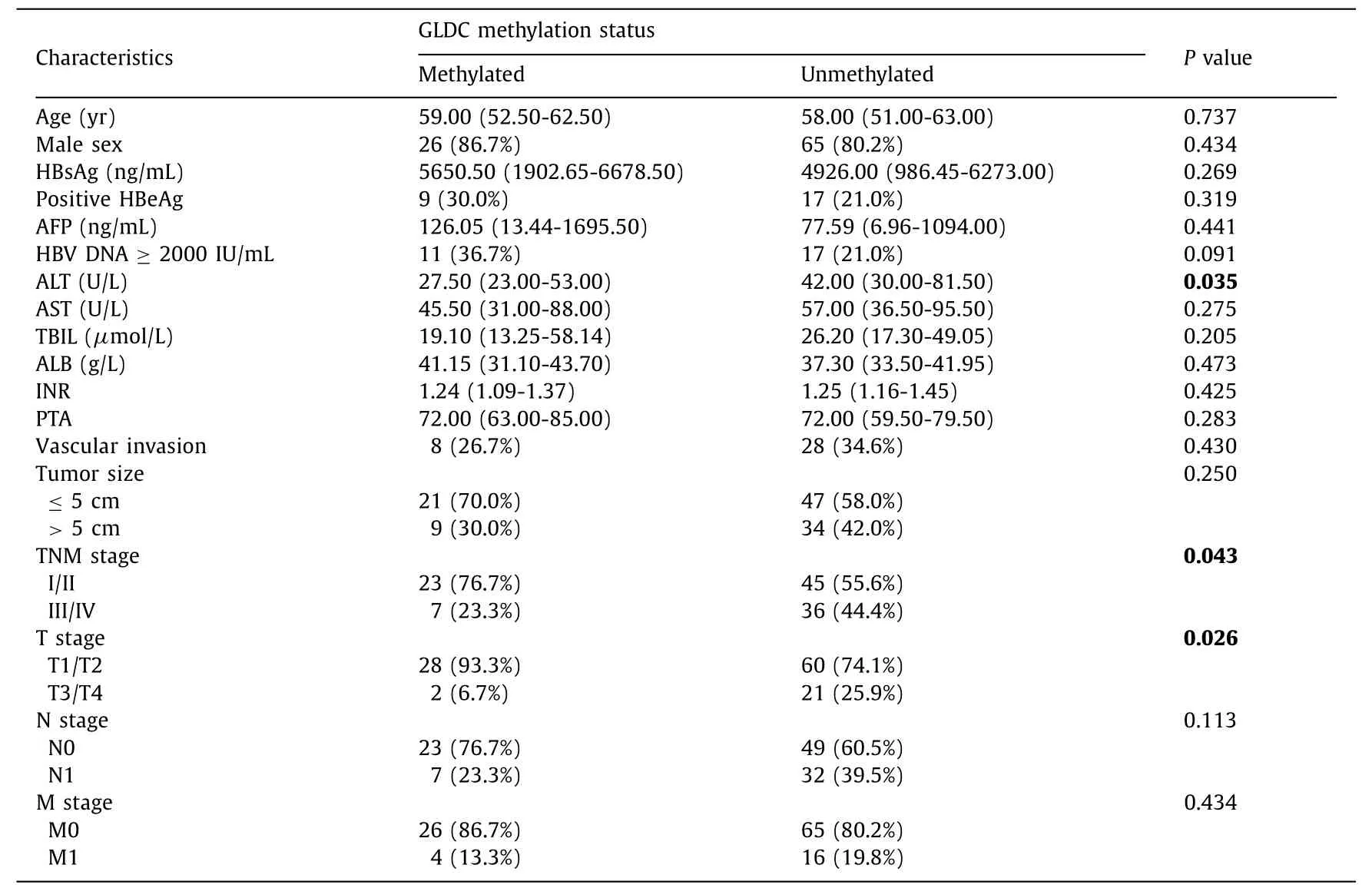
Table 2Association between basic characteristics and GLDC promoter methylation status in HBV-HCC.

Table 3Multivariate logistic regression of clinicopathological features and GLDC promoter methylation in HBV-HCC.
GLDC mRNA expression levels in PBMCs from the subjects
Our real-time qPCR showed that GLDC mRNA levels were significantly higher in the HBV-HCC group than in the CHB group(0.0 096 vs.0.0 026,P=0.022) and HCs (0.0096 vs.0.0006,P<0.001).GLDC mRNA levels were obviously higher in the CHB group than in the HCs group (0.0 026 vs.0.0006,P<0.0 01,Fig.3 A).In methylated HCC patients,the expression of GLDC mRNA was lower than that in unmethylated HCC patients (0.0026 vs.0.0230,P=0.003,Fig.3 B).In the HBV-HCC group,methylation status of GLDC promoter directly related to the mRNA levels (rs=-0.237;P=0.012).Then the association between GLDC mRNA expression and clinicopathological features in HBV-HCC patients was determined.As shown in Fig.3 D,GLDC mRNA expression was negatively correlated with tumor size.And GLDC mRNA levels were not related to vascular invasion (Fig.3 C),as well as other clinicopathological characteristics including HBsAg,HBeAg,HBV DNA,ALT,ALB,AFP,TNM stage,etc.
