Recent Advances in Self-Supported Transition-Metal-Based Electrocatalysts for Seawater Oxidation
Qian Wu ,Qingping Gao ,Bin Shan ,Wenzheng Wang ,Yuping Qi ,Xishi Tai ,Xia Wang ,*,Dongdong Zheng ,Hong Yan ,Binwu Ying ,Yongsong Luo ,Shengjun Sun ,Qian Liu ,Mohamed S.Hamdy ,Xuping Sun ,,*
1 Department of Chemistry and Chemical Engineering,Weifang University,Weifang 261061,Shandong Province,China.
2 Department of Chemical Engineering,Weifang Vocational College,Weifang 262737,Shandong Province,China.
3 Institute of Fundamental and Frontier Sciences,University of Electronic Science and Technology of China,Chengdu 610054,China.
4 College of Chemistry,Chemical Engineering and Materials Science,Shandong Normal University,Jinan 250014,China.
5 Institute for Advanced Study,Chengdu University,Chengdu 610106,China.
6 Catalysis Research Group (CRG),Department of Chemistry,College of Science,King Khalid University,61413 Abha,Saudi Arabia.
Abstract: Seawater electrolysis is a promising and sustainable technology for green hydrogen production.However,some disadvantages include sluggish kinetics,competitive chlorine evolution reaction at the anode,chloride ion corrosion,and surface poisoning,which has led to a decline in activity and durability and low oxygen evolution reaction (OER) selectivity of the anodic electrodes.Benefiting from the lower interface resistance,larger active surface,and superior stability,the self-supported nanoarrays have emerged as advanced catalysts compared to conventional powder catalysts.Self-supported catalysts have more advantages than powder catalysts,particularly in practical large-scale hydrogen production applications requiring high current density.During electrolysis,due to the influx of bubbles generated on the electrode surface,the powdered nanomaterial is peeled off easily,resulting in reduced catalytic activity and even frequent replacement of the catalyst.In contrast,self-supported nanoarray possessing strong adhesion between the active species and the substrates ensures good electronic conductivity and high mechanical stability,which is conducive to long-term use and recycling.This minireview summarizes the recent progress of selfsupported transition-metal-based catalysts for seawater oxidation,including (oxy)hydroxides,nitrides,phosphides,and chalcogenides,emphasizing the strategies in response to the corrosion and competitive reactions to ensure high activity and selectivity in OER processes.In general,constructing three-dimensional porous nanostructures with high porosity and roughness can enlarge the surface areas to expose more active sites for oxygen evolution,which is an efficient strategy for improving mass transfer and catalytic efficiency.Furthermore,the Cl- barrier layer on the surface of catalyst,particularly that with both catalytic activity and protection,can effectively inhibit the competitive oxidation and corrosion of Cl-,thereby delivering enhanced catalytic activity,selectivity,and stability of the catalysts.Moreover,developing super hydrophilic and hydrophobic surfaces is a promising strategy to increase the permeability of electrolytes and avoid the accumulation of large amounts of bubbles on the surface of the self-supported electrodes,thus promoting the effective utilization of active sites.Finally,perspectives and suggestions for future research in OER catalysts for seawater electrolysis are provided.In particular,the medium for seawater electrolysis should be transferred from simulated saline water to natural seawater.Considering the challenges faced in natural seawater splitting,in addition to designing and synthesizing self-supported catalysts with high activities,selectivity,and stability,developing simple and low-cost natural seawater pretreatment technologies to minimize corrosion and poisoning issues is also an important topic for the future development of seawater electrolysis.More importantly,a standardized,feasible evaluation system for self-supported electrocatalysts should be established.In addition,factors such as the intrinsic activity,density of accessible active sites,size,mass loading,substrate effects,and test conditions of the catalyst should be fully considered.
Key Words: Seawater electrolysis; Self-supported nanoarray; Transition metal-based catalyst; Anti-corrosion;Oxygen evolution reaction
1 Introduction
Along with the global crisis over energy and environmental issues,the development of renewable and clean energy is highly required1–3.In this regard,carbon-free hydrogen with high energy density is considered as a promising energy carrier4,5.However,at present,hydrogen production is still mainly based on non-renewable fossil fuels,such as natural gas,coal and oil,which are unsustainable.Meanwhile,hydrogen produced by these traditional processes,also known as “gray or blue hydrogen”,is inevitably accompanied by CO2emissions6.Water electrolysis driven by renewable energy is a pollution-free and carbon-free way to produce “green hydrogen”,which is crucial to future hydrogen-powered development.Currently,commercial water electrolysis such as alkaline electrolyzer and proton exchange membrane electrolyzer mainly rely on fresh water to achieve hydrogen production7.It is widely known that the fresh water is facing pollution and being depletion with the deterioration of our environment,which hinder its practical application.Alternatively,as the most abundant resource in the world,seawater accounts for more than 96% of the global water resources8.Through rational utilizing seawater resource,a large amount of fresh water can be saved and its shortage issue can be alleviated to some degree.Moreover,owing to the intrinsic characteristics such as higher conductivity than pure water and high salinity,seawater is regarded as a natural electrolyte9.A salinity of about 3.5% (i.e.,0.5 mol∙L-1NaCl) allows seawater to be directly electrolyzed with little or no additional conductive electrolytes (usually KOH,NaOH or H2SO4),thereby reducing the cost of hydrogen production10.Consequently,seawater electrolysis has become an appealing approach for hydrogen production and attracted growing attention in recent years.
Water electrolysis involves two half-reactions: the hydrogen evolution reaction (HER) at the cathode and the oxygen evolution reaction (OER) at the anode.The whole electrolytic reaction is a non-spontaneous energy-consuming process.Thermodynamic calculation shows that a theoretical voltage of 1.23 V at 25 °C and 101325 Pa is required to drive water splitting11.Compared with HER,a fast two-electron transfer process,OER has become the bottleneck of water splitting resulting from the sluggish kinetics with four-electron transfer.Consequently,the high overpotentials are introduced in the practical OER12.Beyond the above kinetics problem,in fact,seawater splitting face more challenges,such as the competitive chlorine evolution reaction (ClER) with OER at the anode13,14,toxic corrosion resulting from the high concentration of chloride ions (Cl-)15,reduction of catalytic activity caused by insoluble substances covering active sites of the electrode surface16.All these issues lead to low energy conversion efficiency,thus hindering the possibility of large-scale hydrogen production from seawater electrolysis.Therefore,development of selective and robust OER catalysts is urgently required to promote the overall efficiency of seawater splitting,especially at large current densities.
