Application of ultrasonography-elastography score to suspect porto-sinusoidal vascular disease in patients with portal vein thrombosis
Stefni Gioi ,Adrino De Sntis ,Giuli d’Amti ,Silvi Nrdelli ,Alessndr Spgnoli ,Arinn Di Roo ,Lorenzo Ridol ,Oliviero Riggio
a Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
b Department of Radiological, Oncological, and Pathological Sciences, Sapienza University of Rome, Rome, Italy
c Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy
Keywords: Non-cirrhotic portal hypertension Porto-sinusoidal vascular disease Chronic portal vein thrombosis Liver stiffness Portal hypertension Acoustic radiation force impulse
ABSTRACT Background: Porto-sinusoidal vascular disease (PSVD) and portal vein thrombosis (PVT) are causes of portal hypertension characterized respectively by an intrahepatic and a pre-hepatic obstacle to the flow in the portal system.As PVT may be a consequence of PSVD,in PVT patients at presentation,a pre-existing PSVD should be suspected.In these patients the identification of an underlying PSVD would have relevant implication regarding follow-up and therapeutic management,but it could be challenging.In this setting ultrasonography may be valuable in differential diagnosis.The aim of the study was to use ultrasonography to identify parameters to discriminate between PSVD and “pure” PVT and then to suspect PVT secondary to a pre-existing PSVD.Methods: Fifty-three patients with histologically proven PSVD and forty-eight patients affected by chronic PVT were enrolled and submitted to abdominal ultrasonography with elastography by acoustic radiation force impulse (ARFI).Results: ARFI was higher and superior mesenteric vein (SMV) diameter was wider in PSVD patients than in PVT patients.Thus,a prognostic score was obtained as linear combinations of the two parameters with a good discrimination capacity between PSVD and PVT (the area under the curve=0.780;95% confidence interval: 0.690-0.869).Conclusions: A score based on ARFI and SMV diameter may be useful to suspect an underlying PSVD in patients with PVT and to identify a subgroup of patients to be submitted to liver biopsy.
Introduction
Porto-sinusoidal vascular disease (PSVD) is a vascular liver disease characterized by histological lesions involving portal venules and sinusoids with or without portal hypertension,in the absence of cirrhosis [1].
In about 40% of PSVD patients,a prothrombotic condition is associated,which may have a pathogenic role in the obliteration of the portal venules favoring the obliterative portal venopathy,one of the main lesions of the disease [2].Moreover,the presence of a procoagulant condition may contribute,in addition to portal flow slowdown secondary to portal hypertension,to the occurrence of an extrahepatic portal vein thrombosis (PVT).In fact,30%-40% of PSVD patients complicated with PVT [3,4].Consequently,the association between PSVD and PVT resulted in the elimination of the patency of portal vein among the recent diagnostic criteria of PSVD [1].Therefore,an underlying and undiagnosed PSVD should be suspected in any patient with recent or chronic PVT.However,according to an established diagnostic workup,in patients with recent or chronic PVT,once a prothrombotic state is identified as the possible cause of thrombosis,the possibility of the presence of other predisposing factors and especially of a pre-existing PSVD is usually not considered and consequently liver biopsy is rarely performed.The risk of misdiagnosis of PSVD in PVT patients is high and it may have relevant clinical consequences.This may at least partially explain that the cause of PVT,despite an active search,remains unrecognized in up to 25% of the patients [5].
However,the distinction between PVT due to PSVD and pure PVT is difficult.To date liver biopsy remains mandatory to diagnose PSVD [1]even if its usefulness in establishing a differential diagnosis between the two conditions is debatable [6].One of the histological features of PSVD is obliterative portal venopathy represented by the obliteration and sclerosis of the terminal branches of portal vein [7]in a context of a fibrotic portal tract.Consequently,PSVD is characterized by a certain degree of liver fibrosis,theoretically absent in PVT occurring in patients with no liver diseases [8].
Given this background,this study aimed to use ultrasonography to identify non-invasive parameters able to discriminate these two conditions in order to recognize a subgroup of PVT patients with a high suspicion to be affected by a pre-existing PSVD.We considered liver stiffness a possible parameter [6–10]since PSVD patients have a certain degree of liver fibrosis,and splenic vein (SV) and superior mesenteric vein (SMV) diameters as signs of increased flow from splanchnic area.
