Recent advances in promising drugs for primary prevention of gastroesophageal variceal bleeding with cirrhotic portal hypertension
Ji-Yo Sheng ,Zi-Fn Meng ,Qio Li ,Yong-Sheng Yng ,b,∗
a Department of Hepatobiliary and Pancreatic Surgery, the Second Hospital of Jilin University, Changchun 130041, China
b Jilin Engineering Laboratory for Translational Medicine of Hepatobiliary and Pancreatic Diseases, the Second Hospital of Jilin University, Changchun 130041, China
Keywords: Cirrhotic portal hypertension Target drug Primary prevention Bleeding
ABSTRACT Background: Gastroesophageal variceal bleeding is one of the most severe complications of patients with cirrhosis.Although primary prevention drugs,including non-selective β-blockers,have effectively reduced the incidence of bleeding,their efficacy is limited due to side effects and related contraindications.With recent advances in precision medicine,precise drug treatment provides better treatment efficacy.Data sources: Literature search was conducted in PubMed,MEDLINE and Web of Science for relevant articles published up to May 2022.Information on clinical trials was obtained from https://clinicaltrials.gov/ and http://www.chictr.org.cn/.Results: The in-depth understanding of the pathogenesis and advances of portal hypertension has enabled the discovery of multiple molecular targets for promising drugs.According to the site of action,these drugs could be classified into four classes: intrahepatic,extrahepatic,both intrahepatic and extrahepatic targets and others.All these classes of drugs offer advantages over traditional treatments in prevention of gastroesophageal variceal bleeding in patients with cirrhotic portal hypertension.Conclusions: This review classified and summarized the promising drugs,which prevent gastroesophageal variceal bleeding by targeting specific markers of pathogenesis of portal hypertension,demonstrating the significance of using the precision medicine strategy to discover and develop promising drugs for the primary prevention of gastroesophageal variceal bleeding in patients with cirrhotic portal hypertension.
Introduction
Portal hypertension is a clinical syndrome characterized by elevated portal venous pressure,and cirrhosis is the most common cause of portal hypertension [1].The complications of portal hypertension include gastroesophageal variceal hemorrhage,ascites,hepatorenal syndrome,hepatic encephalopathy,and bacterial peritonitis [2,3].Among them,gastroesophageal variceal bleeding is the most serious complication and a leading cause of death in patients with cirrhosis,with a mortality rate of as high as 15%–20% [4].Because of the poor prognosis in patients with variceal bleeding,early and effective prevention is important to decrease morbidity and mortality.The prevention strategies recommended in the current guidelines are divided into two stages: primary and secondary prevention.Primary prevention intends to prevent the formation and progression of gastroesophageal varices to decrease the occurrence of first hemorrhage and improve survival rates,while secondary prevention intends to avoid the risk of rehemorrhage [5].Furthermore,effective primary prevention is very important which can reduce the incidence of initial bleeding by approximately 50% [6].At present,the two main treatment strategies of primary prevention include endoscopic variceal ligation and drugs.Current guidelines recommend non-selectiveβ-blockers(NSBBs) as first-line primary preventive drugs for gastroesophageal variceal bleeding [7,8].There is a consensus that NSBBs have great clinical efficacy similar to that of endoscopic variceal ligation.Patient characteristics,adverse events,and financial situations should be considered when we choose treatment strategies [7,8].The advantages of NSBBs include low cost,noninvasiveness and convenience.NSBBs can reduce portal venous blood flow by decreasing cardiac output and splanchnic blood flow,and decrease portal venous pressure.However,because these drugs do not precisely target the liver tissue or the portal vein,they have side effects,such as the inhibition of cardiac function,and hypotension.With increasing understanding of the mechanism and targets of portal hypertension,new and safer preventive drugs have been developed with superior clinical efficacy compared with traditional medicines such as NSBBs.Among them,precise drugs such as simvastatin have been used in clinical practice to treat portal hypertension.
Pathogenesis of cirrhotic portal hypertension and gastroesophageal varices
Almost 90% of patients with liver cirrhosis eventually develop portal hypertension [9].As a result,cirrhotic portal hypertension is associated with poor prognosis of cirrhotic patients.The primary mechanism of portal hypertension is increased intrahepatic vascular resistance (backward theory) and portal blood flow (forward theory),as shown in Fig.1.The functional vascular unit of the liver is the hepatic sinusoid,which comprises the sinusoidal endothelial cells (SECs) and hepatic stellate cells (HSCs).Both cells play important roles in regulating sinusoidal blood flow [10,11].In liver fibrosis,HSCs are activated,whereas SECs are inhibited.In addition,the structural changes of hepatic lobule and pseudolobule regeneration lead to hepatic microcirculation disorders.The combination of long-term action of intrahepatic cells and molecules,hepatic sinusoidal capillarization,hepatic vascular remodeling,and increased intrahepatic circulation resistance initiates portal hypertension [12].The imbalance between vasorelaxation and vasoconstriction factors also plays an important role in portal hypertension [13].Consequently,collateral vessels open and dilate,and visceral neovascularization forms,leading to increased portal blood flow and hyperdynamic circulation,further aggravating portal hypertension [14].Portal vein collateral vessels,especially gastroesophageal veins,are remodeled and dilated to adapt to increased blood flow and hyperdynamic circulation [15].These changes result in increased luminal pressure and thinning of the vessel wall,increasing the risk of rupture and bleeding [5].Therefore,reducing portal pressure is an important therapeutic strategy for primary prevention of gastroesophageal varices bleeding.