With the deep understanding of the seawater electrolytic process and the progress of relevant technologies,various nanostructured anode materials have been reported with high activity and stability for seawater OER catalysis in recent years.However,most of them are prepared in powder form,which are usually adhered to the current collector through the polymer binders,thus being used as the work electrodes17.Such operation tends to breed a series of adverse impact on the performance of catalysts,for example,the increase in resistance,the covered active sites,and the undesirable interface,which hamper the electron transport17,18.In addition,due to the high surface energy,nanoparticles are easy to gather,resulting in a large amount of dead volume19.Suffering from the low adhesion between the catalyst and substrate,the powdery coated materials is easily detached from the current collector during the long-time or high-current catalytic process20.The practical seawater electrolysis,however,usually requires high current density,which is obviously difficult for powder nano catalysts.
Compared with powder nanoparticles,benefiting from the inherent advantages,the self-supported electrocatalysts exhibit excellent OER performance for seawater splitting.In morphology,the self-supported catalysts are mostly nanoarray where the catalytic active speciesinsitugrow on the conductive substrates21,22.This binder-free growth method may effectively avoid the shedding of active species under high current density,accelerate the charge transfer,and enhance the mechanical stability of catalysts during long-term continuous seawater splitting23,24.Moreover,as the flexible nanostructured engineering,the catalytic active surface areas of the selfsupported materials may be significantly expanded and favor the exposure of more active sites25,26.Also,the array structure with enough open space ensures gas-phobic property of the electrode,so that the generated bubbles can be smoothly removed from the electrode surface.This also makes the active sites fully contact with electrolyte,thus improving reaction kinetics27,28.Based on these advantages,the self-supported electrocatalysts have been widely used in many related energy devices,including seawater electrolysis,and have shown great potential in future practical hydrogen production.
As the most advanced electrocatalysts,noble metal-based materials are restricted to widely use due to the scarcity and high cost.In contrast,the transition metal (TM) based catalysts have attracted more attention owing to the earth abundance and the diversity in their structure and components.To date,the selfsupported TM-based OER catalysts for seawater electrolysis have made considerable progress,but a comprehensive review is still lacking.Based on the principle and challenges of OER in seawater splitting,the present review focuses on the progress made in response to the corrosion resistance and competitive reaction inhibition by these self-supported OER catalysts (Fig.1).In addition,by comparing and discussing the representative works from seawater electrolysis conditions,design,synthesis,and performance of self-supported catalysts,we strive to have a more comprehensive understanding for OER in seawater electrolysis.Finally,the challenges and opportunities for future development of such electrocatalysts are highlighted.
2 Fundamentals and challenges of seawater oxidation
In the past few decades,aiming at the sluggish kinetics of OER,many efficient catalysts have emerged and significantly reduced the overpotential,improving the energy conversion efficiency of freshwater electrolysis.However,in seawater electrolysis,OER faces more complex challenges than just intrinsic kinetic disadvantage,which are impossible to be solved by only relying on high-performance electrocatalysts.Due to the existence of plenty of Cl-with electrochemical activity,the chlorine chemical reactions may occur on the anode,interfering with OER (Fig.2).As shown in Fig.2,several possible reactions may occur depending on pH,applied potential and concentration of Cl-.ClER is easier to occur at low pH (< 3),and hypochlorite formation usually takes place at high pH (> 7.5),which are the main reactions competing with OER.At high anode potential,HOCl formation occurs in 3 < pH < 7.5.OER can be defined by Eqs.(1,2).ClER in acidic electrolyte and hypochlorite formation in alkaline environment occur as shown in Eqs.(3,4),respectively.
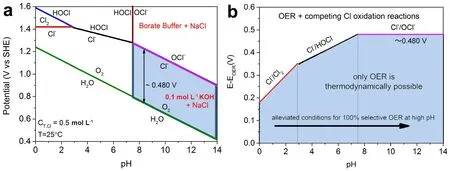
Fig.2 (a) Pourbaix diagram for artificial seawater model.(b) Maximum allowed overpotential of OER catalysts to ensure 100% selective water splitting.
Hypochlorite formation reaction
As seen from these equations,OER with the lower standard redox potentials is thermodynamically favored compared with ClER or hypochlorite formation reaction.However,ClER and hypochlorite formation are all two-electron transfer processes,which possess faster kinetic above the four-electron OER.In fact,chlorine or hypochlorite formed in electrolytic cell is corrosive and difficult to treat,which will seriously damage the stability of electrode materials during the seawater splitting.Therefore,the key to solve this problem is to make seawater electrolysis possess exclusive OER selectivity.Compared with the development of highly selective catalysts for oxygen evolution,it is more feasible and effective to control the seawater electrolysis environment to achieve selective OER over Cloxidation29.Based on the pourbaix diagram,in order to shield Cl–oxidation,the overpotential applied in OER should be lower than that of chlorine chemical reaction (Fig.2)14.It also reveals that the potential difference between OER and Cl–oxidation reaction is related to the pH of the electrolyte.When pH < 3,the potential difference between the two oxidation reactions is only 180–350 mV,which means that it is even more challenging for OER to achieve high current density without chloride ion oxidation under the applied overpotential.Notably,in alkaline seawater,the thermodynamic overpotential of hypochlorite formation reaction is always 480 mV higher than that required for OER,and does not change with pH (Fig.2).In other words,as long as the afforded overpotential of the OER catalyst is controlled below 480 mV under alkaline conditions,the Cloxidation can be entirely avoidable in theory14.
Besides improving the OER selectivity,chlorine-corrosion and insoluble matters are other critical challenges to be addressed in seawater electrolysis.Even if OER electrocatalysts have high performance in alkaline solution,aggressive Cl-can cause corrosion to electrode materials.Especially for transition metal catalysts,Cl-can react directly with these electrondeficient metals and change the physical and chemical properties of materials15.What’s worse,Cl-may even infiltrate into the inner layer of metal compounds and bind to the lattice to induce its distortion,resulting in catalyst deactivation and poor durability29–31.Therefore,toxic corrosion of Cl-is also an important reason for the reduction of cycling stability of electrocatalysts.Additionally,as mentioned above,an overpotential lower than 480 mV under alkaline electrolyte should be applied in seawater oxidation to achieve exclusive OER selectivity.However,when pH is higher than 9.5,the hydroxide precipitation induced by Ca2+and Mg2+in seawater may cause electrode passivation32.Their hydroxides will be adsorbed on the surface of the catalyst to prevent full contact with electrolyte and the rapid release of the gas bubbles,causing the active sites to be blocked.Furthermore,since seawater is an unbuffered electrolyte,the local pH near the electrodes will inevitably change during seawater electrolysis.At the anode,the consumption of OH-due to the continuous OER process causes a decreasing in pH,which accelerates the transition of the reaction to H2O oxidation,thereby resulting in a further migration of pH to the acidic side33.Such acidic environment inducing from local pH change would lead to the hypochlorite production as well as the reaction rate and electrode degradation14.From this point of view,maintaining a constant pH working environment and selectivity are essential for achieving efficient seawater electrolysis and inhibiting the side reactions.In addition,insoluble microorganisms in seawater will also cover and deactivate many active sites,leading to the surface poisoning and poor catalytic performance of OER electrocatalysts34.In a word,it is of great significance to develop highly stable,selective and efficient anode catalysts for hydrogen production from seawater electrolysis.