Methods
One hundred and one patients affected by non-cirrhotic portal hypertension were included in the study.Among them,48 had chronic PVT and 53 had histologically proven PSVD.All the patients of both groups were characterized by the presence of portal hypertension defined by the presence of splenomegaly and oesophageal varices or other portal-systemic collaterals.Patients were included in the chronic PVT group when the presence of cavernomatous transformation of the portal vein was radiologically identified.According to the Sarin’s classification,in all the patients the thrombosis was occlusive and involved the trunk of portal vein (type I and III) [11].Neoplastic portal vein obstruction was ruled out by radiology and by the absence of known neoplasia.
Patients were included in the PSVD group based on liver biopsy [1].Moreover,portal and hepatic veins occlusion was excluded by imaging techniques.
Cirrhosis was ruled out on absence of chronic liver disease,normal liver protein synthetic capacity,and lack of advanced fibrosis on liver elastography.
Patient initial evaluations
Data retrieved from medical charts including sex,age,liver biochemistries,and signs of portal hypertension were recorded.At first evaluation,patients were also submitted to a screening for any underlying prothrombotic disorder due to hereditary and acquired thrombophilia,and to an upper endoscopy to assess the presence of esophago-gastric varices.When present,esophageal varices were defined as small or large [12].Abdominal ultrasound was used for the measurement of longitudinal diameter of spleen,SV and SMV diameter.SV and SMV were measured at its largest point in transverse and longitudinal scans 2 cm before the confluence into the portal vein.When visible,the diameter was recorded also in case of splenic or mesenteric vein thrombosis.SV and SMV were measured in 87 patients,and 14 patients (4 PSVD patients and 10 PVT patients) were not measured because not visible for the intense meteorism in 7 patients,for the presence of splenic and mesenteric vein thrombosis in 4 patients,and for the technical limitation in 3 patients.
Liver stiffness assessment
In all the patients,acoustic radiation force impulse (ARFI) were measured using a Siemens Acuson S2000 ultrasound system.Each patient was placed in the supine position and underwent ARFI on B-mode imaging during deep breath.A region of interest (fixeddimension 1.0×0.5 cm box;maximum evaluable depth,8.5 cm)in the liver parenchyma,free of large blood vessels,was selected using the intercostal approach.Liver stiffness was measured in the right lobe of the liver,almost 1 cm below the liver capsule,using the intercostal approach.Ten consecutive successful measurements were performed in each patient,and mean value in kilopascal (kPa) was recorded.Liver stiffness was assessed as close as possible to the time of the diagnosis of PSVD or to the first clinical assessment.
The purpose of the study was clearly explained to all the patients before obtaining their written informed consent.This study was approved by Ethical Committee of The Sapienza University of Rome (5068/2018).
Statistical analysis
Data were expressed as median and interquartile range (IQR)for continuous variables and frequencies for categorical ones.Comparison between groups was performed by Chi-square test or Mann-Whitney test,as appropriate.
Pearson’s correlation index was used to assess relationship between SV and SMV diameters.The diagnostic performances of ARFI elastography and SMV diameter were assessed by the area under the receiver operating characteristic (ROC) curves (AUC) that were designed to differentiate between the PSVD group and the chronic PVT group.Optimal cut-off values were chosen to maximize the sum of sensitivity and specificity.In order to evaluate the improvement in prediction performance of PSVD with the addition of the SMV diameter to ARFI alone,the net reclassification index (NRI)was computed.
A logistic regression model was estimated,and the beta coefficients (logarithm of the odds ratios) obtained were used to derive weighting factors of a new prognostic score [UltraSonography-Elastography (USE) score].The score was evaluated by means of the AUC for the ROC curve.Internal validation was obtained by means of 10000 bootstrap replicates.
All tests were two-tailed,and a value ofP<0.05 was considered statistically significant.Analyses were performed using R version 4.0.1 (The R Project for Statistical Computing).