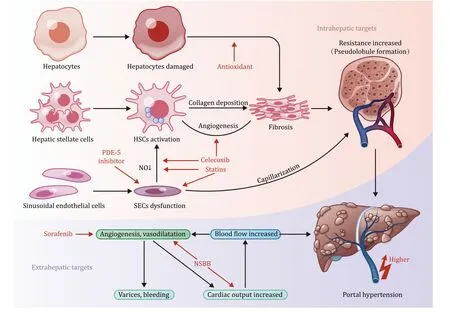
Fig.1. Pathophysiology of cirrhotic portal hypertension and some acting targets of promising drugs.The red arrows indicate the acting targets.NO: nitric oxide;HSCs:hepatic stellate cells;SECs: sinusoidal endothelial cells;NSBB: non-selective β-blocker;PDE-5: phosphodiesterase-5.
Traditional medicine for the treatment of portal hypertension
NSBBs reduce cardiac output and visceral blood flow by blocking adrenergic receptors.Studies have found that blocking adrenergic receptors causes visceral vasoconstriction and reducing portal collateral blood flow [16,17].Moreover,NSBBs have been widely used in the primary prevention of portal hypertension.However,they are difficult to use [18].Because of the effects of NSBBs on heart and blood pressure,we should reduce resting heart rate to 55–60 beats/min and check heart rate,blood pressure,and clinical tolerance at each outpatient service to ensure drug safety.Moreover,NSBBs are associated with a high risk of hypotension,adverse inotropic effects and many side effects,including fatigue,weakened exercise ability,and sexual dysfunction [18].The liver metabolizes NSBBs,whereas patients with cirrhosis often have poor liver function.Therefore,NSBBs need to be used with caution accordingly in patients with liver insufficiency or hepatic failure.An observational study found that a high dose of NSBBs in refractory ascites worsened liver diseases and resulted in elevated bilirubin and higher mortality [19].On the other hand,a retrospective study by Mandorfer and colleagues investigated 607 patients with spontaneous bacterial peritonitis and found that NSBBs cause severe hypotension or severe acute kidney injury in this population [20].
Carvedilol blocks both alpha and beta receptors in intrahepatic cells.Compared with propranolol,carvedilol showed higher efficacy in preventing gastroesophageal varices bleeding and more efficiently decreased the hepatic venous pressure gradient[HVPG][21].In addition,carvedilol achieved a hemodynamic response rate of 56% in propranolol non-responders [22].However,carvedilol has been associated with an increased risk of hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis [23].
Although NSBBs have many advantages in treating and preventing portal hypertension,they are less selective,resulting in many side effects.Moreover,portal hypertension is part of compensation mechanism to confer elevated portal blood pressure,which is indispensable for maintaining liver perfusion.When NSBBs and other clinical drugs were used to decrease the pressure of portal vein,liver damage was aggravated because of decreased blood perfusion and nutrient supply [16].In the long run,the prognosis may be deteriorated,which may be the reason why NSBBs or carvedilol has higher mortality rates compared with controls [24].Therefore,the development of new and safer drugs is necessary to improve clinical outcomes.
Promising drugs for the treatment of portal hypertension
Some clinical trials on promising targeted drugs for cirrhotic portal hypertension are listed in Table 1.The molecular targets involved in the pathogenesis of portal hypertension are shown in Fig.1.These targets include intrahepatic targets expressed on SECs or HSCs affecting intrahepatic resistance,extrahepatic targets expressed on peripheral vascularization,which affect peripheral vascularization and forward blood flow.These targeted drugs can slow or inhibit the progression of portal hypertension by acting at different sites along its progression.In addition,these targeted drugs prevent systemic hemodynamic changes and other side effects suggesting their potential value in the treatment of portal hypertension,prevention of gastroesophageal variceal bleeding and other malignant complications.These promising drugs are classified according to their mechanisms and action sites.
