Nutrient retranslocation strategies associated with dieback of Pinus species in a semiarid sandy region of Northeast China
Chng Liu,Ki Wng,Hongzhng Kng,Boming Du,Rishng Zhng,Shnshn Ti
a Liaoning Technical University, Fuxin, 123000, China
b College of Environmental Sciences and Engineering, Liaoning Technical University, Fuxin 123000, China
c School of Design, Shanghai Jiao Tong University, Shanghai 200240, China
d Qingyuan Forest, National Observation and Research Station, Shenyang 110016, China
e School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China
f Liaoning Institute of Sandy Land Control and Utilization, Fuxin 123000, China
Keywords:Nutrient use strategy Nutrient limitation Seasonal retranslocation Needle age Shelter forest decline
ABSTRACT In the semiarid sandy region of Northeast China, Mongolian pine (Pinus sylvestris var.mongolica) suffers dieback after the age of 35, while Japanese red pine (Pinus densiflora) and Chinese pine (Pinus tabuliformis) stay healthy.Foliar nutrient retranslocation reflects the nutrient conservation and utilization mechanism of plants in response to their habitats.However, the nutrient retranslocation strategies employed by three Pinus tree species to cope with nutrient limitations remain largely unknown.For this study, we investigated the seasonal variations in nitrogen(N)and phosphorus(P)concentrations of Mongolian pine,Japanese red pine,and Chinese pine plantations in terms of the green needles of all ages, senesced needles, and soil.Further, the N retranslocation efficiency(NRE), and P retranslocation efficiency (PRE), and correlations between the N:P ratios of needles and soil were analyzed.The results showed that,except for the spring NRE in 1-year-old needles of Mongolian pine,the spring NRE and PRE in 1-and 2-year-old needles of the three tree species were greater than zero.The autumn PRE was higher than zero for Mongolian pine, but lower than zero for Japanese red pine and Chinese pine.Among the three Pinus species,Mongolian pine showed greater spring PRE in 2-year-old needles,and PRE from 1-to 2-yearold needles, and from 2-year-old needles to litter.However, Japanese red pine had higher P concentrations and lower N:P ratios in senesced needles,while greater PRE was found in Chinese pine litter.Significant relationships between the N:P ratios were found in the current year and 1-year-old needles and soil in the Mongolian pine plantation, while there was an insignificant relationship between the N:P ratios of the needles and soil in the Chinese pine plantation.Thus, for Mongolian pine, the removal of P from needles in autumn, and higher P translocation from older needles under P-deficient soil may have contributed to the tree dieback.In contrast,Japanese red pine and Chinese pine stored P in their needles during autumn.Japanese red pine returned more P to the soil via litter, while Chinese pine maintained N:P homeostasis and increased P withdrawal prior to needle abscission.
1.Introduction
To date, China's Three Northern Protected Forest Program is the world's largest afforestation program, which has effectively controlled wind and sand hazards and soil erosion, steadily increased forest productivity, carbon sequestration, and grain production, and achieved improved ecological and economic benefits in northern China over the last 40 years(Gao and Huang,2020;Zhai et al.,2023).This program was initiated in 1976,and expansive plantations have been established in arid and semi-arid areas ever since.Drought resistant evergreen coniferous trees with tolerance against nutrient-poor soils were selected as the preferential species for afforestation.Mongolian pine(Pinus sylvestris var.mongolica) was initially introduced to the Horqin Sandy Land for sand fixation in the 1950s(Zhu et al.,2008).Due to its relatively high initial growth and seedling survival rates, it has been extensively used for afforestation and has propagated to other regions of northern China(Dang et al., 2022).Its plantations cover a total area of more than 800,000 ha, and large-scale afforestation projects are still ongoing (Song et al., 2022).However, in the southeast of the Horqin Sandy Land,Mongolian pine began to decline after ~35 years of age,as indicated by observed sparse crowns,low growth rates,and dieback(Zhu et al.,2008).This led to a partial loss of protective functions and serious decrease in ecological benefits (Sun et al., 2022).In contrast, Japanese red pine(Pinus densiflora)and Chinese pine(Pinus tabuliformis)grew healthily and even accelerated growth after ~30 years of age in the same regions(Zhang,2017).However,they were seldom planted in sandy land due to their high death rate and slow growth at the seedling stage.Therefore,it is necessary to select suitable tree species for sustainable development of the Three Northern Protected Forest Program.
Afforestation often involves tremendous changes in plant and soil nutrient cycling processes, which may cause soil nutrient deficiencies(Zeng et al., 2009; Wang et al., 2021b).This is particularly the case in pure plantations,which is due to the long-term uptake of single nutrients from the soil.Nitrogen (N) and phosphorus (P) comprise the main limiting factors for forest growth and productivity in Horqin Sandy Land(Chen et al., 2006; Zeng et al., 2009).Since the content of soil N is deficient,trees often suffer N limitation in northern China(Zhang et al.,2018), while some trees may transfer to P limitation with forest development (Wang et al., 2021a).Consequently, elucidating the nutrient conservation and utilization mechanisms of the three Pinus tree species(Mongolian pine, Japanese red pine, and Chinese pine) under nutrient limitation will contribute to the prudent selection of afforestation species,plantation management,and the estimation of vegetation dynamics(Gao and Huang,2020;Lin et al.,2022).
Nutrient retranslocation (resorption) involves a nutrient removal process from one organ to another in the newly growing or stored organs of plants.This can improve internal nutrient use and recycling efficiencies that contribute to plant survival, growth, and development(Aerts, 1996; Wang et al., 2021a), as well as community diversity and productivity (Zhang et al., 2023).The retranslocation process is critical for plant nutrient storage, supply,balance,and turnover(Vergutz et al.,2012), and its investigation might likely assist with elucidating the nutrient conservation and utilization kinetics that plants employ to cope with nutrient deficiencies(Fife et al.,2008;Wang et al.,2021b).Foliage is the primary repository of nutrients in trees, with >60% of foliar nutrients being retranslocated or reabsorbed(Freschet et al.,2010),which is typically higher than that from branches, stems, or roots (Brant and Chen, 2015).Foliar nutrient retranslocation varies with the season and leaf longevity (age), which reflect nutrient conservation and adaptive strategies of plants as relating to their habitats(Du et al.,2021).
Nutrient retranslocation occurs not only during leaf senescence, but throughout the growing season (Fife et al., 2008).Significant seasonal variations in foliar nutrient retranslocation have been well documented,especially for evergreen tree species(de las Heras et al.,2017),which are induced by seasonal dynamics in tree growth and climatic conditions.In spring,the demand for nutrients to support new tissue generation may be satisfied by root uptake from the soil and/or the remobilization of internal stores.In many forests, soil available nutrients are unstable and can change dramatically with the season.Thus, leaf residing nutrients serve as a stable primary source of nourishment for the generation of new tissues, in contrast to those that are newly absorbed from the soil(Nambiar and Fife,1991;de las Heras et al.,2017).In autumn,nutrients are transferred from the leaves to sink organs once leaf growth has ceased, where the stored nutrients increase frost tolerance over the dormant winter period(Chapin and Kedrowski,1983),to be used for new tissue production the following spring (Proe et al., 2000).The level of nutrient remobilization may be driven by the size and strength of the storage pool(Millard et al.,2001),and to some extent is a determinant of the initial growth of plants and their capacity for interspecific competition (Wyka et al., 2016).The seasonal dynamics of leaf nutrient retranslocation is mainly determined by phenology, the supply of soil nutrients,and strength of the foliar sink(de las Heras et al.,2017;Seidel et al.,2019).Thus,it is essential to explore the seasonal retranslocation strategies of various tree species to determine how they cope with nutrient deficiencies.
