Introducing Novel,Multiple Cd Coordination Modes into Gold Nanoclusters by Combined Doping for Enhancing Electrocatalytic Performance
Zhen Liu ,Xiangfu Meng ,Wanmiao Gu ,Jun Zha ,Nan Yan ,Qing You ,Nan Xia ,Hui Wang ,Zhikun Wu ,*
1 Key Laboratory of Materials Physics,Anhui Key Laboratory of Nanomaterials and Nanotechnology,CAS Center for Excellence in Nanoscience,Institute of Solid State Physics,Hefei Institutes of Physical Science,Chinese Academy of Sciences,Hefei 230031,China.
2 Science Island Branch,Graduate School of University of Science and Technology of China,Hefei 230026,China.
3 Institute of Physical Science and Information Technology,Anhui University,Hefei 230601,China
4 High Magnetic Field Laboratory,Hefei Institutes of Physical Science,Chinese Academy of Sciences,Hefei 230031,China.
5 Hefei National Laboratory for Physical Science at Microscale,Department of Materials Science and Engineering,University of Science and Technology of China,Hefei 230026,China.
Abstract: In recent years,gold nanoclusters have been widely used in catalysis,and alloying has become one of the most important methods for improving the catalytic performance of gold nanoclusters.As for the electrocatalytic reduction of CO2 (CO2RR),although many gold nanoclusters show fairly good Faraday efficiencies through Cd-doping,they still exhibit low current density.Furthermore,as an increasing number of Au-Cd alloy nanoclusters are reported,there is a growing interest in understanding the correlation between Cd coordination and catalysis performance.In most cases,Cd atoms are typically doped in the outer staples and connect with Au atoms through S coordinations.Are there any other unreported Cd coordination modes? Can novel or numerous Cd coordination modes be introduced into gold nanoclusters to increase the current density in the CO2RR? This study investigates these questions.
Key Words: Metal Nanocluster; Combined doping; Cd-coordination mode; Structure-property correlation;Electrocatalytic reduction of CO2
1 Introduction
Metal nanoclusters have received extensive interest in a wide range of areas such as catalysis1–4,chemical sensing5–7and biomedicine8–10in recent years.Especially,metal nanoclusters have unique advantages in exploring the reaction mechanism and the relationship between cluster structures and catalytic performances at the atomic level,owing to their well-defined compositions and structures11–14.As a series of clusters with different sizes and structures have been reported15–20,alloying gradually becomes a major trend,aiming to manipulate the geometric and electronic structures of metal nanoclusters by doping heteroatoms21,22,so as to improve catalytic performance through synergistic effects23–25.Interestingly,it is found that the IIB group cadmium (Cd) is the only active metal thus far which can be facilely doped into gold nanoclusters for enhancing electrocatalytic reduction of CO2to CO26–28.For example,Zhuangetal.obtained a Au47Cd2(SR)31(SR = thiolate)nanocluster by precisely doping Au44(SR)28with cadmium using the two-phase anti-galvanic method,and applied it in the electrocatalytic reduction of CO2to CO with high Faradaic efficiency up to 96% at -0.57 V achieved26; Jinetal.reported that doping Cd into [Au23(SR)16]-nanocluster induced the replacement of two adjacent Au atoms in the surface staple with one single Cd atom,thus producing a novel alloy[Au19Cd2(SR)16]-cluster,and the doped nanoclusters lifted the selectivity of the electrocatalytic reduction of CO2to CO to 90%–95% at the potential from -0.5 to -0.9 V,which was doubled of that for the undoped Au23catalyzed reduction27.Nonetheless,the current density in these catalysis reactions still needs to be greatly improved.One resolution could be to introduce more Cd for providing more dense active sites,and another could be to improve the doped Cd site activity.For the latter,improving the Cd coordination or even forming novel Cd coordination (e.g.Cd-Cd coordination) could effect.By far,it is found that most doped Cd atoms tend to exist in the outer shell staples of the clusters and basically be connected with Au atoms through S coordination in μ3or μ4modes in the forms such as“paw-like” Cd(S-Au-S)3,Cd(S-Au-S)2S2,(S-Au-S)2(CdS)2(SAu-S) and (S-Au-S-Au-S)2(CdS)2(S-Au-S) (Table S1,Supporting Information)26,29–33.The question naturally arising is whether one can introduce novel,multiple Cd coordination modes into the gold nanoclusters for enhancing current density in the electrocatalytic reduction of CO2to CO,as well as for the Cd coordination-activity correlation,which inspires our enthusiasm for investigation.