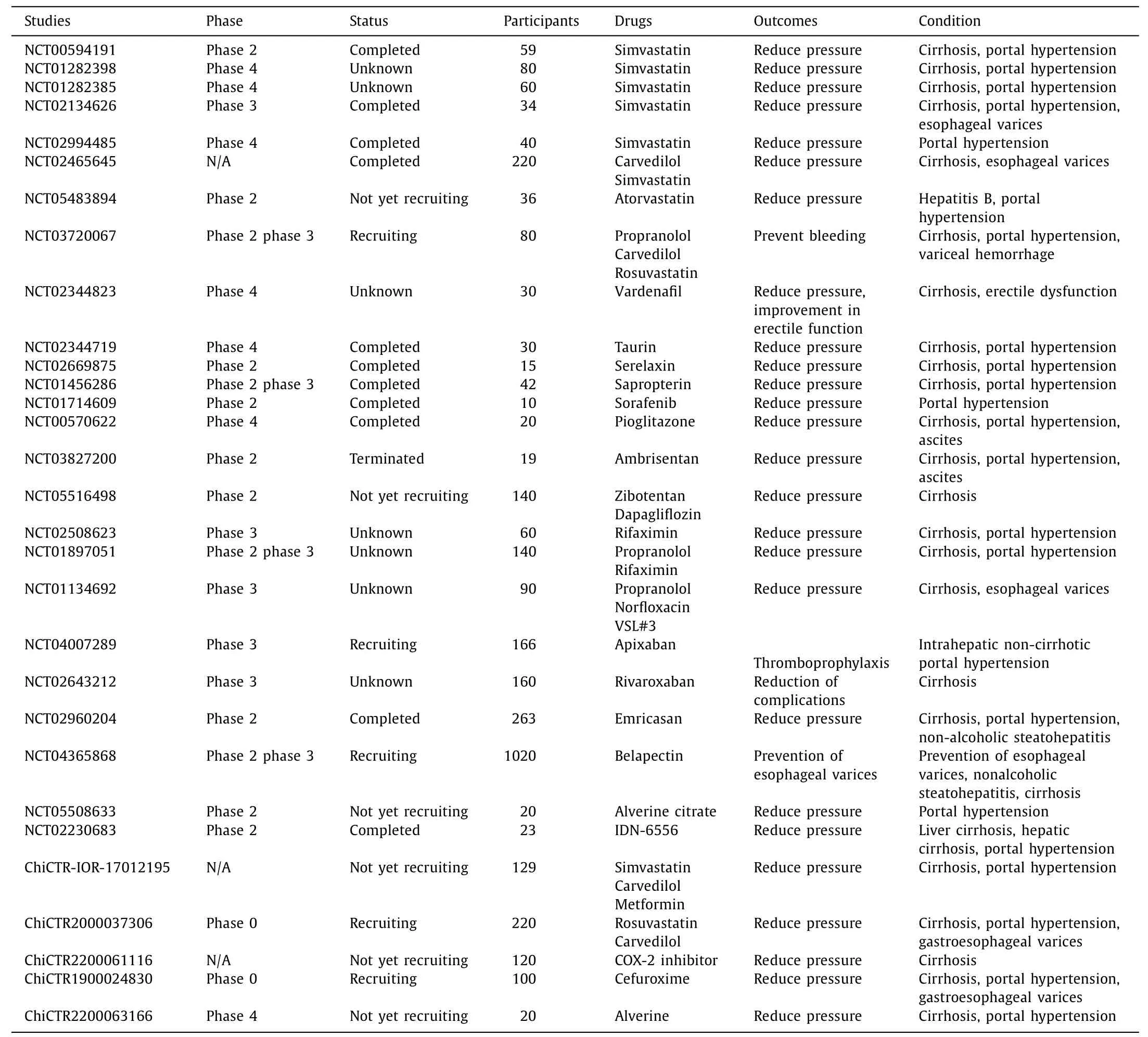
Table 1Clinical trials of promising drugs for cirrhotic portal hypertension.
Drugs acting on intrahepatic targets
Statins
Statins are hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors that effectively reduce low density lipoprotein cholesterol levels by inhibiting HMG-CoA reductase,the ratelimiting enzyme in cholesterol biosynthesis.Therefore,statins are widely used as the primary treatment for dyslipidemia [25].Marrone et al.found that statins can slow the progression of liver fibrosis and prevent portal hypertension.Mechanistically,statins activate Kruppel-like factor 2 (KLF-2) and endothelial nitric oxide synthase (eNOS),deactivate HSC,and selectively reduce intrahepatic resistance without affecting peripheral blood flow [26].
KLF-2 is a key component of SECs that inhibits liver fibrosis and improves endothelial cell function.Dekker et al.found that KLF-2 is specifically expressed on SECs,which prevents inflammation and angiogenesis as well as regulates vascular microenvironment homeostasis and protects blood vessels [27].In early cirrhosis and portal hypertension,KLF-2 can alleviate endothelial dysfunction and angiogenesis and regulate microenvironmental homeostasis [28].However,as the disease progresses,increased KLF-2 expression cannot efficiently antagonize the pathological changes caused by portal hypertension.Vargas et al.found that simvastatin can upregulate KLF-2 on SECs to repair injured endothelial cells and inhibit angiogenesis [29].Through this mechanism,it protects injured blood vessels,maintains hemodynamic stability,and improves portal hypertension.
Statins also prevent the activation of HSCs,the primary extracellular matrix-producing cells in the damaged liver.The activated HSCs promote and aggravate the liver injury and fibrosis [30].Trebicka et al.reported that atorvastatin upregulates KLF-2 and inhibits the activation of HSCs [31].This slows liver fibrosis progression and alleviates intrahepatic pathological resistance in the bile duct ligation-induced cirrhotic animal model.
In addition,statins activate eNOS.The imbalance between intrahepatic vasoconstrictive and vasodilative substances aggravates portal hypertension.eNOS synthesizes vasodilator nitric oxide (NO)and dilates intrahepatic blood vessels,thereby reducing intrahepatic resistance.In portal hypertension,eNOS activity is low and NO production is inhibited [32].Abraldes et al.reported that simvastatin increases the activity of eNOS by regulating RhoA/Rho kinase and PI3K/Akt pathway [33].The RhoA is expressed on HSCs,and its downstream effector protein Rho-kinase is associated with liver fibrosis and portal hypertension [34].Akt phosphorylation causes an increase in Akt-dependent eNOS phosphorylation,which promotes NO synthesis and bioutilization in the liver to reduce intrahepatic vascular resistance [35].