3 Assessment of electrocatalysts for OER
Currently,various standard indicators have been used to evaluate the performance of OER catalysts,which is crucial for the development of new electrocatalysts.Unfortunately,the current methods for evaluating the performance of electrocatalysts are non-standard and unsystematic,which makes objective and fair activity comparisons extremely difficult.In addition,due to the diversity of catalyst preparation methods,composition,and structure,as well as the different dynamic change trend on the catalyst surface under catalytic conditions,strict comparison of electrocatalytic activity is further limited35.To address this issue,Peugeotetal.pointed out that intrinsic activity can truly reflect the performance of catalysts,which requires normalization of the current density through the effective electrochemical surface area (ECSA)36.The intrinsic activity is closely related to the accessible active sites of the catalyst.Furthermore,owing to the different composition and morphology of nanomaterials,the interaction between the catalyst and surrounding electrolyte is different,resulting in significant differences in electrochemical activity and the density of accessible active sites of the electrocatalyst.In this case,to correlate the total OER activity to the intrinsic activity of each active site,they suggest using ECSA measurement in combination with pre-OER oxidation wave analysis to estimate the density of accessible active sites36.Such fusion analysis using these two complementary methods can provide a qualitative comparison of the intrinsic activity of electrocatalysts for OER.
Additionally,in order to meet the industrial application requirements of seawater electrolysis,oxygen evolution needs to be carried out at a higher current density,and it is also expected that the electrocatalyst can exhibit excellent performance.Therefore,parameters such as overvoltage,Tafel slope,Faraday efficiency,turnover frequency (TOF),and long-term stability at high current density are considered important indexes for evaluating the performance of OER catalysts,especially selfsupported ones.Through Tafel analysis,the reaction mechanism and kinetics can be deeply understood.In particular,the accumulation of bubbles on the electrode surface at high current density can severely impede mass transfer and increase resistance,leading to an increase in the Tafel slope value36,37.Faradaic efficiency is also a common metric for electrocatalytic systems.The steady-state Faraday efficiency of pure water oxidation under alkaline conditions is basically uniform because it does not involve the oxidation of other species36,37.However,for seawater electrolysis involving chlorine oxidation side reactions14,Faradaic efficiency decreases due to energy loss and by-product production.
4 Self-supported TM-based electrocatalysts for seawater OER
Rather than aiding by polymer binder for powder catalysts,self-supported nanoarrays couple the catalytic active species with the conductive substrates through chemical bonds or physical forces.To address above issues,a lot of selective and robust self-supported transition metal-based electrodes have been reported and widely applied in seawater oxidation.In general,the performance of electrocatalysts can be optimized by modulating their composition,morphology,size distribution,and element dispersion employing different synthesis strategies38.The main methods widely used in selfsupported nanoarray fabrication include hydro/solvothermal methods,electrochemical deposition,and template-directed synthesis.Note that the preparation of self-supported nanoarray is often based on the multi-step synthesis by combining different methods.Hydro/solvothermal synthesis is a simple and effective approach for preparing various nanoarray with fine structures.Based on the induction effect of different substrates,the heterogeneous crystal nucleation of catalysts is anchored on the substrate by one-step hydro/solvothermal synthesis,resulting in the uniformly covered and firmly bonded nanoarray38.Electrochemical deposition typically relies on standard two- or three-electrode system,using chronoamperometry (CA),chronopotentiometry (CP),or cyclic voltammetry (CV)techniques to deposit nano catalysts on conductive substrates in a very short time (no more than a few minutes)39.In this approach,benefiting from the strong versatility of electrode potentials as redox agents,almost all chemical reactions can be driven38.Consequently,electrochemical deposition methods are suitable for the preparation of most transition metals and their compounds.Template-directed methods are mainly employed to construct highly ordered 1D nanoarray,such as nanowires,nanorods,and nanotubes.The conventional templates include anodic aluminium oxide (AAO),ZnO and Si38.Notably,the easy operability and strong controllability of this method provide chances for exploring the template directed synthesis of multidimensional nanoarray structures.For example,the fabrication of NiSx/NiFe catalyst supported on Ni foam (NF) is achieved by electrodepositing NiFe layered double hydroxide (LDH) ontoinsituformed NiSxnanoarray40.In addition,further thermal conversion can be performed based on precursor array to prepare expected nanoarray possessing more catalytic activity with well preservation of the original structure,such as the fabrication of Ni3FeN@C/NF41and NiTe-NiFeN42.
In practical large-scale hydrogen production applications,industrial electrolytic cells require long-term operation at high current density (> 500 mA∙cm-2) and low overvoltage (< 300 mV).During the electrolysis process,strong bubbles will be generated on the electrodes,which can cause serious attacks on the catalyst.In this situation,the powder catalyst may peer off easily,resulting in reduced catalytic activity and even frequent replacement of the catalyst.By comparison,the active species of the self-supported catalyst can be in direct contact with the substrate,and the strong adhesion between them ensures the good electronic conductivity,which is conducive to maintaining the high mechanical stability of the catalyst for long-term use and recycling.Therefore,it is crucial to develop self-supported nano catalysts that can operate stably and efficiently for a long time under high current density.According to the types of the self-supported TM-based catalysts,this review focuses on the measures to deal with chlorine corrosion and improve OER selectivity and catalytic performance of these materials in seawater electrolysis.