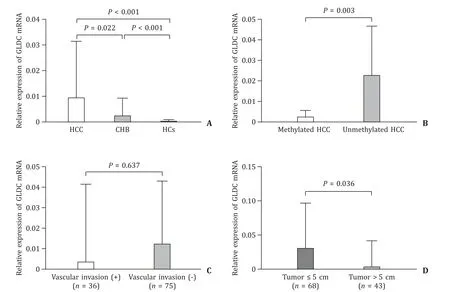
Fig.3.A: Relative expression levels of GLDC mRNA in three groups.B: GLDC mRNA levels were lower in methylated HCC than in unmethylated HCC.C: There was no statistical difference in GLDC mRNA expression between patients with vascular invasion and without vascular invasion.D: GLDC mRNA was significantly higher in patients with tumor size ≤5 cm than in patients with tumor size > 5 cm.HCC: hepatocellular carcinoma;CHB: chronic hepatitis B;HCs: healthy controls.
Diagnostic performance of GLDC promoter methylation and AFP in HBV-HCC
The sensitivity of GLDC promoter methylation to distinguish HBV-HCC from CHB was 72.97% (81/111) and the specificity was 68.63% (35/51).The sensitivity of AFP in discriminating HBV-HCC from CHB was 37.84% and the specificity was 92.16%.In distinguishing HBV-HCC from CHB,there was no statistical difference between the AUC of GLDC promoter methylation and AFP levels(0.708 vs.0.630,P=0.176,Fig.4 A).The AUC of the combination of two markers to identify CHB and HCC was significantly higher than the AUC of AFP alone (0.782 vs.0.630,P<0.001,Fig.4 B).
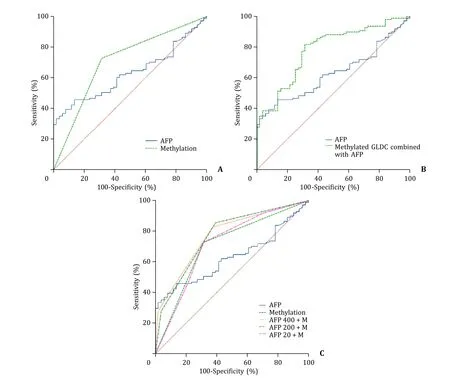
Fig.4.A: ROC curves of serum AFP (AUC: 0.630) and GLDC promoter methylation (AUC: 0.708) in differentiating HBV-HCC from CHB (P=0.176).B: ROC curves of GLDC methylation combined with AFP (AUC: 0.782) and serum AFP (AUC: 0.630) in differentiating HBV-HCC from CHB (P < 0.001).C: ROC curves of the biomarkers in differentiating HBV-HCC from CHB.AFP: serum AFP level;M: plasma GLDC promoter methylation;AFP 400+M: serum AFP (cut-off value of 400 ng/mL) combined with GLDC promoter methylation;AFP 200+M: serum AFP (cut-off value of 200 ng/mL) combined with GLDC promoter methylation;AFP 20+M: serum AFP (cut-off value of 20 ng/mL) combined with GLDC promoter methylation.AFP: alpha-fetoprotein;HBV-HCC: hepatitis B virus-associated hepatocellular carcinoma;CHB: chronic hepatitis B;ROC:receiver operating characteristic;AUC: area under the curve.
The combination of AFP level (cut-off value of 400 ng/mL) and methylation status of GLDC promoter gave an AUC of 0.773,sensitivity of 82.88% and specificity of 62.75%.The results showed that the combination of AFP (cut-off value of 400 ng/mL) and methylation status of GLDC promoter improved the diagnostic performance compared to AFP level alone (P<0.001).The AUC of the combination of AFP and GLDC promoter methylation was 0.770 with a sensitivity of 85.59% and specificity of 60.78% when 200 ng/mL was used as the cut-off value for AFP level.The combination was also considered to have a higher diagnostic value than the AFP level alone (P=0.004).When the cut-off value was 20 ng/mL,the AUC of the combination of AFP and GLDC promoter methylation was 0.715 with a sensitivity of 91.89% and specificity of 29.41%.The combination of AFP (cut-off value of 20 ng/mL) and GLDC promoter methylation showed similar diagnostic performance to AFP level alone in identifying HBV-HCC from CHB (P=0.094,Table 4,Fig.4 C).