A recent meta-analysis demonstrated that statins can significantly improve the hepatic portal hemodynamic parameters,reduce the risk of related complications,including hepatocellular carcinoma and decrease mortality rate [36].A proof-of-concept study found that HVPG was significantly lower in patients who received atorvastatin and propranolol compared with those in the propranolol alone group.This indicates that statins have additive/synergic effect with NSBBs on portal pressure [37].However,a study by Pose et al.found that higher statin causes rhabdomyolysis [38].Therefore,further clinical studies are needed to confirm the therapeutic value and adverse reactions of statins on portal hypertension.
Celecoxib
Celecoxib,a selective cyclooxygenase-2 (COX-2) inhibitor,is used to relieve osteoarthritis and rheumatoid arthritis symptoms and acute pain episodes.In a normal liver tissue,a dynamic balance exists between the production of reactive oxygen species(ROS) and eNOS activation [39].ROS can remove NO through superoxide disproportionation.Overexpression of ROS leads to excessive NO clearance and aggravates portal hypertension.Cyclooxygenase is a key rate-limiting enzyme involved in the conversation of arachidonic acid to prostaglandins.A previous study reported that COX-2 can promote inflammation and oxidative stress by promoting intravascular ROS production [39].They also showed that COX-2 was overexpressed in cirrhotic portal hypertension and activated ROS-NO pathway.The selective COX-2 inhibitor,celecoxib,was also shown to increase the bioavailability of NO by inhibiting COX-2 in hepatic SECs,improving endothelial function and reducing portal vein pressure.In addition,Gao et al.found that celecoxib can activate the vascular endothelial growth factor receptor 2 (VEGFR-2)receptor and inhibit oxidation of hepatic SECs,which is believed to inhibit intrahepatic fibrosis and angiogenesis [40].Meloxicam,another selective COX-2 inhibitor,was found to reduce liver fibrosis in rats by inhibiting collagen deposition and reduce oxidative stress by inhibiting the production of various proinflammatory factors [41].Therefore,since this drug can reduce intrahepatic resistance and portal vein pressure,it is a potential treatment for portal hypertension in cirrhosis.This drug is expected to enter clinical trials to explore its efficacy and adverse reactions.
Phosphodiesterase-5(PDE-5)inhibitor
As a vasodilator,NO has therapeutic effects on portal hypertension,and is mainly regulated by the NO-cGMP pathway.In addition,NO generated by eNOS activates soluble guanylate cyclase(sGC),catalyzes the conversion of GTP to cGMP and changes the gated calcium channels to play a vasodilatory role.PDE-5 is a key enzyme that catalyzes the conversion of active cGMP to inactive 5’-GMP [42].In portal hypertension,PDE-5 is overexpressed which can break down cGMP and decrease bioavailability of cGMP,leading to hepatic sinusoidal contraction and increase of portal vein pressure.However,in visceral vessels,the expression of PDE-5 is decreased,while the expression of cGMP is increased,and vascular dilatation occurs.This increases portal venous blood flow and aggravates portal hypertension [43].Therefore,using the PDE-5 inhibitor to inhibit the metabolism of cGMP and maintain the high biological activity of cGMP increases the activity of the NOcGMP signaling pathway.This reduces intrahepatic resistance and improves portal hypertension.Sildenafil,a PDE inhibitor,was administered to a rat model of thioacetamide-induced liver fibrosis/cirrhosis.This downregulated PDE-5 expression in cirrhotic tissues.In addition,the content of cGMP was significantly increased whereas portal pressure in the experimental group decreased by 19% [44].Earlier experimental studies demonstrated that sildenafil reduced portal pressure in cirrhotic rats [45,46].These findings demonstrate the potential value of sildenafil in the treatment of cirrhotic portal hypertension.Another study found that sildenafil reduced portal pressure in patients with compensated liver cirrhosis in the acute phase.A risk assessment with NSBB showed that sildenafil treatment improved cardiovascular function and there was no symptomatic hypotension nor increased heart rate [47].This suggests that PDE-5 inhibitors have a stress-reducing therapeutic effect and may have milder cardiovascular side effects than NSBB.Another clinical study found that PDE-5 inhibitors have long-term treatment effects on cirrhotic portal hypertension,reducing portal pressure and risk of gastroesophageal varices bleeding [48].In conclusion,PDE-5 inhibitors have significant therapeutic value in the acute bleeding or prevention of chronic portal hypertension in cirrhotic patients.In addition,PDE-5 inhibitors have fewer side effects than traditional NSBB drugs,indicating high clinical value in portal hypertension management.Evidence from animal experiments has also shown that the sGC has important roles in the progression of portal vein pressure [49].For instance,the sGC agonist riociguat reduces portal vein pressure in cholestatic rats by promoting hepatic sinusoidal vasodilatation and reducing fibrosis.Therefore,riociguat has the potential to treat portal hypertension.However,the specific efficacy and side effects need to be further studied.