4.1 Transition metal (oxy)hydroxides
It is well accepted that the catalysts will be reconstructed in the OER process and partially or completely converted into(oxy)hydroxides,which generally act as the real active species43.Based on this,intensive attention is paid to design and synthetize the metal (oxy)hydroxide catalysts for seawater oxidation.For the chlorine-corrosion and the surface poisoning induced by insoluble solids in seawater electrolysis,researchers further found that surface modification could alleviate this dilemma by increasing the surface hydrophilicity/wettability of OER electrocatalysts44–46.The high concentration of Na+,Mg2+and Ca2+in seawater leads to the weakened electrostatic attraction,which enhances the hydrophobicity of the catalyst surface and is not conducive to the water adsorption47,48.Therefore,the hydrophilicity of the electrode should be improved by establishing the hydrophilic surface to overcome the weakening of adsorption caused by the increasing ionic strength in seawater29,48.Wang’s group employed Ni foam as the substrate to perform the surface modification on FeOOH/NF to construct a self-supported Fe(Cr)OOH/Fe3O4/NF with the flat heterointerface (Fig.3a–e)45.Strong coupling between Fe(Cr)OOH and Fe3O4on Ni foam led to an exceptional OER activity with low overpotential of 278 mV at 500 mA∙cm-2in alkaline seawater (Fig.3f).This design provided a superhydrophily of the electrode,thus improving the electrolyte penetration.The incorporation of Cr further enhanced the electron transfer during the OER process45.
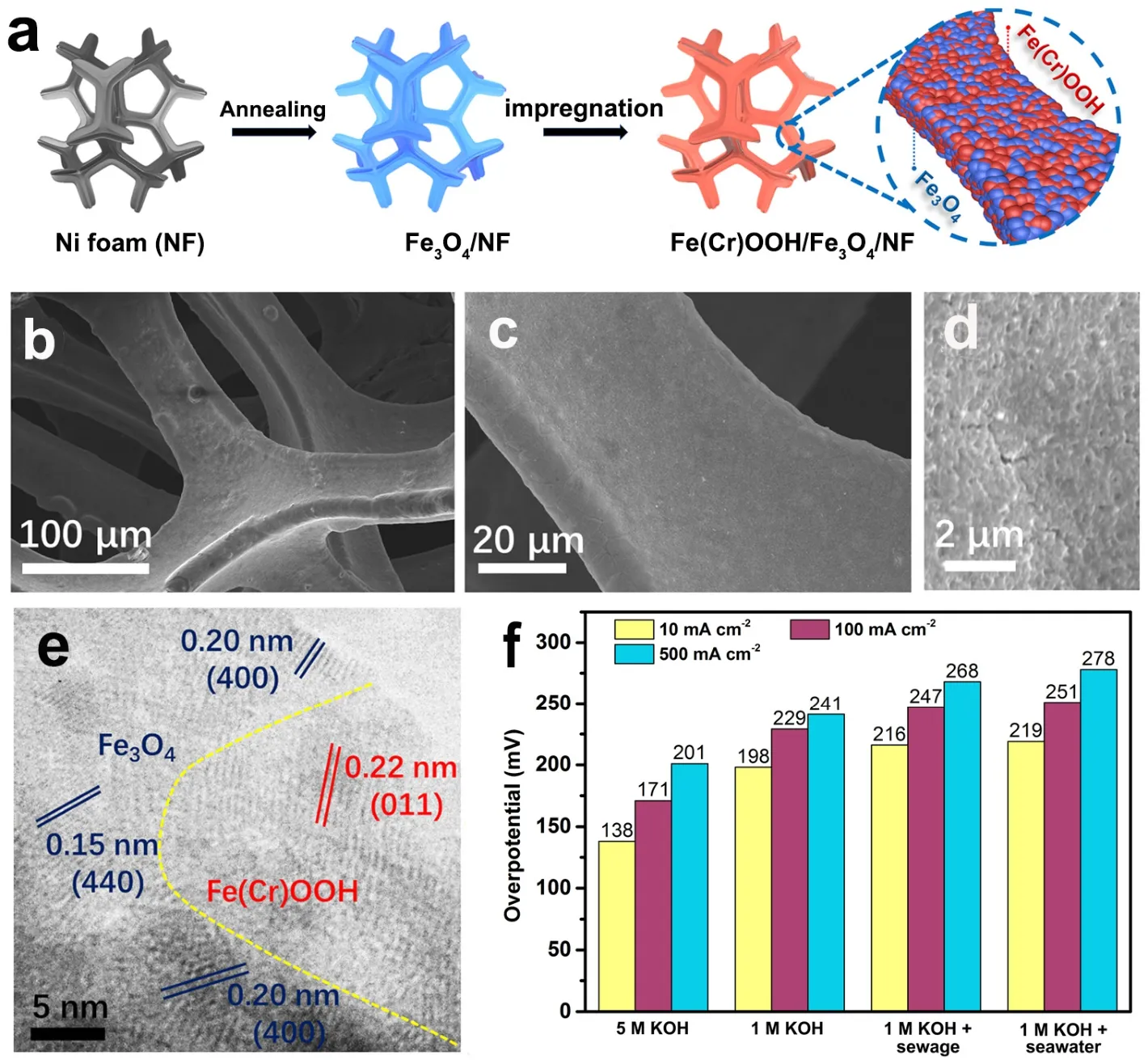
Fig.3 (a) Preparation schematic of Fe(Cr)OOH/Fe3O4/NF.(b–d) SEM and (e) HRTEM images of Fe(Cr)OOH/Fe3O4/NF.(f) Comparison of the overpotentials required at 10,100,and 500 mA∙cm-2 for Fe(Cr)OOH/Fe3O4/NF.
Layered double hydroxides (LDHs),a special kind of layered hydroxides,is formed by overlapping two or more metal hydroxides layers and the interlayer areas consisting of anions and water49.Such stacked ionic layered structure makes LDHs have large interlayer space and flexible chemical property,enabling full exposure of active sites and great promotion of electronic kinetics50–53.Inspired by these inherent advantages,numerous LDHs have been explored as seawater oxidation electrocatalysts in recent years44,52–54.For example,Ren’s group proposed a one-step spontaneous growth method driven by Fe2+to fabricate NiFe LDH on Ni foam at room temperature55.It was found that the OER activity of the resultant NiFe LDH was significantly higher than those prepared by electrodeposition and hydrothermal approaches.In natural seawater (1 mol∙L-1KOH and seawater) electrolyte,this NF/NiFe LDH electrode exhibited good corrosion resistance55.