As leaves develop, their foliar structures, functions, and chemical compositions undergo significant changes(Niinemets,2016),while their nutrient concentrations and contents vary with age (Yuan et al., 2018;Seidel et al., 2019).The withdrawal of nutrients from older leaves is interpreted as internal recycling that accompanies senescence processes.In younger leaves, nutrient levels are fully or partially restored after nutrient translocation, which represents a reserve drawdown and replenishment cycle (Han et al., 2008; Wyka et al., 2016).The remobilization of nutrients from older leaves,or nutrient allocation within them,contributes to age-related variations in nutrient retranslocation.However, it is uncertain that, among various leaf age classes, the levels of nutrient retranslocation with increasing leaf age may increase(Fife et al.,2008), decrease (Yuan et al., 2018), or unchanged (Han et al., 2008),which may be due to leaf life span,soil nutrients,and adaptation of tree species to the habitats(Achat et al.,2018).Consequently,it is crucial to quantify nutrient retranslocation levels for various leaf age classes of different tree species.
The three Pinus trees under study(Mongolian pine,Japanese red pine,and Chinese pine)all possessed needles of various ages in Horqin Sandy Land.The objectives of this study were to 1)determine seasonal N and P retranslocation of the three tree species; 2) compare N and P retranslocation of various leaf ages of the three tree species; 3) elucidate the nutrient retranslocation strategies employed by the three Pinus tree species to cope with soil nutrient limitations.To achieve the above goals,we examined seasonal variations in N and P concentrations in all-aged green needles, senesced needles, and soils, as well as N retranslocation efficiency (NRE) and P retranslocation efficiency (PRE) of Mongolian pine, Japanese red pine, and Chinese pine plantations in the Horqin Sandy Land,China.We hypothesized that in comparison to Japanese red pine and Chinese pine, Mongolian pine has lower nutrient retranslocation efficiency (NuRE) at seasonal and leaf age levels under soil nutrient deficiency conditions that may contribute to its growth decline.
2.Materials and methods
2.1.Study site
This study was conducted at the Zhanggutai Experimental Base(42°32′-42°51′N,121°53′-122°35′E)of the Liaoning Institute of Sandy Land Control and Utilization, located in the southeastern region of the Horqin Sandy Land, China.The study area has an elevation of 208.3-253.4 m and a semiarid climate, with an average annual temperature of 6.8°C, precipitation of 474 mm (mostly during June-August), and potential evaporation of ~1,580 mm.The zonal soil of this region is arenosols that derives from an eolian parent material, and the distributions of soil salinity, texture, and structures are homogeneous(Zhu et al.,2008).The main vegetation type is psammophytes;however,the original vegetation has almost disappeared due to long-term anthropogenic and natural disturbances.Since the 1950s, a large area of shelterbelts was planted in this region for sand fixation and agricultural production.The main tree species include P.sylvestris var.mongolica and Populus spp., interspersed with small patches of P.densiflora,
P.tabuliformis and Ulmus pumila(Wang et al.,2021a).
2.2.Experimental design
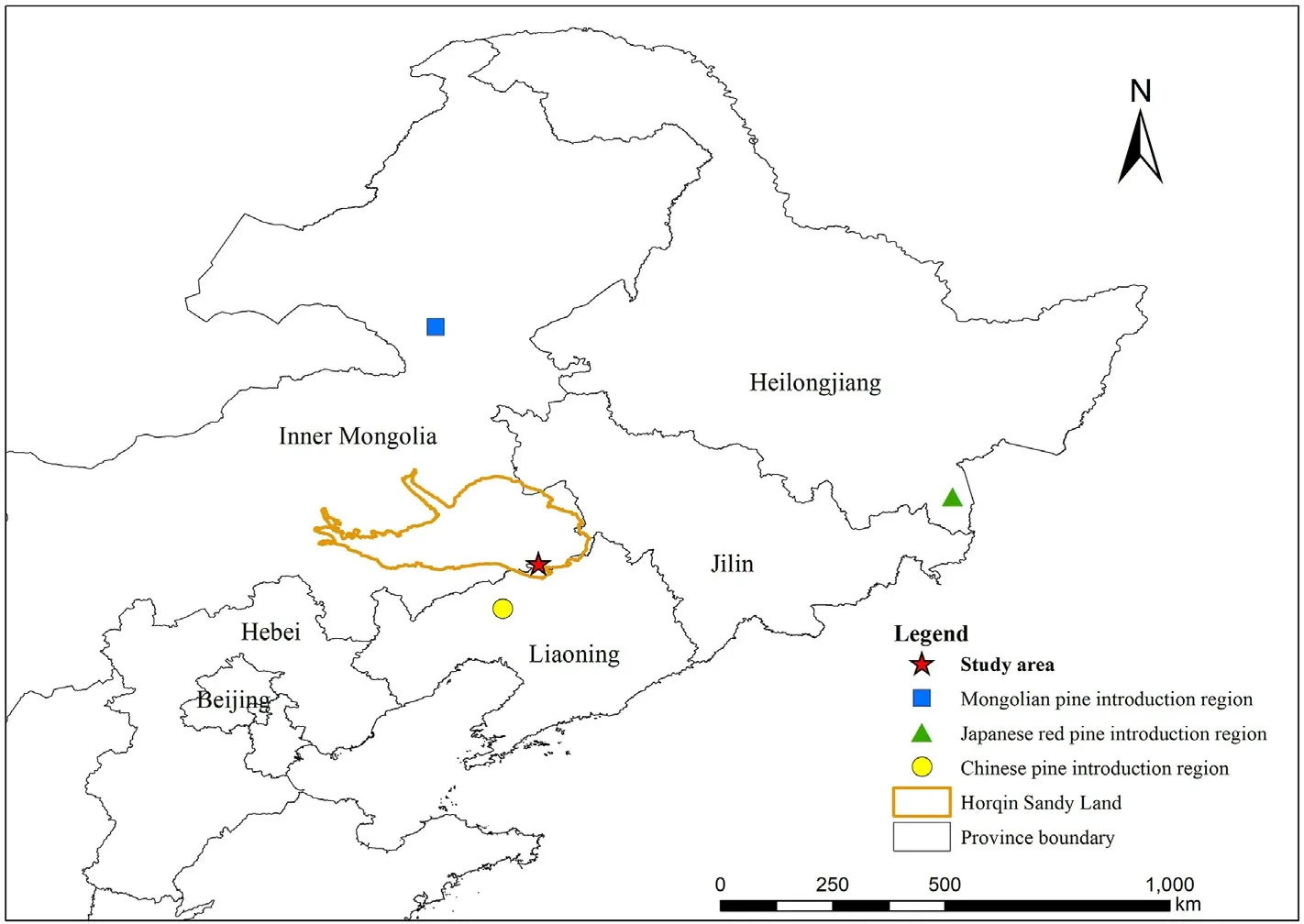
Fig.1.Geographical research site and Mongolian pine,Japanese red pine,and Chinese pine introduction region for this study.(For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The pure plantations of selected Mongolian pine,Japanese red pine,and Chinese pine were established during the 1970s.The seeds of the three Pinus trees came from Honghuaerji of the Hulunbuir sandy plains,the Inner Mongolia Autonomous Region (47°35′-48°36′N,118°58′-120°32′E), Dongning City of Heilongjiang Province(43°25′-44°35′N, 129°53′-131°18′E), and Haitang Mountain of Fuxin City, Liaoning Province (41°56′N, 121°52′E), respectively (Fig.1).These plantations were established from non-vegetated sandy lands,and the soil properties were homogeneous across the non-vegetated sandy lands based on previous investigations(Zeng et al.,2009).Thus,the three plantations had similar site conditions and land management histories prior to afforestation.In 2017, the three plantations at ~40 years old were investigated at the Zhanggutai Experimental Base,where three plot replicates(20 m×20 m)were established in Mongolian pine,Japanese red pine, and Chinese pine plantations, respectively.All plots were within 10 km to ensure similar soil types and climatic conditions,and the selected plantations shared similar topographies (slope <5°), and elevations.The air temperature and rainfall data(2017)were obtained from a weather station located at the study site (Fig.2).The tree density,height,and stem diameter at breast height(DBH),soil organic C,total N,total P concentrations, and pH values of the plots were investigated at sampling time(Table 1).