Taurine
Taurine is a simple sulfur-containing amino acid found in the human body.It combines with bile acids to exert antioxidant and anti-fibrosis effects and can attenuate alcohol-induced liver injury by inhibiting HSCs activation [50].Taurine-binding bile acids have also been reported to activate eNOS,protect endothelial function,and promote NO synthesis [51].A study investigating the effect of natural taurine on portal hypertension in cirrhotic rats found that administration of natural taurine to model rats for six weeks reduced portal resistance and pressure,increased mean arterial pressure,and altered NO and eNOS activities [52].This shows that taurine can exert portal hypotensive effects by activating intrahepatic eNOS activity and inhibiting extrahepatic eNOS.Thus,it is expected to be adopted in the treatment of cirrhotic portal hypertension.A subsequent clinical study in which taurine was administered orally to patients with cirrhosis and varicose veins for four weeks showed a significant reduction in HVPG in the taurine-treated group with no effect on systemic hemodynamics compared with placebo [53].Taurine is a potential drug for the management of patients with alcoholic liver injury.However,its mechanism of action,long-term efficacy and side effects should be further studied.
AMP-activatedproteinkinase(AMPK)pathwayactivator
AMPK plays an important role in the regulation of bioenergy metabolism [54].Hu et al.found that the AMPK pathway activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) promotes the expression of eNOS and the production of vasodilator NO in bile duct ligation and CCl4fibrotic rats,and reduces portal pressure without affecting systemic hemodynamics [55].Another experimental study found that AICAR inhibits the intrahepatic NFκB-inducing kinase pathway and reduces liver inflammation and fibrosis in rats.These results show that AICAR has dual roles,i.e.,anti-fibrosis and dilatation of hepatic sinusoids when applied to cirrhotic portal hypertension [56].This molecule is expected to be a potential treatment for portal hypertension.
Solubleepoxidehydrolase(sEH)inhibitor
Epoxy-fatty acids (epFAs,including epoxyeicosatrienoic acids,epoxyoctadecenoic acids,epoxyeicosatetraenoic acids,and epoxydocosapentaenoic acids) have anti-inflammatory and anti-fibrotic effects,and sEH catalyzes epFAs.Therefore,sEH inhibition has antiinflammatory and anti-fibrotic effects [57].An experimental study in CCl4-induced liver injury in rats found that t-TUCB (an sEH inhibitor) decreases portal pressure,liver fibrosis and improves hepatic endothelial dysfunction in cirrhotic rats [58].In addition,t-TUCB activates eNOS,increases the bioavailability of NO,improves endothelial function and reduces intrahepatic vascular resistance.As a promising drug,sEH inhibitor has potential anti-fibrotic effects in many diseases.Researchers have documented the efficacy of sEH inhibitors and reported very few side effects in patients with cirrhotic portal hypertension [59].Although no clinical research has been carried out on portal hypertension,its potential therapeutic value is still recognized.
FarnesoidXreceptor(FXR)agonist
FXR is highly expressed in the liver and small intestine and plays a role in bile acid metabolism,regulates liver fibrosis,intestinal flora,and vasomotion [17].Sorribas et al.found that FXR protects the gut-vascular barrier by inhibiting bacterial translocation in cirrhosis and portal hypertension,thereby reducing complications and mortality [60].An experimental study on the effect of the selective FXR agonist PX20 60 6 showed that administration of PX20 60 6 up-regulates FXR,reduces bacterial translocation and the expression of intrahepatic pro-fibrotic proteins,and increases the expression of eNOS,leading to hepatic sinusoidal expansion and the reduction of intrahepatic resistance [61].The results show that FXR agonists can reduce liver fibrosis,vascular remodeling,and endothelial dysfunction.Therefore,it can be applied in patients with cirrhotic portal hypertension,especially cholestatic cirrhosis.Another experiment with the FXR agonist obeticholic acid (OCA) revealed that OCA reduced portal pressure by alleviating intrahepatic vascular resistance and had no effect on mean arterial pressure in model animals [62].This suggests that the mechanism of OCA action may be liver-selective,which is currently lacking in traditional drugs.In addition to the FXR target,another target of bile acid action,TGR5,has been reported.TGR5 is widely expressed in SECs and the function of TGR5 is vasodilatation.However,there are few preclinical studies on this target.Therefore,its efficacy in the treatment of portal hypertension should be studied [63].
Relaxin
Relaxin is a peptide hormone first discovered in the circulating blood of pregnant women which facilitates the fetus delivery.Relaxin has since been used to prevent and treat cardiovascular diseases because of its vasodilator function.Fallowfield et al.found that relaxin reduces portal vein pressure and intrahepatic vascular resistance by enhancing the activity of the intrahepatic NO pathway and reducing the contraction of myofibroblasts but does not cause systemic hypotension [64].In addition,Ezhilarasan et al.reported that relaxin has antifibrotic effects,possibly because it can reshape deposited extracellular matrix and inhibit the transduction of pro-fibrotic transforming growth factorβ[65].Therefore,the vascular and antifibrotic effects of relaxin can potentially treat portal hypertension and have unique advantages in managing acute varicose bleeding.However,a phase II clinical study on serelaxin found that short-term application of relaxin had no significant effects on portal vein pressure,although it was safe,well tolerated,and did not affect systemic hemodynamics [66].Therefore,further studies are needed to explore and confirm the molecular mechanism,long-term efficacy,and adverse reactions of relaxin in reducing portal vein pressure.
Terutroban
In portal hypertension,vasoconstrictor substances,such as thromboxane A2,increase the bioactivity in the liver and act on HSCs and SECs.This increases intrahepatic resistance and portal hypertension.Terutroban,a selective antagonist of the thromboxane receptor,blocks thromboxane induced vasoconstriction and reduces portal vein pressure [67].This study also found that terutroban acts on the Rho pathway and reduces liver Rho-kinase activity,thereby decreasing intrahepatic vascular resistance.In addition,terutroban reduces transforming growth factorβand extracellular matrix deposition to play an anti-fibrotic role.However,due to the limited experimental research data,the specific feasibility of the application of terutroban to portal hypertension and its side effects still need to be studied.