Constructing amorphous structure to lower crystallinity of OER catalyst is also an important measure to resist chlorine corrosion and enhance catalytic activity56,57.The disordered structure and relatively loose atomic arrangement of amorphous materials allow for abundant permeation channels,which are not only conducive to the electrolyte diffusion,but also beneficial to corrosion resistance53,58.Tuetal.researched the adsorption behaviors of Cl-and OH-at the prepared NiFe-LDH supported on Ni foam consisting of numerous nano crystal faces surrounded by an amorphous phase59.It was thought that Ni3+active sites existing at the boundaries/defects between the crystalline facets and amorphous phase were the main reason for competitive adsorption of Cl-and OH-.On the basis of the hardsoft-acid-base (HSAB) principle,OH-as the harder base was more easily adsorbed on Ni3+sites than Cl-,which endows the electrode with excellent resistance ability to Cl-corrosion59,60.Benefiting from its partially amorphous structure,NiFe-LDH offered a low OER overpotential of 257 mV to deliver 500 mA∙cm-2current density in alkaline saline solution.Similarly,the resistance of NiSx/NiFe catalyst40to Cl-corrosion may also be explained by the above principle.A NiSxlayer was firstlyinsitugenerated on the surface of Ni foam by solvothermal method,and then NiFe LDH was uniformly electrodeposited on top of the NiSxlayer.The as-fabricated amorphous NiSx/NiFe catalyst after activation possessed sharply increased Ni3+active sites for OER,which would preferentially absorb OH-rather than Cl-in an alkaline simulated seawater electrolyte according to HSAB theory59,60.Furthermore,it was found that the anodic etching of the NiSxlayer followed by activation resulted in the generation of sulfate ions,which intercalated into the NiFe LDH together with carbonate ions existing in KOH solution.This polyanion effect of anode may repel Cl-from reaching the electrode,thus enhancing the corrosion resistance of the catalyst40.Notably,the resultant NiSx/NiFe catalyst exhibited an outstanding durability,and only required a low overpotential of ~0.38 V to achieve a large OER current density of 1500 mA∙cm-2in simulated alkaline seawater40.
4.2 Transition metal nitrides (TMNs)
Owing to the excellent conductivity and corrosion resistance,TM nitride-based catalysts have become potential candidates for high current density seawater electrolysis61,62.The contraction of the metaldband caused by the metal―N bond in nitrides improves the OER kinetics,endowing the electrodes with high catalytic performance63,64.Obviously,the most direct and effective way to address chlorine corrosion and OER selectivity issues in seawater electrolysis is to build a protective layer for the catalyst.Especially,as the diffusion barrier of Cl-,a protective layer with catalytic activity can not only enhance the corrosion resistance,but also promote the performance of the electrocatalysts.This,together with the synergistic effect of inherent anti-corrosion ability of nitrides,ensured both good OER selectivity and durability during the seawater splitting.Wang and colleagues prepared self-supported Ni3FeN/NF microsheets coated with a carbon layer (Ni3FeN@C/NF)(Fig.4a)41.By coating the carbon layer,the resultant Ni3FeN/NF array achieved porous structure which enlarged the surface area and enriched the active sites (Fig.4a1–c2).Moreover,the superhydrophilic and superaerophohic surface coupling with the synergetic effect between the Ni3FeN and carbon layer facilitated mass transfer and shortened the electrolyte diffusion distance,and thus enhanced the electrocatalytic performance of Ni3FeN@C/NF for seawater oxidation (Fig.4d,e).Notably,such carbon layer coated on Ni3FeN surface not only inhibited the collapse of microsheets,but also effectively blocked Cl-and insoluble sediments in seawater from corroding the interior Ni3FeN41.
Constructing heterostructure is widely recognized as an effective method to develop high performance electrodes65,66.The heterostructure coupling with the synergism between TMNs and other species can not only modulate the electronic states to enhance charge/mass transport,but also optimize the absorption/desorption of the catalytic intermediates,thus delivering high electrocatalytic performance67–69.For instance,Li and coworkers treated self-supported NiTe nanorods through electrodeposition and nitridation successively to obtain a heterostructured NiTe-NiFeN grown on Ni foam (Fig.5c)42.The composite of NiFeN nanosheets and NiTe nanorods (Fig.5d,f)provided a “hydroxide enrichment” environment resulting from local electric field enhancement,as evidenced by the simulated results that the OH-concentration in the Helmholtz layer was twice that of Cl-in seawater (Fig.5g–i),and finally imparted the OER electrodes significant corrosion resistance in alkaline seawater solution.Benefiting from the interior NiTe array and the interfacial synergy between NiFeN and NiTe,the prepared NiTe-NiFeN catalyst presented advantages such as abundant active sites,enhanced wettability,shorter charge/mass transport path,rapid gas release,further promoting its electrocatalytic activity in seawater splitting42.
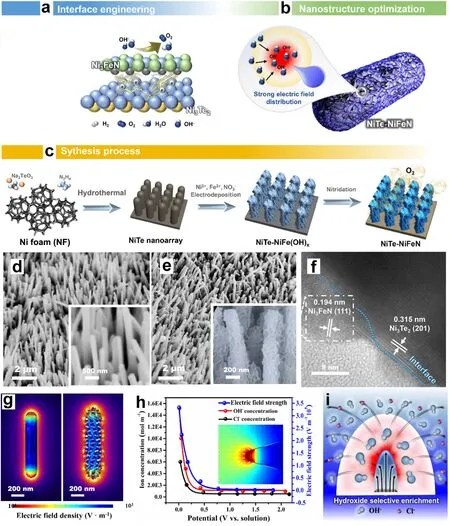
Fig.5 (a,b) Electrocatalytic activity improvement mechanism schematics.(c) Synthetic schematic of NiTe-NiFeN.(d–f) SEM images of NiTe,NiTe-NiCoN,and NiTe-NiFeN.(g) Comparison of electric field distributions with different nanostructures.(h) The relationship of the anion concentration distributions (Cl- and OH-) versus potential under different electric field strength (inset: the calculation area highlighted in the model).(i) Seawater OER mechanism schematic.
4.3 Transition metal phosphides (TMPs)
TMPs have attracted more attention from researchers especially in HER applications for water electrolysis because of the better conductivity compared with their oxides and sulfides70–74.In contrast,the OER activity of phosphides is slightly inferior.In fact,recent study has shown that TMPs possessed the most robust OER catalytic activity,followed by sulfides and oxides containing the same transition metal75.This is mainly attributed to the better electrical conductivity of TMPs,especially the metal-rich phosphides with some metallic properties75.In recent years,some strategies,such as heteroatom doping76,77,heterostructuring78–81,and vacancy engineering82,83,have been proved to be significant effective in promoting the OER performance of self-supported TMPs.Meanwhile,more and more self-supported phosphide-based catalysts with high electrocatalytic activity have been successfully synthesized for seawater OER process84–88.For example,Yuetal.introduced Mo atoms into CoPxto prepare Mo-CoPx/NF catalysts for seawater splitting employing the hydrothermal-phosphorylation method (Fig.6)87.The nanoarray structure and the bimetallic synergy between Mo and Co atoms led to the enhancement of reaction kinetics,good electronic conductivity,and full exposure of active sites for OER catalysis,further promoting the electrocatalytic performance in seawater splitting87.