2.3.Plant and soil samples
In each plot, three representative trees with average DBH were selected for sample collection.Green needles and soil samples were collected in mid-April, June, August, and October (i.e., prior to the beginning of growth, early-growing, fast-growing, and late growth periods) (Zheng et al., 2020).Seasonal climate variabilities produced phenological changes in the three pine needles,which were formed by a set of 0-2 year old needles growing in needle cohorts, with the 0-year needle samples representing the current year.Two or three needle age classes were present on the trees at any given sampling time,i.e.,current year needles produced in June of 2017, and 1- and 2-year-old needles produced in 2016 and 2015,respectively.As not all needle cohorts were present on the trees for the four sampling periods, the needle samples were collected for two data subsets.One covered prior to the beginning of the growth period, including the 1- and 2-year-old needles, while the other covered the early-growing,fast-growing,and late growth periods,including the current year,1-and 2-year-old needles.
From each selected tree, four branches with green needles were collected from four opposite compass sides of the top half of the crown using a tree trimmer.The needle cohorts were collected and segregated by age class(0-,1-and 2-year-old),which were composited into a single sample per tree for each age class.The soil samples were simultaneously extracted using a soil auger (Ø 5 cm) at depths of 0-20, 20-40, and 40-60 cm.Four soil cores were randomly extracted within 1 m of the base of each selected tree following the removal of understory plants and surface litter, and then thoroughly mixed to homogenize a sample.To avoid underestimating the nutrient concentrations in senesced needles,those that were completely yellow,dry,and ready to fall from branches with just a gentle shake/touch were directly collected in mid-November(Wang et al., 2021a).Three replicate samples of green and senesced needles, and soils were collected for each plot.

Fig.2.Monthly mean temperature and rainfall in the study area(a),and soil water content at sampling time for Mongolian pine (MP),Japanese red pine (JRP)and Chinese pine (CP) plantations (b).

Table 1Characteristics of the three plantations in the study region.
2.4.Sample measurements
All plant samples were dried at 60°C for 72 h, where 10 green or senesced needles were weighed to calculate the individual leaf mass after each sampling.The remaining samples were ground using a mechanical grinder and passed through a 0.15-mm sieve.The samples were initially digested with H2SO4-H2O2,after which the N and P concentrations were determined via the semimicro-Kjeldahl method with a Kjeldahl autoanalyzer (JY-SPD60, Beijing, China) and colorimetric method with a spectrophotometer (T6, Beijing, China), respectively.Any roots and stones were removed from the soil samples.The fresh soil samples were passed through a 1-mm mesh, following extraction with NaHCO3, after which the soil available N and P concentrations were measured by alkali diffusion and colorimetric methods, respectively, with a spectrophotometer.Some soil samples were dried for 12 h at 105°C to determine the soil water content, while others were air-dried and passed through a 1-mm mesh to measure the pH values in a suspension with a soil/water ratio of 1:2.5.The remaining air-dried soil samples were passed through a 0.15-mm mesh to determine the soil organic C, total N and P concentrations.The soil organic C concentrations were measured using the oil bath-K2Cr2O7titration method.The soil total N concentrations were determined using a Kjeldahl auto-analyzer (JY-SPD60, Beijing, China)after digestion in H2SO4-mixed catalyst.The soil total P concentrations were determined by the colorimetric method with a spectrophotometer(T6,Beijing,China)after digestion in H2SO4-HClO4(Bao,2000).
2.5.Calculations
According to the previous study on Pinus trees (de las Heras et al.,2017),nutrient retranslocation primarily occurred in three phases during the growing season: 1) nutrient remobilization from older leaves in response to new shoot production during spring; 2) nutrient relocation for storage or reutilization before dormancy during autumn; and 3)nutrient resorption from litter prior to leaf fall.Nutrient retranslocation efficiency (NuRE) was used to quantify the nutrient retranslocation,which was calculated as follows:
where NuRE is the percentage of seasonal or annual variations in nutrient (N or P) content per needle.For seasonal NuRE, Nut0and Mt0represent the nutrient concentration and mass per needle of a specific age class (current year, 1- or 2-year old) during a specific growth season(April, August, or October), respectively.Nut1and Mt1represent the needle nutrient concentration and mass for a specific age class in the next sampling season (June, October, or November), respectively.Thus, we designated the percentage of variations in the nutrient content per needle from April to June as the spring NuRE, from August to October as the autumn NuRE,and from 2-year-old needles in October to senesced needles in November as the NuRE in litter.As tree growth peaks in August in this region,the nutrient content in August was used only for calculating the annual NuRE; thus, Nut0and Mt0represented the nutrient concentration and mass per needle of a specific generation,respectively, while Nut1and Mt1represented the nutrient concentration and mass per needle in the needles of the next generation or in litter,respectively.Specifically,we calculated the NuRE in the first year(NuREfirst)from current year to 1-year-old needles, second year (NuREsecond) from 1- to 2-year-old needles, and prior to leaf abscission (NuREfall) from 2-year-old needles to litter(Zheng et al.,2020).
The degree of stoichiometric homeostasis was determined by the following model(Sterner and Elser, 2002):
where y is the N:P ratios of needles with three age classes, and x is the available N:P ratios in the soil that were calculated as the means at 0-60 cm.H and c are obtained through linear regression analysis.If the regression relationship was insignificant(P >0.05),the slope(1/H)was set to 0, which indicated strict homeostasis and that the plant N:P ratio did not change with the soil N:P ratio.If the regression relationship was significant(P <0.05),a slope between 0 and 1 indicated a homeostatic adjustment of the plant N:P ratio,where greater slopes mean higher plant plasticity(Wang et al.,2021b).
2.6.Statistical analysis
Repeated measures analysis of variance was employed to assess the effects of the sampling time, needle age, tree species, and their interactions on green needle N and P concentrations and their ratios, as well as the sampling time,soil layer,tree species,and their interactions on soil N and P concentrations and their ratios.We performed a threeway analysis of variance to test the impacts of season, needle age, tree species, and their interactions on NRE and PRE.One-way analysis of variance was used to compare significant differences in N and P concentrations and N:P ratios,as well as seasonal and annual NRE and PRE.Duncan's test was conducted for post-hoc multiple comparisons.Pearson's correlation analysis was used to examine the relationships between the needle and soil N:P ratios in the three tree plantations.All figures were prepared using SigmaPlot 10.0 software, and all data were analyzed using SPSS 16.0 software for Windows(SPSS Inc.,Chicago,IL,USA).The significance level was set to 0.05.