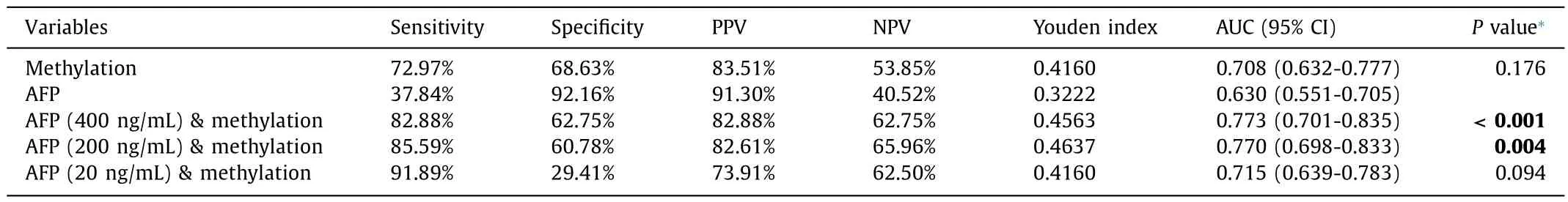
Table 4Results of GLDC promoter methylation in differentiating HBV-HCC from CHB.
GLDC promoter methylation is an independent predictor for overall survival (OS) of HBV-HCC patients
At the end of the follow-up period,41 HBV-HCC patients died.The Cox regression analysis was used to determine whether the methylation status of GLDC can be used to predict the OS.In the univariate analysis,five variables were found to be associated with OS,including ALT,INR,vascular invasion,TNM stage and methylation status of GLDC promoter.In the multivariate regression analysis,ALT (HR=1.003,95% CI: 1.000-1.0 06,P=0.021),TNM stage(HR=3.182,95% CI: 1.336-7.577,P=0.009) and GLDC methylation status (HR=0.284,95% CI: 0.086-0.932,P=0.038) were identified as independent predictors for OS of HCC patients (Table 5).

Table 5Univariate and multivariate Cox regression analysis for overall survival.
Kaplan-Meier (KM) survival curves showed that the GLDC unmethylated group exhibited a worse OS than the GLDC methylated group (P=0.004,log-rank test;Fig.5).The median survival time was 25 months (95% CI: 20.905-29.095) for the unmethylated group.
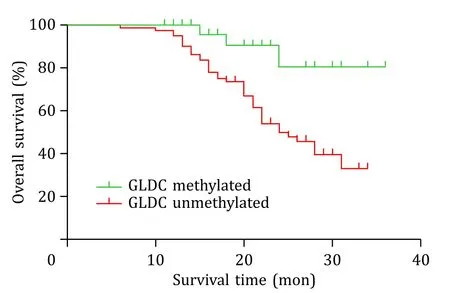
Fig.5. Kaplan-Meier curves for patients with HBV-HCC stratified according to GLDC methylation status.
Discussion
HCC is one of the most common and invasive malignant tumors in the world.HBV infection is one of the primary causes for chronic liver diseases and HCC in China.Most HCC patients do not display significant symptoms in the early stages and therefore they are often diagnosed at a late stage,resulting in an overall dismay prognosis for HCC patients.Recent studies found that altered DNA methylation was a key event in the development of HCC [24,25].In the present study,we found that the GLDC promoter methylation existed in HBV-HCC patients.
DNA hypomethylation is an important mechanism underlying carcinogenesis,and hypomethylation is usually the result of an enzymatic replacement of 5-methylcytosine (5MeC) with cytosine [26].GLDC promoter hypomethylation might result from the interaction with DNA methyltransferases 1 (DNMT1),which was a member of DNMTs [27].Recent studies have demonstrated that noncoding RNAs (ncRNAs) such as ecCEBPα,Dali,Dum,and Dacor1 could interact with DNMT1 to inhibit its methylation activity.These ncRNAs thus indirectly alter local methylation status in different cancers,acting as key tissue-specific epigenetic regulators of gene expression [28–30].Recently,a report described demethylation of spalt-like transcription factor 4 (SALL4) in HBV-related HCC,which might contribute to SALL4 reactivation in HCC [31].In addition,the 10-11 translocator protein iteratively oxidized 5-methylcytosine (5mC) to generate oxidized cytosine bases,which might facilitate DNA demethylation [32].The expression level of 10-11 translocation enzyme 2 (TET2) was decreased significantly in HCC [33],which might be another reason for GLDC promoter hypomethylation.