Results
The characteristics of the patients at entry are summarized in Table 1.PSVD patients were younger and male dominated.Liver function tests were comparable in the 2 groups,but platelets count was higher in chronic PVT patients,probably due to the higher proportion of myeloproliferative neoplasms (MPN) in these patients.A thrombophilia was found in 5 PSVD patients (9.4%) and in 23 chronic PVT patients (47.9%);MPN were diagnosed in 6 PSVD patients and 9 PVT patients.Moreover,in other 12 PSVD patients an associated condition (immunological disease/coeliac disease/cystic fibrosis/exposition to oxaliplatin) was present.
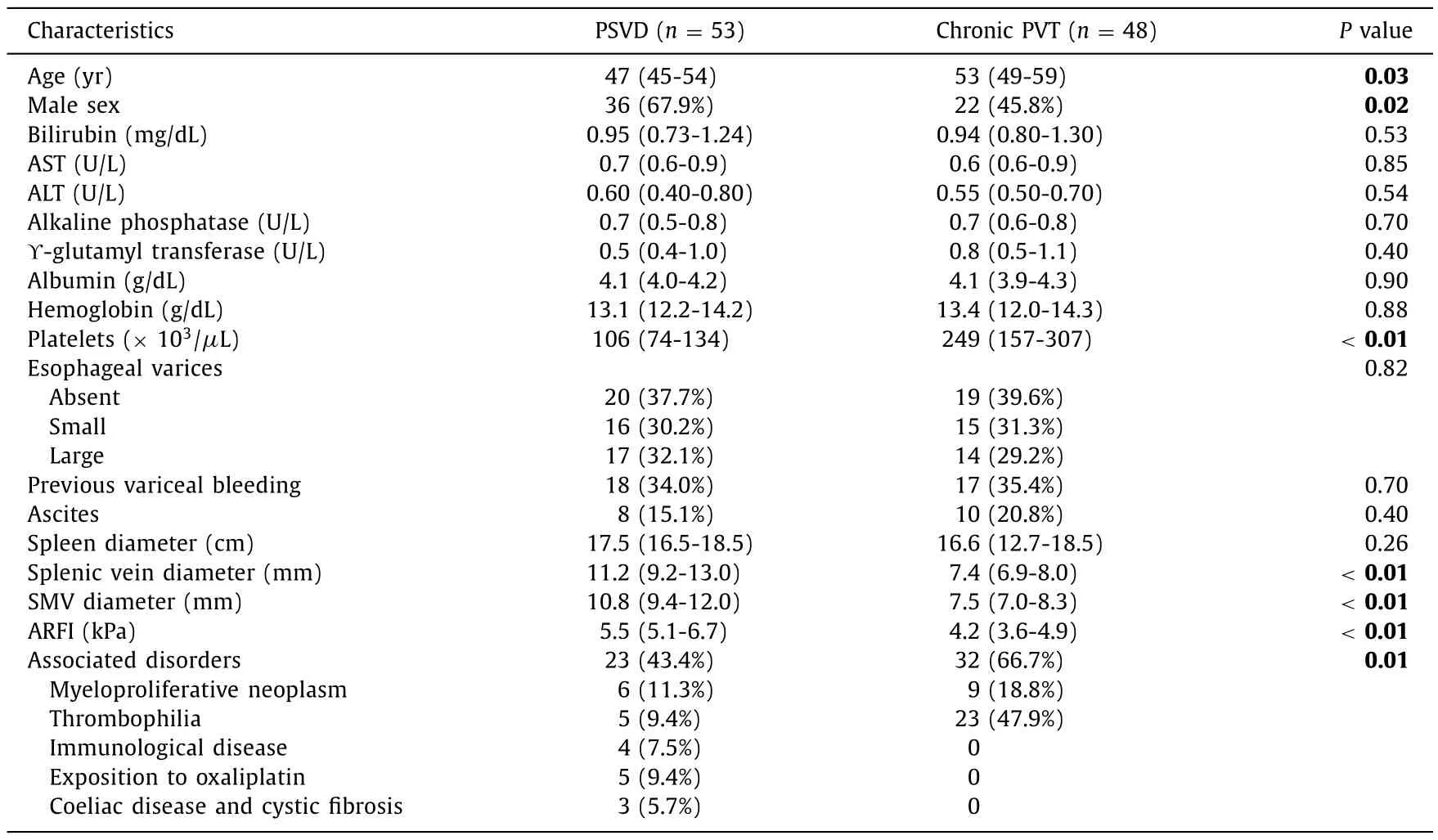
Table 1Characteristics of the 101 patients included in the study.