3.Results
3.1.Seasonal dynamics of needle N and P concentrations and their ratios
The soil organic C,total N and P concentrations,in 0-20 cm soil layer,varied from 3.15 to 5.16, 0.29 to 0.69, 0.087 to 0.129 g·kg-1, respectively, in the three Pinus plantations, with greater C and N for Japanese red pine but lower P for Mongolian pine.The C:N,C:P and N:P ratios in the plantations varied from 7.49 to 12.28,30.25 to 52.54,2.80 to 6.69,respectively, with greater C:P ratios for Mongolian pine but lower N:P ratios for Chinese pine(Table 1).
The sampling time, tree species, and interactions between the sampling time and needle age,as well as between the sampling time and tree species significantly affected the needle N and P concentrations and N:P ratios(Table 2).For Mongolian pine,the N concentrations in the current year,1-and 2-year-old needles showed a trend of initially increasing and then decreasing over time.The P concentrations in the needles of all ages decreased from August to October,and they decreased from April to June in the 1-and 2 year-old needles.The N:P ratios in the 2-year-old needles initially increased and then decreased over time.For Japanese red pine and Chinese pine, the N concentrations in the current year needles initially increased and then decreased over time, while they decreased from April to June,and from August to October in the 1-and 2-year-old needles.Generally,the P concentrations in the needles of all ages tended to initially decrease and then increase over time, while the N:P ratios increased from June to August and then decreased from August to October.Additionally, the N and P concentrations and N:P ratios in senesced needles were significantly lower than those of the two-year-old needles in October for the three tree species(Fig.3).
3.2.Seasonal and annual variations in NuRE
Season, needle age, and tree species and their interactions significantly affected the NRE and PRE(Table 3).The spring NRE in 2-year-old needles,the spring PRE in 1-and 2-year-old needles,the autumn NRE in the needles of all ages, and the NRE and PRE in litter of the three tree species were greater than zero, while the spring NRE in 1-year-old needles of Mongolian pine and the autumn PRE in current year, 1- and 2-year-old needles of Japanese red pine and Chinese pine were lower than zero (Fig.4).For Mongolian pine, the spring NRE and PRE were significantly greater in the 2-year-old than 1-year-old needles, while there were no significant differences between the autumn NRE in 2-yearold needles and NRE in litter,and between the spring PRE in 2-year-old needles and PRE in litter (Fig.4a and b).For Japanese red pine, there were no significant differences in the spring NRE and autumn NRE and PRE among the needle age classes,while the spring PRE was significantly greater in the 1-year-old than 2-year-old needles (Fig.4c and d).ForChinese pine, the spring NRE was significantly greater in the 1-year-old than 2-year-old needles; however, the spring PRE showed an inverse trend, while the autumn NRE and PRE were higher in current year needles and lower in 1-year-old needles (Fig.4e and f).Furthermore, the NRE and PRE in litter were higher than those in spring and autumn for Japanese red pine and Chinese pine(Fig.4).
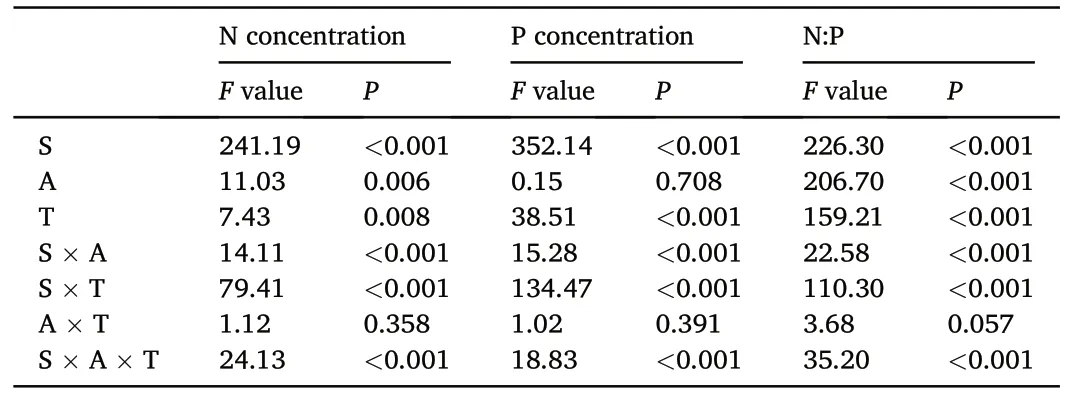
Table 2F values and statistical significance of repeated measures analysis of variance on the effects of sampling time (S), needle age (A), tree species (T), and their interactions on needle N and P concentrations and their ratios.
The NuREfallwas significantly higher than NuREfirstand NuREsecondfor the three tree species (Fig.5).NREfirstwas lower than NREsecondfor Mongolian pine and Japanese red pine,but an opposite trend was found for Chinese pine (Fig.5a).PREfirstwas lower than PREsecondfor Mongolian pine,while there were no significant differences between PREfirstand PREsecondfor Japanese red pine and Chinese pine(Fig.5b).Japanese red pine exhibited lower NREfirstbut greater NREsecondand NREfall,whereas Mongolian pine showed lower PREfirstbut greater PREsecondand PREfall,compared with the other tree species(Fig.5).
3.3.Soil nutrients and stoichiometric homeostasis in the three pine plantations
The soil available N and P concentrations and N:P ratios were significantly affected by the sampling time and soil layer in the three pine plantations (Table 4).Generally, the soil available N and P concentrations tended to initially increase and then decrease over time with the maximum in June, and decreased with deeper soil layers.The soil N:P ratios exhibited an upward trend from June to October, particularly in the deeper soil layers of the three pine plantations(Fig.6).
Both the current year and 1-year-old needle N:P ratios correlated significantly with the soil N:P ratios of the Mongolian pine plantation(Fig.7a), while a significant relationship was found only between the current year needle and soil N:P ratios in the Japanese red pine plantation (Fig.7b).There was an insignificant relationship in the N:P ratios between the needles and soils in the Chinese pine plantation (Fig.7c).
4.Discussion
4.1.Seasonal N and P retranslocation strategies of the three Pinus tree species
In this study, the average surface soil organic C, total N and P concentrations were 4.34, 0.46, and 0.11 g·kg-1, respectively, in the three Pinus plantations (Table 1), which were lower than average values in China (24.6, 1.9, and 0.8 g·kg-1, respectively, Tian et al., 2010).These results suggest that soil C,N,and P in Horqin Sandy Land were deficient due to the long severe erosion.We found a similar C:N ratio but greater C:P and N:P ratios in the plantations compared with the average values in Horqin Sandy Land as a whole(9.5,28.5,and 3.0,respectively,Cao et al.,2023), implying decreased P mineralization but unchanged N mineralization in the soils.Thus, P limitation to tree growth enhanced after the conifer afforestation.In disagreement with our hypothesis, in comparison to Japanese red pine and Chinese pine, Mongolian pine showed greater autumn PRE,and spring PRE in 2-year-old needles,and PREsecondand PREfallunder soil P deficiency,which may contribute to tree dieback.