Antioxidant
The production of intrahepatic oxides is an important driver of HSC activation and SECs dysfunction.As a result,applying antioxidants can reduce liver damage and fibrosis and play a role in treating portal hypertension.Resveratrol,a polyphenolic substance present in various fruits,improves endothelial cell dysfunction and reduces liver fibrosis and portal pressure in cirrhotic rats [68].Mitoquinone is a mitochondria-targeted antioxidant that inactivates HSCs in cirrhotic rats and reduces oxidative stress in the liver.As a result,mitoquinone reduces intrahepatic vascular resistance,liver fibrosis,and portal pressure [69].The antioxidant recombinant human manganese superoxide dismutase [70],diammonium glycyrrhizinate [71]and glutamine [72]have been shown to reduce liver fibrosis and portal vein pressure by anti-oxidative stress.Antioxidants have unique advantages in treating and preventing portal hypertension.Therefore,further studies need to be conducted to confirm the efficacy of antioxidants.
Tetrahydrobiopterin(BH4)
BH4is an important cofactor of eNOS.BH4deficiency in portal hypertension uncouples eNOS leading to dysfunction of SECs and the increase of portal hypertension [73].Experimental studies have shown that BH4supplementation can protect hepatic sinusoidal endothelial function and effectively reduce portal vein pressure in cirrhotic rats [73,74].However,administration of BH4to patients with chronic liver disease does not significantly affect HVPG.Similarly,the concentration of BH4in liver tissue was not correlated with the severity of portal hypertension [75].The results of clinical experiments have been inconsistent with those from animal models,which may be due to differences in targets and mechanisms between the two.Therefore,use of BH4in the treatment and prevention of portal hypertension needs to be further studied.
Drugs acting on extrahepatic targets
Tyrosinekinaseinhibitor
Tyrosine kinase inhibitors are widely used in the treatment of cancer,especially lung,kidney,and liver cancer [76].These drugs inhibit VEGFR and platelet-derived growth factor receptor(PDGFR).Adnane et al.found that inhibiting VEGFR and PDGFR inhibits angiogenesis in tumor tissues,which inhibits tumor growth [77].Palmer et al.reported that tyrosine kinase inhibitor sorafenib significantly inhibits visceral angiogenesis,liver inflammation,and fibrosis and improves collateral circulation in portal hypertension model rats [78].This is because VEGFR,PDGFR,and other pro-angiogenic factor receptors are overexpressed in portal hypertension,and angiogenesis is active,especially in mesenteric collateral branches.These factors lead to the systemic hyperdynamic circulation and increase of prehepatic blood flow.Sorafenib inhibits the expression of VEGFR,PDGFR,and angiogenesis which results in the reduction of portal vein pressure [79,80].Mejias et al.applied sorafenib to rats with portal hypertension and found that sorafenib reduces the expression of eNOS in the viscera in rats which reduces the portal blood flow (forward theory) [81].A prospective study of lenvatinib in patients with advanced hepatocellular carcinoma showed that lenvatinib significantly improves hemodynamics in patients with portal hypertension,providing favorable clinical evidence that tyrosine kinase inhibitors reduce blood flow in portal vein [82].
Other studies have demonstrated that sorafenib regulates intrahepatic resistance by acting Rho kinase signaling,which inhibits the activation of hepatic stellate cells [83,84].Thus,tyrosine kinase inhibitors have great potential in treating portal hypertension.In addition,some clinical studies have shown that sorafenib is more effective in treating patients with primary hepatocellular carcinoma or alcoholic cirrhosis complicated with portal hypertension [85].Long-term administration of sorafenib could reduce portal vein pressure and improve prognosis.Otherwise,a study by D’Amico et al.found that the combination of sorafenib and propranolol had better efficacy than either of the drugs alone.Therefore,combining sorafenib and propranolol can significantly reduce portal pressure and angiogenesis in rats with portal hypertension [86].However,due to the limitations of current experimental and clinical studies,adverse reactions such as arterial hypertension and rash caused by sorafenib cannot be ruled out.More basic and clinical studies are needed to explore the potential application of tyrosine kinase inhibitors in portal hypertension.
Thalidomide
Thalidomide,once widely used as a sedative and analgesic to treat nausea and vomiting during pregnancy,has been banned after tens of thousands of malformations occurred in children.Although thalidomide has several side effects,recent evidence shows that it can reduce portal vein pressure in cirrhotic rats by inhibiting tumor necrosis factor-alpha (TNF-α) and mesenteric angiogenesis and vasodilatation.In addition,studies show that thalidomide reduces portal vein pressure by reducing peripheral NO synthesis and extrahepatic blood flow [87,88].However,the updated study observed no increase in TNF-αand no change in eNOS level after thalidomide administration in the rat model of liver cirrhosis.Still,there was a decrease in the mean arterial pressure and portal vein pressure [89].This suggests that the mechanism of thalidomide treatment for portal hypertension may be independent of TNF-αand the traditional NO pathway.In conclusion,thalidomide has the potential effect of reducing forward blood flow in the treatment of portal hypertension,but its specific mechanism and adverse reactions still need more studies.