Recently we realized the local surface structure reconstruction by sulfur doping34–36,thus we supposed that the combination of S and Cd doping could lead to novel,multiple Cd coordination modes since sulfur itself could also coordinate with Cd,which was indeed confirmed by our experiments.Fortunately,we developed a synthesis method and obtained a novel Au-Cd alloy cluster with multiple and even novel Cd coordination modes for enhancing CO partial current density (120 mA·cm-2at -0.9 V)and higher Faradaic efficiency (99.3% at -0.7 V) in the electrocatalytic reduction of CO2to CO.Below we will show the details.
2 Experimental and computational section
2.1 Chemicals
Tetraoctylammonium bromide (TOAB,98%),tretrachloroauric(III) acid (HAuCl4∙4H2O,99.99%),Cd(NO3)2∙4H2O (99.5%),benzyl mercaptan (PhCH2SH,99.9%),sodium borohydride (NaBH4,99.8%),methanol,dichloromethane (DCM),petroleum ether,toluene and acetonitrile were all purchased from Sinopharm Chemical Reagent Co.,Ltd.All chemicals are of analytical grade and used as received.The ultrapure water (18.2 MΩ∙cm) used in all experiments was produced by a Milli-Q NANO pure water system.
2.2 Synthesis of Au41Cd6S2(SCH2Ph)33 and Au38(SCH2Ph)24 nanoclusters
The synthesis steps of the Au41Cd6S2(SCH2Ph)33cluster are described in Fig.S1 (Supporting Information).Briefly,150 mg of TOAB,40 mg of Cd(NO3)2and 100 mg of HAuCl4∙3H2O were dissolved in 12 mL of methanol solution.After evenly stirring for 20 min,120 μL of benzyl mercaptan (PhCH2SH) was added into the flask (benzyl mercaptan can not only provide protecting for gold nanoclusters,but also offer the sulfur source as reported previously35).40 min later,100 mg of NaBH4dissolved in deionized water was added and the solution turned black immediately.The reaction solution became transparent and the precursor was attached to the flask and magneton after stirred for 4 h.The as-obtained polydisperse AuxCdy(SR)znanoclusters were washed by methanol for 3 to 4 times.In the second step of etching,the precursor dissolved in 3 mL of toluene containing 100 μL of benzyl mercaptan was stirred in an oil bath under 80 °C for 2 h.After washed with excess methanol solution for multiple times,the as-obtained crude product was then separated by preparative thin-layer chromatography(PTLC) and the target Au-Cd alloy clusters were isolated.The yield of Au41Cd6S2(SCH2Ph)33is about 1.2%.Single crystals were grown in a toluene/acetonitrile mixed solution under -4 °C in about two weeks.
Au38(SCH2Ph)24was also synthesized by a two-step method.Typically,HAuCl4∙4H2O (100 mg) dissolved in 2 mL of water was added to 10 mL of CH2Cl2solution containing TOAB (150 mg).After vigorously stirring for 15 min,the water layer was removed,and 100 μL of PhCH2SH was added into the remaining solution.After continuously stirring for 20 min,an aqueous solution of NaBH4(50 mg,dissolved in 2 mL of cold water) was added all at once,resulting in the immediate darkness of the reaction mixture.After 1 h reaction,the crude product was washed with methanol,dissolved in 3 mL of toluene containing 200 μL of benzyl mercaptan,and then stirred in an oil bath under 60 °C for 2 h.After the product was washed and precipitated with excess methanol,Au38(SCH2Ph)24nanoclusters were isolated by preparative thin-layer chromatography (PTLC).