Seasonal nutrient retranslocation is critical for trees in terms of supplying nutrients for new tissue flushes and storing nutrients for the survival of adverse environments; thus, it is a key adaptative strategy for elevating nutrient use efficiencies under nutrient deficient conditions(Nambiar and Fife,1991;Zheng et al.,2020).Except for the spring NRE in 1-year-old needles of Mongolian pine, we found that the spring NRE and PRE in 1-and 2-year-old needles of the three tree species was greater than zero(Fig.4).The decreased N and P concentrations in older needles from April to June(Fig.3)coincided with the flush of new growth during spring.These results suggested that the N and P mobilized from living needles were used to support new needle and shoot production for
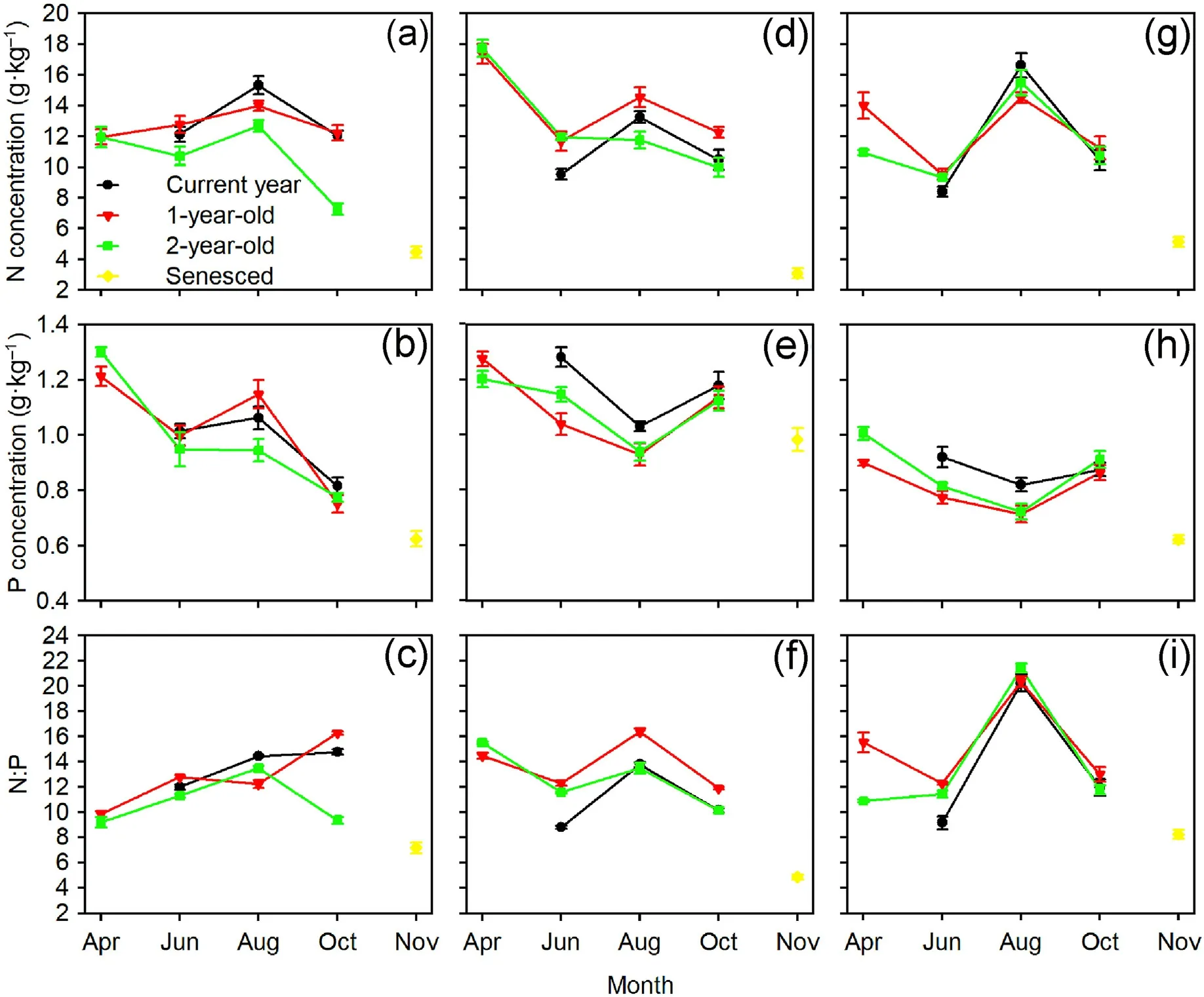
Fig.3.Seasonal variations of N and P concentrations and N:P ratios in needles of Mongolian pine (a, b, c), Japanese red pine (d, e, f), and Chinese pine (g, h, i).

Table 3F values and statistical significance of three-way analysis of variance on the effects of sampling time(S),needle age(A),tree species(T),and their interactions on N and P retranslocation efficiencies.
P.sylvestris var.mongolica,P.densiflora and P.tabuliformis.Similar results were found for other Pinus species,such as P.sylvestris(Wyka et al.,2016)and P.halepensis (de las Heras et al.,2017).The mobilization of N from older needles to new growth was confirmed by the tracking of an15N label in Pinus contorta(Mead and Preston,1994),and the removal of old needles inhibited the production of new needles(Millard et al.,2001).In fact, the formation of new needles is facilitated by the P pools stored in older needles from the previous growing season(Fircks et al.,2001).As photosynthetic activities are relatively weak during spring, only a small proportion of energy may be utilized for nutrient uptake by roots (Yan et al., 2016).For many evergreen coniferous trees, nutrient remobilization from needles for new growth at the beginning of the growing season occurs prior to the utilization of nutrients taken up by roots(Millard and Grelet,2010).Thus,living needles of the three Pinus species may serve as important N and P sources for the support of new growth.
All autumn NRE of the three Pinus species were greater than zero(Fig.4), and a significant decrease in N concentrations was observed from August to October(Fig.3).These results may be due to dilution by added structural materials, hydrolysis of organic N, and then the retranslocation of breakdown products(Chapin and Kedrowski,1983).In autumn,when N was not required for tissue growth,it cycled back from leaves and shoots to roots (Imsande and Touraine, 1994).Thus, the needles of the three Pinus species did not store N in autumn.
In this study,the autumn PRE was above zero for Mongolian pine but below zero for Japanese red pine and Chinese pine (Fig.4), which reflected the various P conservation strategies of different tree species.P concentrations in the needles of Mongolian pine declined from August to October,which indicated that P was transferred from the needles to other tissues in autumn.Decreased P concentrations induced the accumulation of carbohydrates, inhibition of photosynthesis, reduction of frost tolerance, and accelerated leaf senescence (Güsewell, 2004).The concentrations of P in needles of all ages for Japanese red pine and Chinese pine increased from August to October (Fig.3), which suggested that P accumulated and was stored in living needles during autumn.These needles served as P sinks and maintained high P levels prior to the onset of winter, which may have been related to the phospholipids that increased water mobility through membranes.This was advantageous for enhancing frost tolerance and delaying needle abscission (Chapin and Kedrowski,1983;Yuan et al.,2018).For boreal evergreen Pinus species,needle senescence typically occurs in autumn once aboveground growth has ceased (Ueda and Tokuchi, 2013).Although the three Pinus species possessed needles of three age classes in the Horqin Sandy Land, there were differences in their longevity.We observed that most needles of Japanese red pine and Chinese pine did not drop until the 3rd-growing season after November, however, some current year and 1-year-old needles of Mongolian pine were lost during winter.Needle P exports in autumn may be one of reasons that partial needles wither and fall off early.