Pioglitazone
The peroxisome proliferator-activated receptorγ(PPARγ) is a ligand-dependent nuclear receptor that can be activated by pioglitazone.The PPARγregulates lipid and glucose metabolism,which has been applied in treating diabetes [90].In addition,PPARγis anti-inflammatory,antioxidant,angiogenic,and reduces collateral circulation.Schwabl et al.found that the activation of PPARγwith pioglitazone in rats with portal hypertension reduces portal vein pressure,neovascularization,and portosystemic shunt and plays an anti-inflammatory role to a certain extent [91].Another study on pulmonary hypertension in liver cirrhosis found that pioglitazone downregulates peripheral eNOS and vascular endothelial growth factor activities and reduces shunting and angiogenesis without affecting systemic hemodynamics [92].This demonstrates the unique advantages of pioglitazone in the treatment of portal hypertension.However,further studies on the mechanism and long-term effects of pioglitazone should be conducted before using it in portal hypertension.
Drugs acting on both intrahepatic and extrahepatic targets
CB2agonists
A previous study demonstrated that the cannabinoid receptor 2 (CB2) agonists,JWH-133 and GP1a,increases heme oxygenase 1(HO-1) expression and reduce portal hypertension [93].According to the study,CB2 agonists increased the expression of HO-1 and inhibited the production of TXB2 by activating Kupffer cells.The CB2 agonists did not affect the mean arterial pressure or heart rate,suggesting a potential advantage in treating portal hypertension with inflammatory liver injury.This study showed that the highly selective CB2 agonist GP1a could reduce portal pressure by 21% within minutes of intraperitoneal injection,suggesting that GP1a could be used to prevent and treat bleeding in acute gastroesophageal varices.CB2 agonists exert anti-portal hypertensive effects by im proving visceral hemodynamics and reducing neovascularization.Furthermore,an experimental study revealed that rats treated with JWH-015 showed significant reduction of portal vein pressure,decrease of blood flow in the superior mesenteric artery,portosystemic shunt,and angiogenesis [94].Moreover,Qin et al.reported that CB2 agonists reduced portal blood flow and decreased vasoconstriction by inhibiting the expression of HO-1 and nuclear factor-E2-related factor 2,thereby reducing portal vein pressure [95].Taken together,these studies reveal that CB2 agonists reduce portal vein pressure by regulating systemic hemodynamics and reducing visceral angiogenesis.In addition,CB2 agonists have anti-inflammatory and anti-fibrotic effects,suggesting a potential therapeutic role in portal hypertension.
Endothelin-1 (ET-1)
ET-1 is a vasoconstrictor.Activated HSCs in portal hypertension produce ET-1 and other vasoconstrictive substances,which affect the hepatic sinusoidal blood flow,thus aggravating intrahepatic vascular resistance [96].Therefore,ET-1 antagonists could be exploited as novel therapeutic agents in portal hypertension.The selective ETAantagonists were found to be more effective than non-selective ET-1 antagonists in cirrhotic portal hypertension [97,98].A previous study showed that ambrisentan and atorvastatin had synergistic effects,which could significantly reduce portal hypertension and improve endothelial function [99].Ambrisentan was shown to block ET-1,thus inhibiting the vasoconstriction induced by the activation of HSCs.In addition,ambrisentan increases the phosphorylation of eNOS in hepatic sinusoids.Hsu et al.showed that the non-selective ET-1 antagonist and the selective ETAantagonist ambrisentan reduced portal vein pressure in cirrhotic rats,by decreasing portosystemic shunt and mesenteric angiogenesis.Ambrisentan showed no effects on systemic hemodynamics [100].Therefore,ET-1 antagonists offer a good therapeutic value in portal hypertension.
Others
Antibiotics
Abnormal bile metabolism in liver cirrhosis impairs enterohepatic circulation,which causes the imbalance of intestinal microbiota and the overgrowth of small intestinal bacteria.Bacterial endotoxins can damage hepatocytes directly,while bacterial translocation can aggravate intrahepatic infection and decompensation [101].Furthermore,intestinal bacterial translocation and endotoxin production increase portal pressure in cirrhosis,especially in alcoholic hepatitis or concurrent spontaneous peritonitis [102].Therefore,intestinal decontamination with antibiotics can alleviate the severity of portal hypertension.Kemp et al.found that norfloxacin combined with propranolol effectively reduced portal venous pressure in decompensated cirrhosis [103].In addition,rifaximin,an orally administered antibiotic with minimal absorption in the gastrointestinal tract,has been shown to prevent the development and progression of complications in liver cirrhosis.Furthermore,Caraceni et al.found that rifaximin alone or in combination with other drugs can prevent complications,reduce portal vein pressure,and have a good therapeutic value for cirrhotic portal hypertension [104].The 5-lipoxygenase pathway and the Toll-like receptor have been found to participate in bacterial translocation and liver injury resulting in activation of KC and dysfunction of SECs [105].Therefore,inhibitors of these targets could be therapeutically valuable in cirrhotic portal hypertension.