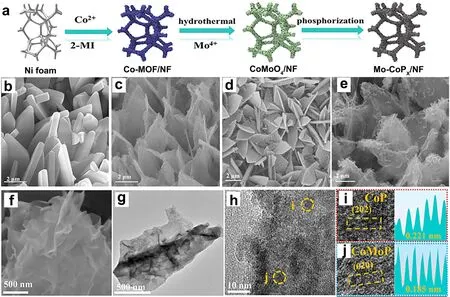
Fig.6 The materials of Mo-CoPx/NF electrocatalysts for seawater splitting.(a) Synthetic schematic.(b–e) SEM images of Co-MOF/NF,CoMoO4/NF,CoPx/NF,and Mo-CoPx/NF.(f–h) TEM and (i,j) HRTEM images of Mo-CoPx/NF.
Ren’s group fabricated the core-shell CoPx@FeOOH supported on Ni foam with a large surface area and high conductivity for seawater oxidation using a three-step method88.By coupling with the CoPxcore,CoPx@FeOOH exhibited remarkable Cl-corrosion resistance,as evidenced by the higher corrosion potential,lower corrosion current density and Zeta potential than those of FeOOH.In addition to the protective effect of CoPxcore,thermodynamically,the alloying of P atoms can efficiently inhibit the metal dissolution,thereby also enhancing the corrosion resistance and durability of the electrode.Moreover,the hydrophilic surface of CoPx@FeOOH/NF benefited electrolyte diffusion and gas release,together ensuring the high performance of catalyst for seawater OER electrolysis.
4.4 Transition metal chalcogenides (TMCs)
Transition metal chalcogenides (TMCs),containing sulfides89–92and selenides93,have been considered as the promising flexible pre-catalysts for OER process in water electrocatalysis.It is well accepted that surface reconstruction of TMCs caused by electro-oxidation give rise to true active species92,93.For example,Wangetal.reported a 3D heterolateral Ni3S2/Co3S4(NiCoS) nanosheets supported on Ni foam and demonstrated that the amorphous NiCoOOH species derived from sulfides provided the real active sites for OER catalysis,which afforded high intrinsic catalytic activity (Fig.7a–c)94.Particularly,due toinsituself-doping derived from sulfur residues,S-doped NiCoOOH surface was obtained,which can modulate the absorption energy of OER intermediates and effectively repel Cl-to endow the electrode with enhanced corrosion resistance (Fig.7d).We reported a NiFeS nanosheet array with enhanced OER catalytic activity by treating NiFe LDH grown on NF through an ion exchange process (Fig.7e–g)95.It was found that NiOOH and sulfate originated from the catalyst reconstruction during the OER process improved the catalytic activity of the electrode.This design achieved the OER current density of 100 mA∙cm-2at 219 and 226 mV overpotentials in 1 mol∙L-1KOH + 0.5 mol∙L-1NaCl and 1 mol∙L-1KOH +seawater electrolytes,respectively (Fig.7h).Such a slight overpotential difference indicated that the self-supported NiFeS electrode had strong corrosion resistance,which was attributed to the effective rejection of Cl-in seawater by the anion-rich(S2-) surface of the catalyst89,95.
Doping has been confirmed as an effective strategy to modulate the surface property and the electronic conductivity of TMCs,thereby delivering high catalytic performance96.Moreover,the passivation layer can effectively inhibit the enrichment of Cl-on the anode surface,thus improving the corrosion resistance of the OER catalyst in seawater splitting10,97.For instance,Chang and colleagues developed a porous Fe,P dual-doped NiSe2nanofilms (Fe,P-NiSe2NFs) grown on carbon cloth with high OER selectivity and Faraday efficiency in seawater electrolysis (Fig.7j)98.It was found that the Fe-doping contributed to the formation of high valence nickel as the active center for OER,and improved the electronic conductivity(Fig.7i).The doped P was easily oxidized during hydrolysis,resulting in the formation of the surface passivation layer containing active P―O species,which not only improved the conductivity but also prevented the dissolution of selenide(Fig.7i,k).Eventually,this Fe,P-NiSe2NFs exhibited a high OER catalytical activity with excellent stability during the seawater electrolysis (Fig.7l)98.
4.5 Other transition metal catalysts
The polyanion effect can effectively prevent Cl-in seawater from reaching the electrode surface,improving corrosion resistance and ensuring the long-term stable operation of the OER catalysts31,89,95.For example,Song and coworkers99fabricated a S-NiFe-Pi/NFF hierarchical hollow electrocatalystviaoxidation,phosphorylation and anion processes.It was found that the phosphate groups tend to gather on the electrode surface more than OH-in alkaline seawater,showing outstanding corrosion resistance.The introduction of S caused the distortion of NiFe-Pi lattice along with the production of many defects,leading to the low local electron concentration around NiFe species and high intrinsic catalytic activity of the electrocatalyst.Consequently,the resultant S-NiFe-Pi/NFF electrode only afford the overpotentials of 241 and 295 mV to deliver the current densities of 100 and 500 mA∙cm-2,respectively,accompanied by high stability in alkaline seawater99.
Featuring excellent conductivity,high anti-chemical property and extensive pH applicability,carbon-based nanostructures has been recognized as the promising materials for electrochemical energy conversion system100,101.To further improve the catalytic activity,the engineering of rational design and synthesis of transition metal-based carbon composites is an attractive strategy.Benefiting from the strong conjugation effect between carbon species and metals,the catalysts can provide higher conductivity and enlarged surface area to further disperse the active phase,which may address the chlorine corrosion issue in seawater electrolysis41,102,103.Lee’s group found that the excellent chlorine corrosion resistance of GO@Fe@Ni-Co@NF electrode for OER catalysis was attributed to the GO layer coating on the active sites of the catalyst,which acted as the interface film between the anode and seawater (Fig.8a–f,h,i)103.Coupled with theinsitu-formed oxidized carbon during OER process,GO layer served as the barrier for Cl-diffusion toward active phase.Meanwhile,the engineering of GO-protective structure can facilitate the permeation of OER intermediates and O2,as well as the synergy effect between metal interface and layers,thus efficiently catalyzing OER and ensuring an outstanding catalytic durability at high current densities during seawater electrolysis (Fig.8g,j).The integration of GO@Fe@Ni-Co@NF as both cathode and anode only required voltages of 1.57 and 2.02 V to achieve current densities of 500 and 1000 mA∙cm-2,respectively,in natural alkaline seawater at 25 °C (Fig.8k)103.