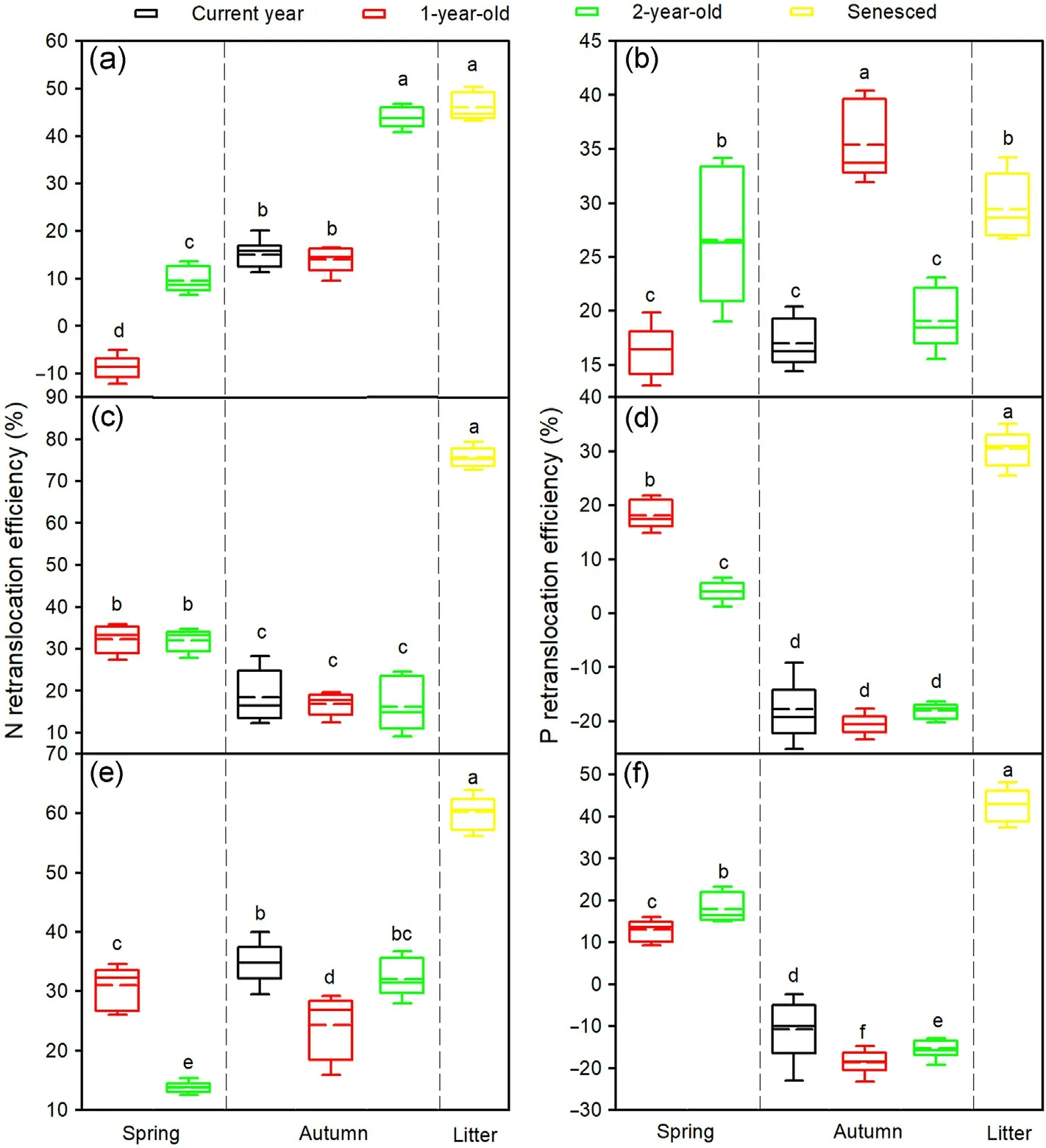
Fig.4.N and P retranslocation efficiencies in needles of Mongolian pine(a,b),Japanese red pine(c,d),and Chinese pine(e,f).Different letters within the same tree species indicate significant differences at the P <0.05 level.(For interpretation of the references to color in this figure legend,the reader is referred to the Web version of this article.)
We measured the NuRE during leaf senescence using matched adjacent pairs of green 2-year-old needles in October and senesced needles in November.This provided a more accurate measure of retranslocation prior to abscission, in contrast to previous research (Nambiar and Fife,1991; Saur et al., 2000).Japanese red pine and Chinese pine exhibited higher NRE and PRE in litter than those in spring and autumn (Fig.4),which implied the greater magnitude of retranslocation prior to needle abscission compared with seasonal retranslocation.However,Mongolian pine exhibited a similar or even greater magnitude of seasonal retranslocation than that prior to needle abscission(Fig.4),which indicated that the amounts of N and P retranslocated from young needles were much greater than that from senesced needles.Thus,the growth of Mongolian pine needles may be more dependent on the supply of seasonal N and P retranslocation from other needles compared with Japanese red pine and Chinese pine.
4.2.Needle age-related N and P retranslocation strategies of the three Pinus tree species
The needles of evergreen trees typically persist across several growing seasons, which lengthens the nutrient residence time; thus, improving the efficacy of nutrient utilization.The nutrient content and ability of retranslocation change with needle age due to nutrient allocation and transport among needles of different age classes(Wyka et al.,2016).We found higher NRE and PRE prior to needle abscission than in the previous years(Fig.5),which indicated that the N and P was more reabsorbed in the year of needle fall.The pattern of nutrient retranslocation among needle age classes contributed to maintaining a higher nutrient content and longer residence nutrient time in living needles;thus,improving the nutrient use efficiencies for internal recycling (Fife et al., 2008).There were differences in the maximum needle N and P contents among age classes for the three pine tree species, which led to variable resorption begin times.For Mongolian pine,N and P began to be resorbed since the needles were current year and 1-year-old, respectively, while for Japanese red pine, they were 1-year-old and current year, respectively.However, both N and P resorption in Chinese pine began in 2-year-old needles.These results were inconsistent with the previous studies on Mongolian pine(13-year-old)(Zheng et al.,2020)and Japanese red pine(80-year-old natural forest in Japan) (Han et al., 2008).Thus, the nutrient retranslocation process among needles of different age classes varied with tree species and age,forest type,and site,by affecting needle development and longevity.