HBV plays a central role in the induction of oxidative and inflammatory responses as well as the development of HCC [34].A previous study reported a significantly higher degree of oxidative damage and antioxidant capacity in HBV-HCC patients compared to that in CHB patients [35].In addition,the potential benefits of antioxidants including superoxide dismutase (SOD) in the treatment of cancer have also been reported [36].These results strongly indicated that oxidative stress might be involved in the development of HBV-HCC.Invitroexperiments showed that oxidative stress-induced DNA damage caused substantial DNA hypomethylation [37].Therefore,HBV-induced oxidative stress might be a prerequisite for GLDC promoter hypomethylation in HBV-HCC.
DNA methylation might be linked to the pathogenesis of HCC.Previous studies have proven that hypomethylation of oncogenes might result in its activation and was associated with a variety of virus-associated human cancers [38–41].GLDC is the key ratelimiting component of the GCS and is a major determinant of plasma glycine levels [42].Glycine-derived 1C units support purine and pyrimidine biosynthesis in HCC.GLDC is involved in purine and pyrimidine biosynthesis in HCC.It also promotes the lipoylation and activity of pyruvate dehydrogenase,which support tumor growth.It was found that the silencing of GLDC in HepG2 slowed cell proliferation and was due to the inhibition of dihydrolipoyl moiety transfer to PDH that is mediated by GCS H-protein,impairing mitochondrial function.Therefore,we concluded that GLDC hypomethylation might promote hepatocarcinogenesis by affecting glycine levels and maintaining mitochondrial protein lipoylation to activate GLDC oncogenes.In our study,GLDC mRNA was highly expressed in the HBV-HCC group and was closely associated with GLDC hypomethylation,which to some extent also verified the above inference.
Serum AFP has been widely used in the diagnosis of HCC,but its specificity and sensitivity are insufficient.DNA methylation of certain genes can be used as biomarkers for diagnosis in human cancers.In this study,we constructed ROC curves to investigate the diagnostic value of GLDC promoter methylation in HBV-HCC.We found that combining AFP levels with GLDC methylation status improved the diagnostic accuracy compared to AFP levels alone.When the two markers were combined,the optimal diagnostic cutoff value for AFP was 400 ng/mL.
In addition,methylation status of GLDC promoter methylation status can be an independent predictor for the prognosis of HBVHCC patients.We also found that GLDC promoter methylation correlated with the TNM stage of HBV-HCC.The frequency of GLDC methylation in the TNM stage III/IV group was significantly lower than that in the TNM stage I/II group.These suggest that GLDC hypomethylation is associated with the progression of HBV-HCC.
Inevitably,our study also has some limitations.First,methylation properties in peripheral blood were detected,but intrahepatic methylation was still unknown.We will detect the methylation status and gene expression in the liver tissues in our further study.Second,the sample size of this study was relatively small and a larger cohort study is needed in further study.Third,MSP is not a quantitative but a qualitative analysis method.Bisufite sequence PCR (BSP) and MethyLight can quantify the methylation values of GLDC promoter in further studies [43,44].Fourth,due to the lack of liver tissues,the relationship between GLDC methylation status in PBMCs and the pathological grading of HCC was not explored.
In conclusion,this study showed GLDC promoter hypomethylation in HBV-HCC patients.In addition,GLDC promoter methylation combined with serum AFP levels improved the diagnostic accuracy of HBV-HCC.
Acknowledgments
None.
CRediT authorship contribution statement
Li-Li Miao:Data curation,Formal analysis,Methodology,Project administration,Writing-original draft.Jing-Wen Wang:Formal analysis,Methodology.Hui-Hui Liu:Formal analysis,Methodology.Shuai Gao:Formal analysis,Methodology,Writing-original draft.Yu-Chen Fan:Formal analysis,Methodology,Writing-original draft.Kai Wang:Conceptualization,Funding acquisition,Supervision,Writing-review &editing.
Funding
This study was supported by grants from the Key Project of the Chinese Ministry of Science and Technology (2017ZX102022022),and National Key Research and Development Program of China(2021YFC2301801).
Ethical approval
The study complied with theDeclarationofHelsinki,and was approved by the Local Research Ethics Committee of Qilu Hospital of Shandong University.All subjects gave written informed consent.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2024年1期
Hepatobiliary & Pancreatic Diseases International2024年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Recent advances in promising drugs for primary prevention of gastroesophageal variceal bleeding with cirrhotic portal hypertension
- Stereotactic body radiotherapy in pancreatic adenocarcinoma
- Application of ultrasonography-elastography score to suspect porto-sinusoidal vascular disease in patients with portal vein thrombosis
- Polydatin ameliorates hepatic ischemia-reperfusion injury by modulating macrophage polarization
- AGK2 pre-treatment protects against thioacetamide-induced acute liver failure via regulating the MFN2-PERK axis and ferroptosis signaling pathway
- Circulating RNA ZFR promotes hepatocellular carcinoma cell proliferation and epithelial-mesenchymal transition process through miR-624–3p/WEE1 axis