2.3 Characterization
The UV-Vis/NIR absorption spectra in the range of 190–900/1200 nm were measured on a UV-2600/3600 spectrophotometer (Shimadzu,Japan) at room temperature.Thermal gravimetric analysis (TGA) was conducted in a N2atmosphere (flow rate ~50 mL∙min-1) on a TG/DTA 6300 analyzer (Seiko Instruments,Japan) at a heating rate of 10 °C∙min-1.Electrospray ionization mass spectra (ESI-MS)were recorded on a Waters Q-TOF mass spectrometer (America)with a Z-spray source.X-ray photoelectron spectroscopy (XPS)measurements were performed with an ESCALAB 250 Xi XPS system (Thermo Scientific,America).The single crystal X-ray crystallography (SCXC) data of Au41Cd6S2(SCH2Ph)33were collected on a Bruker D8 VENTURE CMOS photon 100 diffractometer (Germany) with a Helios mx multilayer monochromator and MoKαradiation (λ= 0.71073 Å) (1 Å = 0.1 nm).Differential pulse voltammetry (DPV) of clusters at 0.01 V∙s-1in degassed CH2Cl2containing 0.1 mol∙L-1Bu4NPF6was performed on a Zahner electrochemical workstation (Germany)with a conventional three-electrode setup,Pt working (1 mm diameter),SCE reference and carbon-rod counter electrodes.
2.4 Electrochemical measurements
The electrocatalytic reduction of CO2(CO2RR) performance tests were conducted in a flow-cell device which was reported previously37.The nanoclusters were dripped on the surface of the hydrophilic layer of carbon paper (1.5 cm × 1.5 cm),and then slowly dried for 24 h in a vacuum oven to be used as the working electrode.The effective area of the working electrode was 1.0 cm × 1.0 cm.The nickel foam electrode and Ag/AgCl electrode were used as the counter electrode and the reference electrode,respectively.The catholyte was 1.0 mol∙L-1KOH and the electrolyte flow was controlled and circulated through the cell by a peristaltic pump at 10 mL∙min-1.During the measurements,CO2gas was directly fed to the cathode at a rate of 20 sccm(standard-state cubic centimeter per minute).All electrochemical studies were conducted using an electrochemical workstation (CHI760E).All potentials in this work were referenced to the reversible hydrogen electrode(RHE) by the following Nernst equation:
E(vs.RHE) =E(vs.Ag/AgCl) + 0.198 + 0.0591 × pH
NMR spectroscopy was used to determine the liquid products on the drug high-throughput screening system (500 MHz superconducting Fourier NMR spectrometer).Product quantification was performedviathe internal DMSO standard.The gas-phase products were identified by gas chromatography(Agilent 7890B Series gas-chromatography system,America),equipped with a packed HeysepD column.Argon (Praxair 99.999%) was used as the carrier gas.The separated gas products were analyzed by a flame ionization detector with a methanizer(for CO concentrations) and a thermal conductivity detector (for H2concentration).The Faradaic efficiency of the gas product was calculated on the basis of the following equation38:
FE =ix/itot=nx×vgas×cx×F/(itot×Vm)
whereixis the partial current of product x;itotis the total current;nxrepresents the number of electrons transferred towards the formation of 1 mol of product x;vgasis the CO2flow rate;cxrepresents the concentration of product x detected by gas chromatography (ppm);Fis the Faraday constant,andVmis the unit molar volume.
2.5 DFT calculation details
All the density functional theory (DFT) calculations were performed using the Vienna ab-initio simulation package(VASP)39–42.The projector augmented wave (PAW) method was used to describe the interactions between ion cores and valence electrons43.The GGA-PBE was employed to describe the exchange-correlation functional44,45.The Kohn-Sham equations were expanded in a plane wave basis set with a cutoff energy of 400 eV.The Brillouin zone sampling was performed using Γ-center Monkhorst-Pack k-point grid46.The clusters were optimized in a 30 Å × 30 Å × 30 Å box.The free energies(G) of the reactants,intermediates,and products during CO2RR and hydrogen evolution reaction (HER) were obtained using the equation:
G=Etotal+ ZPE -TS
whereEtotalis the total energy of the species,ZPE is the zero point energy,andSis the entropy.
As for computational models,the –SCH2Ph ligands in the nanoclusters are replaced with –SCH3groups and this R-group simplification is a commonly used approach to reduce computational cost,without affecting the interfacial bond strength.To investigate the effect of Cd atoms in different coordination environments,–SCH3group is removed from Au-SCH3-Cd or Cd-SCH3-Cd sites of Au41Cd6S2(SCH2Ph)33in the outer motifs,compared with the Au-SCH3-Au site of Au38(SCH2Ph)24.