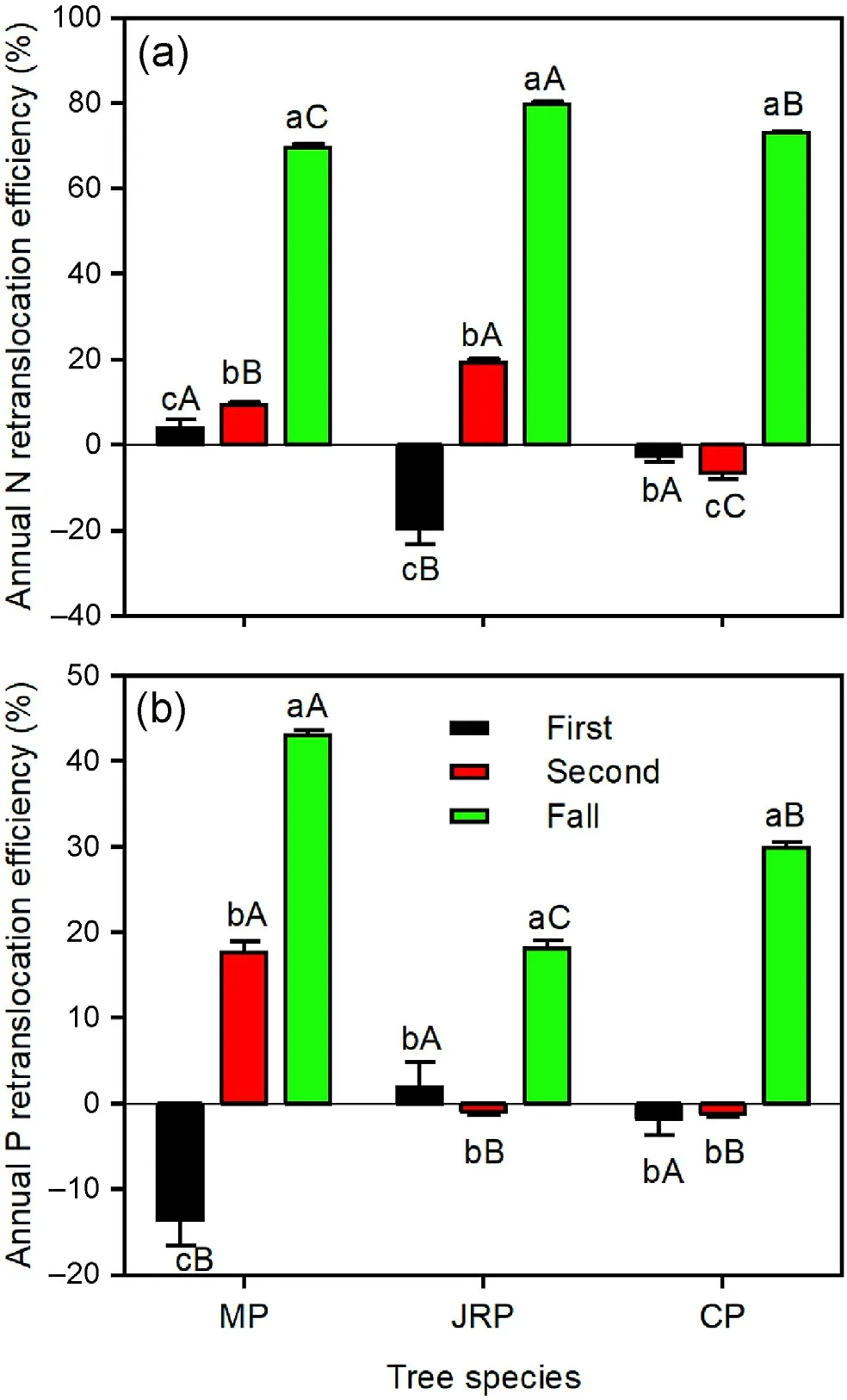
Fig.5.Annual N and P retranslocation efficiencies in needles of Mongolian pine(MP), Japanese red pine (JRP), and Chinese pine (CP).First, second, and fall represent nutrient retranslocation efficiencies.'First'refers to the retranslocation from the current year to the 1-year-old needles, 'second' refers to the transfer from 1-to 2-year-old needles,and'fall'indicates the retranslocation from 2-yearold needles to litter prior to leaf senescence.Different lowercase letters within the same tree species indicate significant differences among years,and different capital letters within the same year indicate significant differences among tree species at the P <0.05 level.(For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)

Table 4F values and statistical significance of repeated measures analysis of variance on the effects of sampling time (S), soil layer (L), tree species (T), and their interactions on soil N and P concentrations and their ratios.
The N and P dynamics with leaf aging were accompanied by seasonal accumulation, retranslocation, and replenishment cycles.Needles of different age classes may play various roles in nutrient consumption,allocation and storage, while nutrient source-sink relationships among needle age classes may vary with the seasons (Seidel et al., 2019).For example,1-year-old needles of Picea abies served as the primary nutrient reservoir for the new flush in spring, but 2- and 3-year-old needles contributed little (Weikert et al., 1989).However, the magnitude of N and P retranslocation in spring and autumn increased with needle age for Mongolian pine (Zheng et al.,2020).In this study, we found similar results with greater spring NRE and PRE in the 2-year-old than 1-year-old needles of Mongolian pine (Fig.4a and b), which indicated that the 2-year-old needles may have served as the main N and P pools for new needle production in spring.However,greater PREsecondand PREfallwere found in Mongolian pine compared with Japanese red pine and Chinese pine, implying that a higher proportion of P was removed from 1- and 2-year-old needles.Evergreen species stored nutrients in needles,whereas deciduous species employed their woody roots and older stems(Millard et al., 2001).The removal of old needles in evergreen species inhibited the generation of new needles.Thus, for Mongolian pine, old needles may contribute to new needle and shoot production in spring.However, to prevent the decline of Mongolian pine at the Zhanggutai town, some old needles and branches are often removed in stand management practice.The unreasonable artificial management would increase the loss of nutrients from trees, affect the spring growth of new needles, and even exacerbate the degradation of Mongolian pine plantation.
4.3.Nutrient use strategies of the three Pinus tree species
Soil available N:P ratio can directly reflect the ability of the soil nutrients to supply for plants(Yu et al.,2011);thus,it is a better indicator for the determination of soil nutrient limitations to plant growth, in contrast to plant N:P ratio (Wang et al., 2021a) and nutrient resorption(Han et al.,2013).Generally,the soil N:P ratio increased from the early growth to late growth periods in the three Pinus tree plantations(Fig.6),which signified that soil P limitation was enhanced with the progression of the seasons.Both current year and 1-year-old needle N:P ratios were significantly correlated with the soil N:P ratios in the Mongolian pine plantation (Fig.7a), implying that the growth of needles was primarily dependent on the supply of soil nutrients.In autumn,soil P limitation led to the removal of P from needles;thus, decreasing the needle P concentration, which may accelerate needle senescence and abscission (Güsewell, 2004).Further, the loss of these old needles of Mongolian pine would inhibit new needle growth in the following spring.These results likely contributed to the slow growth and sparse canopy in the Mongolian pine plantation, which are indicative features of growth decline.
For the Japanese red pine plantation,significant relationships in the N:P ratios were found only between the current year needle and soil(Fig.7b), which implied that the nutrients in young needles, not old needles, changed with the soil.Nutrients were assimilated and simultaneously used for growth in young needles; however,old needles served not only as a nutrient sink but also a nutrient source, thus, they may accumulate and disproportionately store N and P (Tang et al., 2019).Green needles increased P concentrations through retranslocation from other tissues in autumn.It was also observed that there were higher P concentrations and lower N:P ratios in the senesced needles of Japanese red pine compared with Mongolian pine and Chinese pine(Fig.3).These results implied that litter was easily decomposed; thus, more P would return to the soil (Kang et al., 2010), leading to a higher soil total P content in the Japanese red pine plantation than Mongolian pine plantation and Chinese pine plantation (Table 1).According to nutrient budgets, plants with low N:P ratios in the late growth period better retained nutrients during winter, which induced greater biomass in the following season (Güsewell, 2004).It was found that current year and 1-year-old needle N:P ratios in Japanese red pine were lower than those of Chinese pine and Mongolian pine in autumn (Fig.3), indicating that Japanese red pine would grow quickly in mature plantations.This inference was confirmed by Zhang(2017)that the annual volume growth of Japanese red pine was greater after the age of 32 compared with Chinese pine and Mongolian pine.