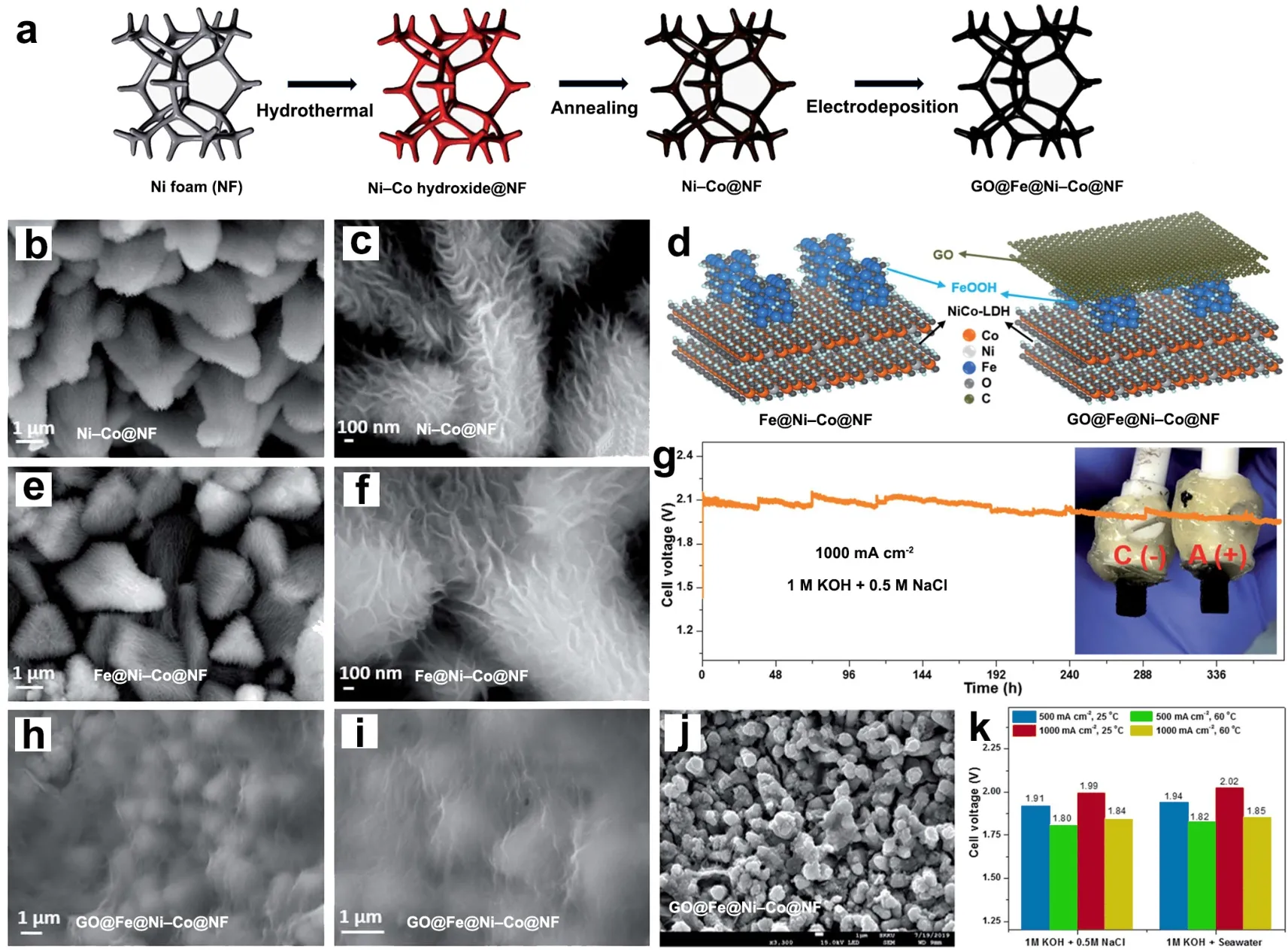
Fig.8 (a) Synthetic schematic of GO@Fe@Ni-Co@NF.(b,c,e,f,h,i) FE-SEM images of Ni-Co@NF,Fe@Ni-Co@NF and GO@Fe@Ni-Co@NF,respectively.(d) Layer structure models of Fe@Ni-Co@NF and GO@Fe@Ni-Co@NF,respectively.(g) Durability test (378 h) curve at a constant current density of 1000 mA∙cm-2 in 1 mol∙L-1 KOH + 0.5 mol∙L-1 NaCl (inset: the photograph of anode and cathode after durability tests).(j) FE-SEM image of the GO@Fe@Ni-Co@NF anode after 378 h testing at 1000 mA∙cm-2.(k) Comparison of the required voltages at current densities of 500 and 1000 mA∙cm-2 for Go@Fe@NiCo@NF(+)//Go@Fe@NiCo@NF(-) electrolyzer in different electrolytes.
Electrochemical reconstruction,the intrinsic and inevitable ending for many catalysts under oxidative potentials,has been confirmed as a powerful protocol to develop highly active electrode materials for water oxidation104,105.Ren’s group achieved the fabrication ofinsituelectrochemically tuned NiO co-doped with Fe and Mo (Fex&Mo-NiO) by CV scanning the corresponding highly active HER catalyst (Fex-Ni&Ni0.2Mo0.8N),accompanied by rapid leaching of most Mo to alkaline electrolyte106.The resultant Fe0.01&Mo-NiO exhibited excellent OER activity in alkaline seawater,although the catalytic activity decreased slightly compared with alkaline freshwater.When coupled with the HER-active nitride,the Fe0.01-Ni&Ni0.2Mo0.8N‖Fe0.01&Mo-NiO pair delivered the current densities of 500 and 1000 mA∙cm-2at voltages of 1.510 and 1.562 V,respectively,in 6 mol∙L-1KOH & seawater at 60 °C with long-term stability106.
In recent years,MOF materials have shown great potential in electrochemical energy application field with its unique advantages107.Incorporating additional functional components is recognized as an effective means to modulate the electronic structure and explore synergistic effects of MOF catalysts,thus delivering enhanced catalytic activity that cannot be achieved with MOF alone108,109.Luo and colleagues synthesized bimetallic CdFe-MOFs supported on NF with remarkable OER performance in alkaline and seawater media compared with Fe-BOC110.Since the introduced Cd atoms were exposed to the surface of MOFs,their dangling bonds were saturated by oxygen atoms,which affected the coordination environment of Fe-BDC,thus resulting in changes in the morphology of Fe-BDC.The synergism between Cd and Fe2+/Fe3+tailored the surface properties and modulated the electron density of active sites,further improving the electrocatalytic oxygen evolution performance.