3 Results and discussion
The as-obtained nanoclusters dissolved in dichloromethane solution exhibits three characteristic absorption peaks at 360,450 and 700 nm,respectively (Fig.1a).Electrospray ionization mass spectrometry (ESI-MS) was used to characterize its composition.Cesium acetate (CsOAc) was added to the nanocluster solution to assist ionization by forming cationic cluster adducts with Cs+.As shown in Fig.1b,a dominant peak at mass/charge ratio (m/z) of 6573.62 acquired in ESI-MS positive mode corresponds to [Au41Cd6S2(SCH2Ph)33+ 2Cs]2+(calculatedm/z: 6573.85,deviation: 0.23).Thermalgravimetric analysis (TGA) result shows a mass loss of 31.17% (w) in nitrogen atmosphere,in well agreement with the theoretical loss of 32.05% (w) of Au41Cd6S2(SCH2Ph)33(Fig.1c) after the evaporation of C,H and S elements.Thus,the atomic composition of the as-prepared Au-Cd alloy cluster is concluded to be Au41Cd6S2(SCH2Ph)33.X-ray photoelectron spectroscopy(XPS) also provides support for this conclusion (Fig.1d): the Au/Cd/S atomic ratio of 41/5.7/34.3 is close to the theoretical 41/6/35 for a composition of Au41Cd6S2(SCH2Ph)33,and no Cl,Br,N and Na elements were detected,which excludes the possibility of the existence of potential counterions such as Cl-,Br-,TOA+and Na+.
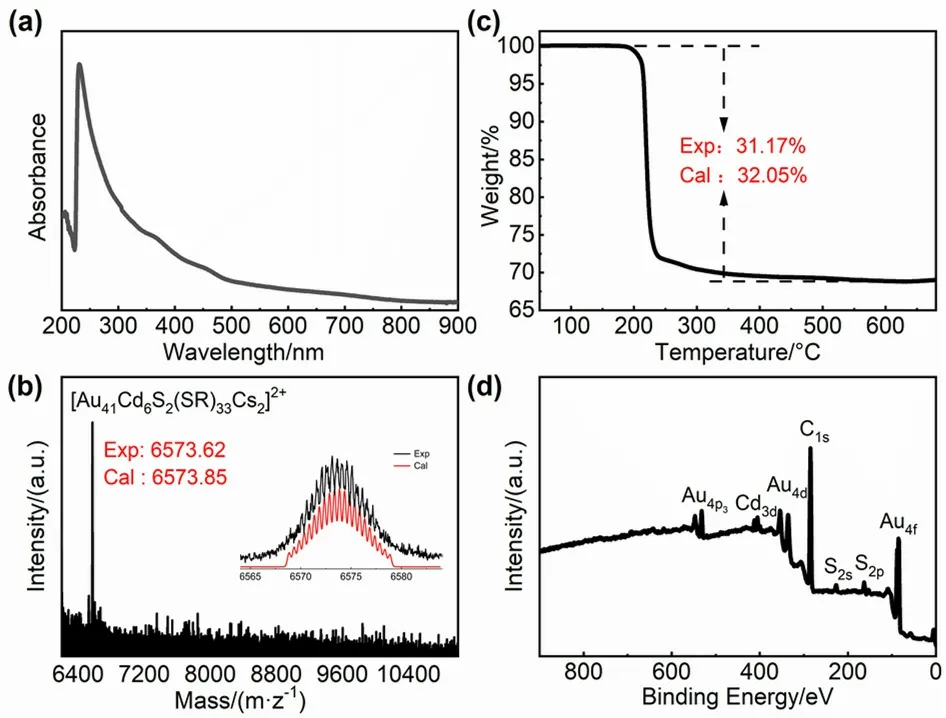
Fig.1 (a) UV-Vis/NIR absorption,(b) ESI-MS,(c) TGA and(d) XPS spectrum of Au41Cd6S2(SCH2Ph)33 nanoclusters.