Anticoagulants
Accumulation of coagulation factors during the decompensated stage of cirrhosis increases the risk of portal vein thrombosis and intrahepatic resistance.Further,portal hypertension increases the risk of gastroesophageal variceal bleeding and thrombosis-related complications.Therefore,anticoagulants can be used to prevent portal hypertension-related complications [106].A previous study reported that enoxaparin reduced liver fibrosis and decreased intrahepatic resistance by inhibiting intrahepatic thrombosis and thrombin-mediated activation of HSCs,thereby alleviating portal pressure in cirrhotic rats [107].Anticoagulants do not increase the risk of gastroesophageal venous hemorrhage.Instead,they increase portal vein recanalization.Therefore,anticoagulants are safe and effective therapeutic agents for the treatment of cirrhotic portal hypertension [108].Contrary to this finding,Fortea et al.reported that enoxaparin treatment did not improve liver fibrosis or reduce portal hypertension in rats with advanced cirrhosis [109].Therefore,there is a need for further studies to investigate the clinical use of anticoagulants in portal hypertension.In addition,rivaroxaban was shown to inactivate HSCs,increase intrahepatically available NO,and reduce fibrin deposition,leading to the suppression of portal vein pressure [110].Anticoagulants show a promising value for the prevention and treatment of portal hypertension.However,the clinical applicability of anticoagulants in portal hypertension requires to be investigated in further studies.
Emricasan
Emricasan is an irreversible pan-caspase inhibitor that improves liver function and inhibits apoptosis in liver fibrosis and cirrhosis.It is well tolerated in most patients,with few reported adverse events.Mechanistically,it acts by inhibiting FAS-induced apoptosis [111].Recent experimental studies showed that emricasan can reduce portal pressure in nonalcoholic steatohepatitis and compensatory cirrhosis with severe portal hypertension [112,113].Although the mechanism of action of emricasan is unclear,it is postulated that it reduces portal vein pressure by inducting antiinflammatory and anti-fibrotic effects.Therefore,emricasan is a potential therapeutic agent for patients with portal hypertension.
Galectin-3inhibitor
The galectin-3 inhibitor,belapectin,has been investigated in clinical management for non-alcoholic steatohepatitis [114].A phase 2 clinical trial showed that belapectin could inhibit liver fibrosis,reduce portal pressure,and decrease the risk of esophageal varices [115].However,only a few preclinical and clinical studies have investigated the benefits of belapectin.Therefore,the pharmacological mechanism and side effects of belapectin in portal hypertension need to be further investigated.
Cysteinyl-leukotrienes(Cys-LTs)inhibitor
Cys-LTs are a class of bioactive substances that constrict blood vessels.The bioactivity of Cys-LTs is increased in portal hypertension following liver cirrhosis.The 5-lipoxygenase enzyme participates in the synthesis of Cys-LTs.Previous studies found that 5-lipoxygenase and Cys-LT inhibitors can reduce intrahepatic vascular resistance,thus decreasing portal vein pressure by lowering the biological activity of Cys-LTs [116,117].However,further studies should be conducted to reveal the molecular mechanism and clinical value of Cys-LT inhibitors in the management of portal vein hypertension.
Summary and future prospects
Portal hypertension and its complications,such as gastroesophageal varices,refractory ascites and hepatic encephalopathy,worsen the prognosis of patients with liver diseases.Currently,it is difficult to prevent and treat portal hypertension due to the lack of effective strategies for reversing the intrahepatic structural changes and peripheral neovascularization.Furthermore,the currently used drugs have several adverse hemodynamic effects.Thus,the emergence of new targeted drugs,such as statins,presents hope for future treatment of portal hypertension,which are shown to reduce intrahepatic resistance or decrease portal vein blood flow,and are expected to become clinically applicable in the management of portal hypertension.Furthermore,precision medicine is expected to offer solutions for the diagnosis and individualized treatment of portal hypertension.Precision medicine can improve the classification and individualize treatments according to different disease subtypes,molecular markers or genomes.Combining targeted therapies and precision medicine offers the most promising therapeutic strategy for patients with portal hypertension.
Acknowledgments
None.
CRediT authorship contribution statement
Ji-Yao Sheng:Funding acquisition,Writing– original draft,Writing– review &editing.Zi-Fan Meng:Data curation,Formal analysis,Writing– original draft.Qiao Li:Investigation,Writing–original draft.Yong-Sheng Yang:Conceptualization,Supervision,Writing– review &editing.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81902484),China Postdoctoral Science Foundation (2020M670864),Youth Support Project of Jilin Association for Science and Technology (202028),Jilin Provincial Health Special Project (2020SCZT039),and Jilin Health and Healthy Youth Science and Technology Training Plan (2020Q017).
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2024年1期
Hepatobiliary & Pancreatic Diseases International2024年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Stereotactic body radiotherapy in pancreatic adenocarcinoma
- Application of ultrasonography-elastography score to suspect porto-sinusoidal vascular disease in patients with portal vein thrombosis
- Polydatin ameliorates hepatic ischemia-reperfusion injury by modulating macrophage polarization
- Hypomethylation of glycine dehydrogenase promoter in peripheral blood mononuclear cells is a new diagnostic marker of hepatitis B virus-associated hepatocellular carcinoma
- AGK2 pre-treatment protects against thioacetamide-induced acute liver failure via regulating the MFN2-PERK axis and ferroptosis signaling pathway
- Circulating RNA ZFR promotes hepatocellular carcinoma cell proliferation and epithelial-mesenchymal transition process through miR-624–3p/WEE1 axis