Currently,in practical seawater electrolysis,commercial electrolytic cell must be desalted before use.Direct electrolysis of seawater that has not been alkalized or acidified remains a major challenge.Fortunately,Ling's group applied the Lewis acid-modified self-supported catalyst (Cr2O3-CoOx) for direct natural seawater electrolysis111,and found that the Lewis acid layer could prevent Cl-from approaching the catalyst surface by capturing a large amount of OH-around the electrode,thus effectively inhibiting chlorine corrosion.Moreover,due to the strong binding between OH-and Lewis acid layer,insoluble precipitation caused by Mg2+and Ca2+cations in seawater are significantly reduced,alleviating the physical blockage of the catalyst.Such local alkaline reaction environment promoted the natural seawater splitting and achieved catalytic performance comparable to that in high purity water electrolysis.In addition,the group also investigated the feasibility of practical application of prepared self-supported electrode in commercial electrolyzer,and constructed the membrane electrode by hot pressing method.Specifically,the Nafion 115 membrane was placed between two Ti fiber mats and pressed for 1 min at 100 °C and 6 MPa to complete the assembly of the membrane electrode,maintaining the structural integrity of the electrode.A flowing natural seawater electrolytic cell was constructed based on this.The results showed that a current density of 1.0 A∙cm-2for industrial hydrogen production can be obtained by applying 1.87 V at 60 °C without purifying/desalting process and strong alkali addition.The catalyst can maintain long-term stability for over 100 h at 500 mA∙cm-2,and the Faraday efficiency of O2is up to~92%,achieving efficient direct seawater electrolysis.
5 Conclusions and perspectives
Seawater electrolysis is a sustainable technology to produce clean hydrogen by utilizing abundant seawater resources.However,many challenges remain,such as corrosion caused by Cl-and pH change,chlorine-related competitive reactions,and poisoning from insoluble substances.The design of OER electrocatalysts with high activity,selectivity and stability is of great significance to promote the development of seawater electrolysis.Generally,seawater electrolysis requires to work under high current density,which is difficult for powder electrodes.While the self-supported electrodes possess lower interface resistance,larger active surface,and superior stability,which is more reasonable and meaningful than the traditional powder electrodes in seawater electrolysis.
In this minireview,we summarized the research progress of transition metal self-supported catalysts for OER process during seawater electrolysis in recent years,focused on the engineering strategies employed to overcome the challenges faced in oxygen evolution,providing a reference for the subsequent design of efficient and stable OER catalysts for seawater electrolysis.In general,the engineering strategy for rationally designing efficient and stable OER catalysts for seawater electrolysis mainly includes the following aspects.Firstly,constructing 3D porous nanostructures with high porosity and roughness can make catalysts have large surface areas and abundant active sites,which is an effective way to improve mass transfer,enhance OER activity,and increase catalytic efficiency.Moreover,the increase in porosity and roughness is beneficial to improving the wettability of the electrode surface.Secondly,a Cl-blocking layer on the surface of catalyst can effectively inhibit the competitive oxidation and corrosion of Cl-.Note that a protective layer that only acts as a barrier to Cl-may lead to a reduction in diffusion and mass transfer,which is unfavorable to the improvement of catalytic activity.Designing a protective layer with both catalytic activity and protection is an effective strategy for promoting catalytic activity,selectivity,and stability of catalysts.Constructing an anion exclusion layer modified with Lewis acid seems to be a good choice.Thirdly,during the industrial seawater splitting at high current density,severe gas production reactions will cause serious attacks on the electrodes,and catalysts may peel off after long-term electrolysis,resulting in reduced catalytic activity and poor stability.In this case,in addition to developinginsitugrown self-supported catalysts with strong adhesion,it is necessary to design catalytic materials with super hydrophilic and super hydrophobic surface to increase the permeability of the electrolyte,promote the effective utilization of active sites,and avoid the accumulation of large amounts of bubbles.Finally,according to previous studies,the alkaline condition at 60 °C is the preferred industrial condition for seawater electrolysis,as it offers great potential space for OER process.
Although the research of electrocatalysts for seawater oxidation has made important progress,it still cannot meet the needs of industrial production,and there are still great challenges in the following aspects.
(1) The medium of seawater electrolysis should be transferred from simulated saline water to natural seawater.At present,most of the self-supported catalysts have shown excellent performance in alkaline (saline) water electrolysis,but still face many challenges in natural seawater splitting.The complication of natural seawater composition will inevitably affect the performance and durability of the catalysts.Therefore,in addition to design and synthesis of catalysts with high activity,selectivity and stability,the development of simple and low-cost natural seawater pretreatment technology to alleviate the corrosion and poisoning issues as much as possible is also an important topic for the future development of seawater electrolysis.
(2) The active sites and real catalytic species for OER need systematic study.As a kind of heterogeneous catalytic reactions,electrocatalytic reaction usually occurs on the surface of electrodes.Consequently,from this point of view,the surface properties of electrodes would largely determine the catalytic activity.Most of the catalysts developed so far are “precatalysts”,that is,the catalysts will be reconstructed in the OER process and partially or completely converted into the real active structures.The catalysts undergoing partial reconstruction may exhibit obvious interfacial effects,which greatly affect the adsorption/desorption of intermediates and electron transport.Therefore,real-time tracking of the reconstruction process is particularly important for confirming the real catalytic active components.With the continuous development of DFT calculations andinsituanalysis techniques,future research should focus on accurately capturing the dynamic reconstruction process,deeply exploring the structure-activity relationship,identifying the true active species,and revealing the catalytic mechanism of catalysts,so as to obtain the maximum possible improvement in the performance and selectivity of electrodes.
(3) The standardized evaluation system of electrocatalysts should be established reasonably.During the catalyst evaluation,factors such as the intrinsic activity,intrinsic activity,catalyst size,mass loading,substrate effects,and test conditions should be fully considered.Especially under high current density,overpotential,exchange current density,Tafel slope,turnover frequency,Faradaic efficiency,long-term stability,electrochemical active surface area and other parameters should be comprehensively evaluated to deeply understand the kinetics and thermodynamics of catalytic reaction.
Author Contributions:Conceptualization,Wu Q.and Sun X.; Methodology,Gao Q.,and Sun S.; Formal Analysis,Yan H.;Investigation,Qi Y.and Zheng D.; Resources,Luo Y.; Writing-Original Draft Preparation,Shan B.,Wang W.and Ying B.;Writing-Review & Editing,Wu Q.and Sun X.; Visualization,Liu Q.; Supervision,Tai X.and Wang X.; Project Administration,Wu Q.; Funding Acquisition,Wang X.,Sun X.and Hamdy M.S.