Single-crystal X-ray crystallography (SCXC) undoubtedly confirmed the composition and revealed the atomic structure of Au41Cd6S2(SCH2Ph)33.As shown in Fig.S2 (Supporting Information),the nanoclusters crystallize in a monoclinic,P21/cspace group and arrange in 4H crystal phase in the crystal.Each single crystal structure consists of four parts: a biicosahedral Au23kernel,two long Au5Cd2S(SR)9staples,two Au3(SR)4trimers and one Au2Cd2(SR)7tetramer,among which the six doped Cd atoms are all located in the outer staples (Fig.2).The Au23kernel (Fig.2a) is composed of two icosahedral Au13units by sharing three vertices in a “face to face” fashion,similar to that of the reported Au38(SC2H4Ph)24cluster47but slightly wider(9.1 Å) than that of the Au38(SC2H4Ph)24(8.4 Å),although they have the same height (5.6 Å) (Fig.S3,Supporting Information).As shown in Fig.2b,two long Au5Cd2(SR)9S staples that have never been reported symmetrically cross-cap the Au23core and there is a single S in the middle of each staple.On both sides of Au41Cd6S2(SCH2Ph)33,one common trimeric staple of Au3(SR)4stays on Au23kernel (Fig.2c).Notably,a unique (S-Au-S)2(CdSS-CdS) tetramer staple with two μ3Cd atoms locates at the bottom of the Au23kernel.To the best of our knowledge,this is the first time that the staple Cd atom directly linked to Cd atomviaS has been found in the shell layer of a cluster.This novel μ3Cd coordination mode provides a new opportunity for investigating the structure-property relationship (ubi infra).

Fig.2 Structure of a Au41Cd6S2(SCH2Ph)33 nanocluster:(a) biicosahedral Au23 kernel; (b) Au33Cd4S2(SCH2Ph)18 unit;(c) Au39Cd4S2(SCH2Ph)26 unit and (d) total structure of Au41Cd6S2(SCH2Ph)33.Color labels: orange = Au; blue = Cd;yellow = S.C and H atoms are omitted for simplicity.
The novel Au41Cd6S2(SCH2Ph)33and the existing Au38(SC2H4Ph)24cluster can be regarded as a pair of “homokernel-hetero-staples” clusters48; that is,the clusters have the same kernel structure but different outer staple structures (Fig.S4,Supporting Information).In order to eliminate the interference of the protective ligand and explore the effect of outer structure on cluster properties,we prepared Au38(SCH2Ph)24nanoclusters with the same thiolate type as that of Au41Cd6S2(SCH2Ph)33,and compared the optical and electrochemical properties of the two clusters.It is found that the optical absorption spectrum of Au41Cd6S2(SCH2Ph)33is distinct from that of Au38(SCH2Ph)24(Fig.S5,Supporting Information).By extrapolating the lowest energy absorption peak to zero absorbance,we obtained the optical energy gaps of Au41Cd6S2(SCH2Ph)33and Au38(SCH2Ph)24,1.43 and 0.94 eV,respectively (Fig.3a,c).Besides,the electrochemical energy gaps of two clusters were also evaluated by differential pulse voltammetry (DPV).As shown in Fig.3b and 3d,the electrochemical bandwidth (the distance between the first oxidation peak O1and the first reduction peak R1) of the Au41Cd6S2(SCH2Ph)33is 1.42 V,larger than the 1.18 V of Au38(SCH2Ph)24cluster.Both UV-Vis/NIR absorption and DPV results indicate that the differences in the outer staples have significant influences on the optical and electronic properties of the clusters.
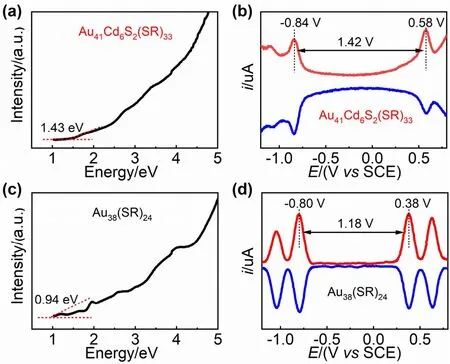
Fig.3 (a) UV-Vis/NIR spectrum on the energy scale and(b) DPV curves of Au41Cd6S2(SCH2Ph)33; (c) UV-Vis/NIR spectrum on the energy scale and (d) DPV curves of Au38(SCH2Ph)24.