The proportion of clinical signs of portal hypertension was similar in the two groups of patients.SV and SMV diameters were larger in PSVD patients compared with those in PVT patients while spleen diameter was similar.Liver stiffness measured by ARFI elastography was also significantly higher in the PSVD group than in the chronic PVT group (Table 1).
On the basis of the results of the comparison between the PSVD and chronic PVT groups,we assessed the accuracy of ARFI and SMV diameter in discriminating between these two conditions.The SV diameter was not included in the analysis because it was considered a covariant of SMV diameter as supported by the high correlation (r=0.76) found in our series between these two parameters.The AUC of ARFI was 0.712 (95% CI: 0.603-0.821) (Fig.1 A).The AUC of SMV diameter was 0.839 (95% CI: 0.754-0.924) (Fig.1 B).An NRI analysis was performed to evaluate the improvement in accuracy with the addition of the SMV diameter to ARFI alone in the prediction of PSVD.The NRI was 0.503 (95% CI: 0.323-0.685,P<0.0001),indicating that 50% of the patients were better classified using the combination of both parameters.
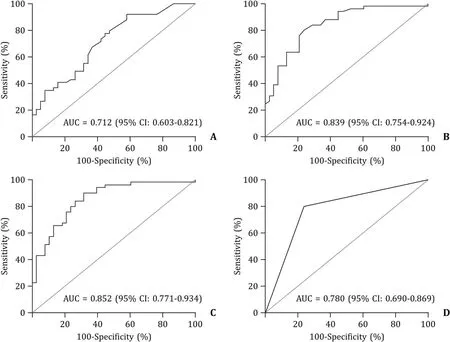
Fig.1. Area under the receiver operating characteristic (ROC) curve of the ability of ARFI (A),SMV diameter (B),ARFI+SMV diameter (C),and USE score (D) to predict PSVD.CI: confidence interval;ARFI: acoustic radiation force impulse;SMV: superior mesenteric vein;USE: UltraSonography-Elastography;PSVD: porto-sinusoidal vascular disease.
Finally,a logistic regression model based on ARFI value and SMV diameter was constructed (Table 2).The AUC of the model was 0.852 (95% CI: 0.771-0.934) (Fig.1 C).
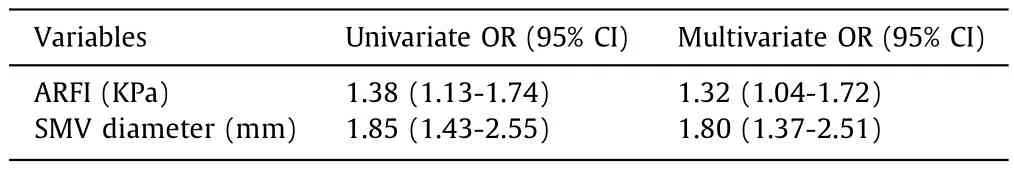
Table 2Logistic regression model based on ARFI value and SMV diameter for the prediction of PSVD.
Prognostic score (USE score) was obtained as linear combinations of the ARFI value and SMV diameter,where the weights were obtained by rounding the logistic regression beta coefficients (USE score=0.3 ARFI+0.6 SMV).Therefore,a prognostic score was assigned to each patient to identify the individual risk of being affected by PSVD according to ARFI and SMV diameter values.The USE score showed a good discrimination with an AUC of 0.780(95% CI: 0.690-0.869) (Fig.1 D).The optimal cut-off for discriminating PSVD and PVT using the USE score was 6.8,with a sensitivity of 81% and a specificity of 76%.The USE score was then internally validated by a bootstrap sampling procedure,which gave an AUC of 0.779 (95% CI: 0.688-0.866).
Discussion
Recently,more and more patients with portal hypertension have been identified as affected by PSVD.In the past,most of these patients were misdiagnosed as affected by cryptogenic compensated cirrhosis [13].Moreover,the presence of PSVD has been also recognized among patients with disorders associated to this condition as primary or secondary immunodeficiency or treated with drugs such as oxaliplatin [14–16].PSVD can also be misdiagnosed in patients first observed because of acute or chronic PVT [17].In these patients,portal hypertension due to PSVD,by reducing the portal flow,may trigger the occurrence of portal vein obstruction.This mechanism favoring the PVT is usually missed as PVT patients are usually not submitted to liver biopsy [18].
In the present study,by comparing PSVD with PVT patients,we tested the capacity of ultrasonographic parameters in distinguishing these two conditions on the hypothesis that ultrasonographic parameters could help identify PVT patients due to a pre-existing PSVD.
Liver stiffness measured by ARFI as well as superior mesenteric vein diameter were significantly higher in patients affected by PSVD than in those affected by chronic PVT.Therefore,a score based on both the ARFI and the SMV values was developed and showed to have a discriminatory ability between PVT and PSVD.In particular,the USE score was obtained as linear combinations of the two parameters (ARFI value and SMV diameter),and the identified optimal cut-off for discriminating PSVD and PVT using the USE score was 6.8.Therefore,in our opinion,a USE score>6.8 should insinuate the suspect of PSVD in PVT patients and suggest performing a liver biopsy to confirm the diagnosis of PSVD.
The identification of an underlying PSVD,particularly in patients with acute PVT,may have relevant therapeutic consequences.In patients with recent PVT and no evident prothrombotic conditions,anticoagulation is usually withdrawn at the resolution of the thrombosis.However,when PVT is due to PSVD,the portal flow slowdown may favor the recurrence of PVT at the withdraw of anticoagulant treatment [6].Thus,in this case a lifelong anticoagulation therapy may be suggested.Moreover,despite achieving a theoretical complete recanalization,some patients with acute PVT develop portal hypertension during follow-up.This observation may support the hypothesis that at least in some patients,acute PVT can be related to a pre-existing PSVD.In addition,although not demonstrated,anticoagulation prolonged after the thrombosis resolution in PVT patients due to PSVD may have a positive impact also on the intrahepatic vascular alterations [19],and on the patients’ prognosis.Finally,a diagnosis of PSVD may also result in the active search of the disease known to be associated to PSVD such as immunodeficiency,coeliac and autoimmune disease,etc [20].
Unfortunately,since our observation and the consequent suggestion of a method to suspect PSVD in PVT patients remains a hypothesis,liver biopsy in all PVT patients should be done to verify this hypothesis.Until then,our data could at least raise the suspicion in the group of PVT patients with the highest USE score independently on an identified systemic pro-thrombotic state.
In conclusion,due to its relevant clinical implications,the existence of a PSVD should be always suspected in PVT patients.Our data support the use of ultrasonography with elastography for this aim in all PVT patients.PSVD can be suspected in patients with USE score>6.8 and always confirmed by liver biopsy.
Acknowledgments
None.
CRediT authorship contribution statement
Stefania Gioia:Conceptualization,Data curation,Investigation,Project administration,Writing– original draft,Writing– review&editing.Adriano De Santis:Conceptualization,Supervision.Giulia d’Amati:Data curation,Visualization.Silvia Nardelli:Data curation,Formal analysis,Writing– review &editing.Alessandra Spagnoli:Formal analysis,Methodology.Arianna Di Rocco:Formal analysis,Software.Lorenzo Ridola:Conceptualization,Visualization.Oliviero Riggio:Conceptualization,Data curation,Supervision,Validation.
Funding
None.
Ethical approval
This study was approved by Ethical Committee of The Sapienza University of Rome (5068/2018).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2024年1期
Hepatobiliary & Pancreatic Diseases International2024年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Recent advances in promising drugs for primary prevention of gastroesophageal variceal bleeding with cirrhotic portal hypertension
- Stereotactic body radiotherapy in pancreatic adenocarcinoma
- Polydatin ameliorates hepatic ischemia-reperfusion injury by modulating macrophage polarization
- Hypomethylation of glycine dehydrogenase promoter in peripheral blood mononuclear cells is a new diagnostic marker of hepatitis B virus-associated hepatocellular carcinoma
- AGK2 pre-treatment protects against thioacetamide-induced acute liver failure via regulating the MFN2-PERK axis and ferroptosis signaling pathway
- Circulating RNA ZFR promotes hepatocellular carcinoma cell proliferation and epithelial-mesenchymal transition process through miR-624–3p/WEE1 axis