The significant differences of electrochemical band gap also prompted us to investigate the electrocatalytic performance of these two clusters.Before catalytic investigation,the stability(including thermal stability and oxidation resistance) of the Au41Cd6S2(SCH2Ph)33nanoclusters was studied.Specifically,the clusters were dissolved in toluene and heated under 80 °C with continuous monitoring by UV-Vis/NIR absorption spectrum,which shows that there is no significant change within 4 h of heating (Fig.4a),indicating the high thermal stability of Au41Cd6S2(SCH2Ph)33.Likewise,the UV-Vis/NIR absorption spectrum was also used to monitor the anti-oxidation of the clusters towards hydrogen peroxide (H2O2) at room temperature.As presented in Fig.4b,the absorption spectrum changes little within 32 h,indicating that the clusters are also oxidationresistant.The good stability of the cluster provides the prerequisite for catalytic investigation.
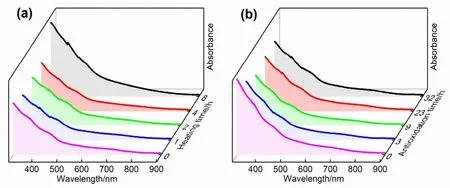
Fig.4 (a) Thermal stability at 80 °C and (b) oxidation resistance towards H2O2 of Au41Cd6S2(SCH2Ph)33 in toluene solution.
Electrocatalytic reduction of CO2(CO2RR) was chosen as the model reaction to explore the influence of surface Cd doping on the electrocatalytic performance.As revealed by the linear sweep voltammetry (LSV) curves (Fig.5a),both Au41Cd6S2(SCH2Ph)33(short for Au41Cd6) and Au38(SCH2Ph)24(short for Au38) clusters show sharp reduction peaks in the CO2environment; while in the Ar environment,the two catalysts only exhibit a slight current-voltage response,indicating their CO2RR activity.The onset potential for CO2RR on Au41Cd6is shifted to a more positive value and a significantly higher polarization current density is obtained on Au41Cd6compared with that on Au38,indicating that the former has higher CO2RR activity and faster CO2RR kinetics than the latter.Gas and liquid products were identified and quantified by online gas chromatography and nuclear magnetic resonance (NMR) spectroscopy,respectively.It is found that CO and H2were the only gas products and no liquid product was detected (Fig.S6,Supporting Information).As shown in Fig.5b,Au41Cd6exhibits an outstanding performance on the Faradaic efficiency of CO(FECO),exceeding 80% at almost all applied potentials,and reaching up to 99.3% at -0.7 V (all potentials are referenced to RHE),while Au38only shows a maximum FECOof 75.6% at the same potential.Compared with various Au-Cd nanoclusters reported in the literature (Fig.S7,Supporting Information)26–28,Au41Cd6has a superior performance and is one of the best nanoclusters for catalyzing CO2to CO in term of the FECO.Furthermore,jCO(CO partial current density) was obtained under different applied potentials after that the total current density and the corresponding FECOwere considered.As seen from Fig.5c,Au41Cd6exhibits ajCOof 89.7 mA∙cm-2at -0.7 V and reaches the highestjCOof 120 mA∙cm-2at -0.9 V in the investigated conditions.In contrast,thejCOon Au38shows only half of that on Au41Cd6at all potentials.Moreover,it is observed that the competing hydrogen evolution reaction (HER) on Au41Cd6is substantially suppressed.The UV-Vis/NIR absorption spectra of both Au41Cd6and Au38were measured and found untouched after the tests of CO2RR (Fig.S8,Supporting Information),indicating their high stability during electrocatalytic reactions.A 14 h continuous electrolysis was additionally performed,and the FECOand the current density had no obvious attenuation (Fig.5d),further demonstrating the catalytic stability of Au41Cd6.
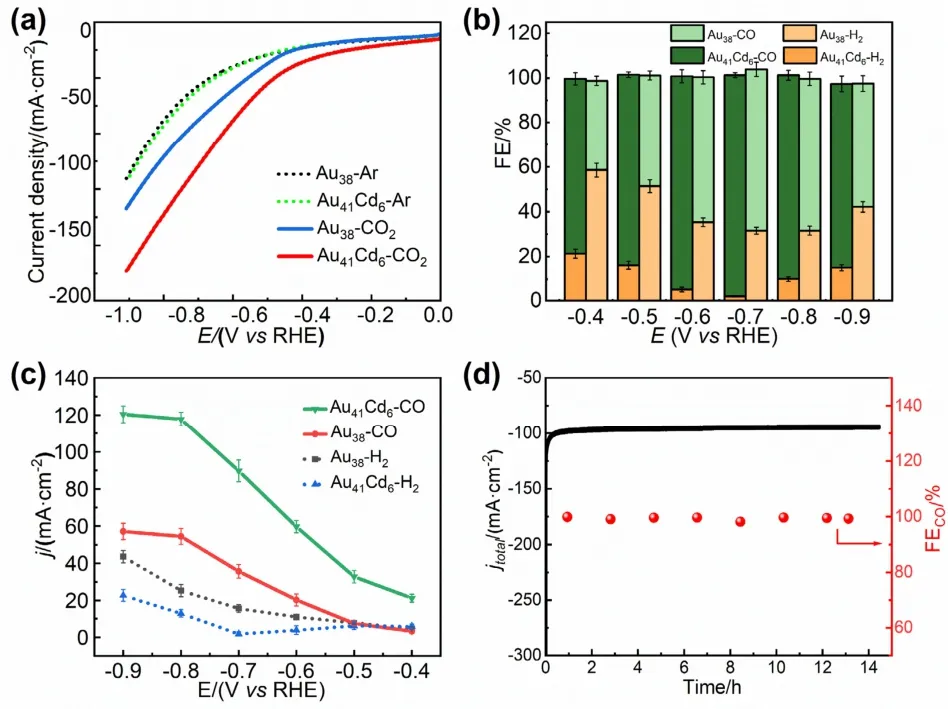
Fig.5 (a) LSV curves of Au41Cd6 and Au38 in an Ar-saturated(dotted line) and a CO2-saturated (full line) 1 mol∙L-1 KOH solution;(b) FEs of CO and H2 products obtained on Au41Cd6 and Au38 clusters with different applied potentials; (c) The corresponding partial current density of CO and H2 on Au41Cd6 and Au38;(d) Stability test of Au41Cd6 at -0.7 V.
To gain insights into the role of various doped Cd atoms in enhancing the CO2RR performance,DFT calculations were performed on Cd-Cd and Au-Cd motif sites (Figs.6d–e) of Au41Cd6,compared with Au-Au staple site (Fig.6a) of Au38.As for the Au nanocluster-catalyzed CO2RR,it mainly involves two intermediate species *COOH and *CO49–52.The corresponding optimized structures of the reaction intermediates involved in the pathways are provided in Fig.S9 (Supporting Information,the Au-Au and Cd-Cd sites are used as examples).As revealed in Fig.6c,the formation of *COOH intermediate is spontaneous and the determination step of all other sites is the last step of CO release except for the Au-Cd1site.Compared with the mentioned Au-Au site,all sites containing Cd atoms reduce the required energy barrier more or less,which is consistent with the experimental fact that the introduction of Cd atoms in Au41Cd6well improves the catalytic performance.Notably,the intermediate *COOH at the Au-Cd3site stabilizes in the form of Cd-O-C(OH)-Au (Fig.S10c,Supporting Information) with a maximum free energy release of 0.89 eV,the same form as found in the Au47Cd2(SR)31catalysis,which is thought to contribute to the excellent catalytic performance of Au47Cd2(SR)3126.The unique Cd-Cd coordination site is the most favorable in the determination step with a significant lowest energy required(0.49 eV only).Nevertheless,the HER theoretical calculation shows that the Cd-Cd site has a poor effect on inhibiting hydrogen evolution while the Au-Cd1site performances well in
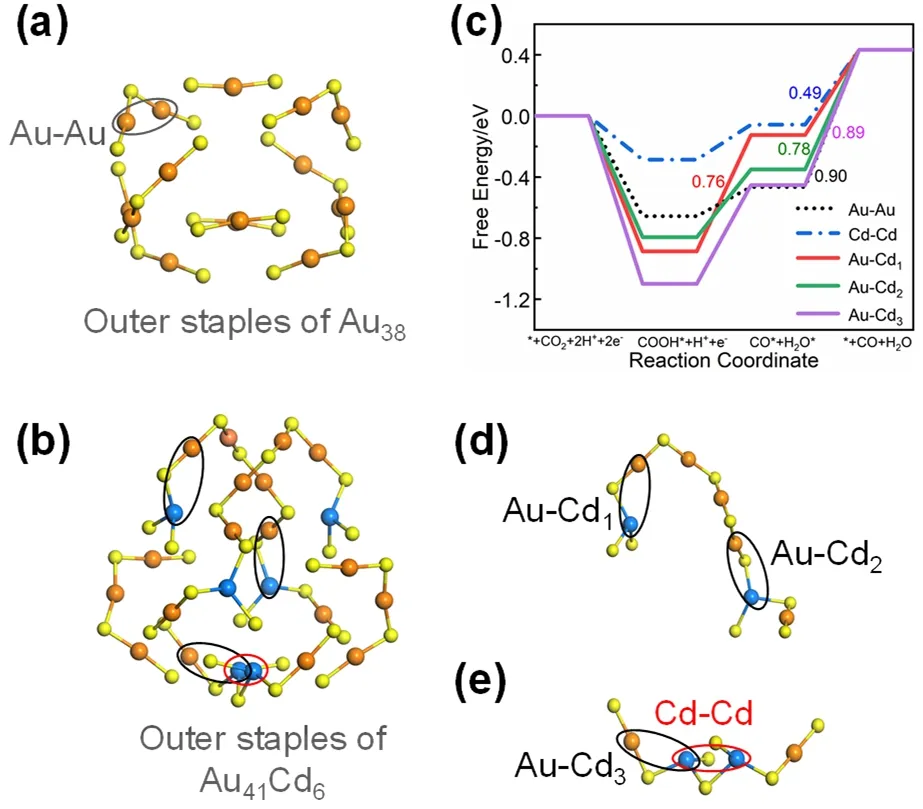
Fig.6 (a) Outer staples of Au38 and the Au-Au site of Au38; (b) outer staples of Au41Cd6; (c) free energy diagram for electrocatalytic CO2 reduction on Cd-Cd,Au-Cd sites of Au41Cd6 and Au-Au site of Au38; (d)Au-Cd sites on the Au5Cd2(SR)9S staple motif; (e) Au-Cd and Cd-Cd sites on the Au2Cd2(SR)7 staple.Color labels: orange = Au; blue = Cd; yellow = S.
doing so (Fig.S11,Supporting Information,).Given the consideration of both CO production and hydrogen evolution inhibition,it is speculated that the Au-Cd1site might play an important role in promoting the catalytic performance of Au41Cd6.Note that,calculations on the single sulfur site show that the energy barrier in the rate-determining step (the formation of *COOH intermediate) is huge,thus the direct role of the single sulfur site in CO2RR can be neglected (Fig.S12,Supporting Information).Therefore,DFT calculations reveal that differently coordinated Cd sites have distinct catalytic activities and selectivities as shown in Fig.6c and S11 (Supporting Information),demonstrating that introducing multiple,various Cd coordination modes in gold nanoclusters is an effective strategy for enhancing gold nanoclusters performance in electrocatalytic reduction of CO2to CO.
4 Conclusions
In conclusion,we introduced novel,multiple Cd coordination modes into gold nanoclusters for greatly enhancing performance of gold nanoclusters in the electrocatalytic reduction of CO2to CO.Here we employed a combination doping strategy,developed a two-step synthesis method and synthesized a novel Au-Cd alloyed nanocluster,whose composition and structure were precisely determined by multiple techniques such as ESIMS,TGA,XPS and SCXC.SCXC revealed that the Au41Cd6S2(SCH2Ph)33nanocluster contains a biicosahedral Au23core protected by two Au3(SR)4trimers,one Au2Cd2(SR)7tetramer and two long Au5Cd2S(SR)9staples,among which,the Au2Cd2(SR)7tetramer and the Au5Cd2S(SR)9staple are newly found in this work.Multiple,various Cd coordination modes endow the alloy nanoclusters high CO partial current density(120 mA∙cm-2at -0.9 V) and Faradaic efficiency (99.3% at -0.7 V) in the electrocatalytic reduction of CO2to CO.DFT calculations provide interpret for its superior catalytic performance in comparison with the “homo-kernel-heterostaples” Au38(SC2H4Ph)24clusters.These novel,nontrivial findings and results have important implications for future research on the synthesis,structure and structure-property correlation of metal nanoclusters and is expected to stimulate more work in the related areas.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.