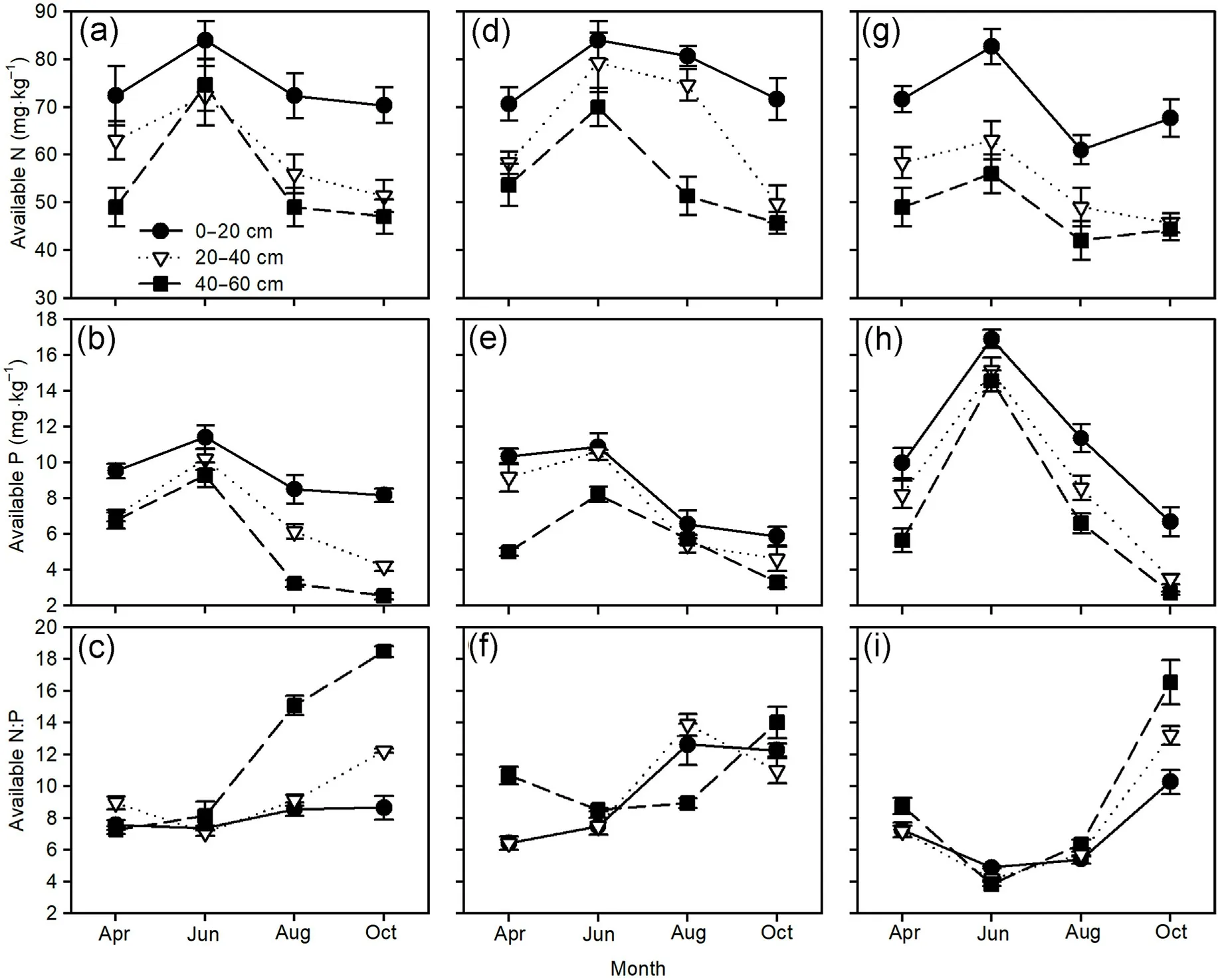
Fig.6.Seasonal variations of soil available N and P concentrations,and available N:P ratios(mean±standard error)of Mongolian pine(a,b,c),Japanese red pine(d,e, f), and Chinese pine (g, h, i) plantations.
Strict N:P homeostasis occurred in the Chinese pine plantation, and there were no significant relationships between the soil and needle N:P ratios (Fig.7c).This result signified that Chinese pine adopted a relatively conservative nutrient use strategy, where the internal N and P cycles of the trees were independent of the soil availability.The maintenance of stable stoichiometric ratios in organisms in arid and nutrientdeficient environments is beneficial for survival and development(Wang et al., 2021b).We found that the mean green needle N and P concentrations of Chinese pine(11.93 and 0.85 g·kg-1)were lower than those of Japanese red pine (12.78 and 1.12 g·kg-1) and Mongolian pine (12.10 and 1.00 g·kg-1).Chinese pine maintained relatively low green needle N and P concentrations by reducing growth (low height and DBH in Table 1), so that the carbon metabolism was not disturbed (Güsewell,2004).It was also found that there were higher mean N:P ratios in green needles(Fig.3)and PRE in litter(Fig.4)for Chinese pine compared with Mongolian pine and Japanese red pine, which implied relatively high P-use efficiency for growth(Güsewell,2004).
5.Conclusion
This study described the seasonal N and P retranslocation in needles of all ages for Mongolian pine,Japanese red pine,and Chinese pine in the Horqin Sandy Land of China.The living needles of the three tree species served as an important N and P source to support annual new growth in spring; however, they were not sinks for the storage of N in autumn.Among the needles of three age classes, the magnitude of N and P retranslocation prior to needle abscission were greater than those in the previous years,which improved the N and P use efficiencies of trees.For Mongolian pine, seasonal N and P retranslocation from old needles played an important role for the growth of new needles.Under enhanced P limitation as the seasons progressed, the three Pinus tree species adopted various nutrient use strategies, which may have contributed to forest decline.The growth of Mongolian pine was essentially dependent on the supply of soil nutrients.A decrease of P in green needles in autumn may have accelerated needle senescence and abscission,while the loss of old needles further inhibited new growth in the following spring.Thus,the phenomenon of slow growth and sparse canopy occurred in Mongolian pine plantation.Japanese red pine increased the green needle P concentrations via retranslocation from other tissues during autumn.High P concentrations and low N:P ratios in senesced needles contributed to improving litter decomposition, and thus the soil P content.Chinese pine maintained N:P homeostasis and relatively low N and P concentrations in green needles, which made the trees less dependent on soil nutrient supply.The trees exhibited more developed internal P cycles by increasing P withdrawal prior to needle abscission and improving high P use efficiency.The old needles of Pinus tree species should not be removed, and the application of P fertilization would alleviate the decline of Mongolian pine plantation.
Data availability
Data are available on request from the authors.
Funding
This work was supported by the National Natural Science Foundation of China(Nos.32271846,31400613),and the Key Program of Education Department of Liaoning Province(No.LJKZZ20220050).
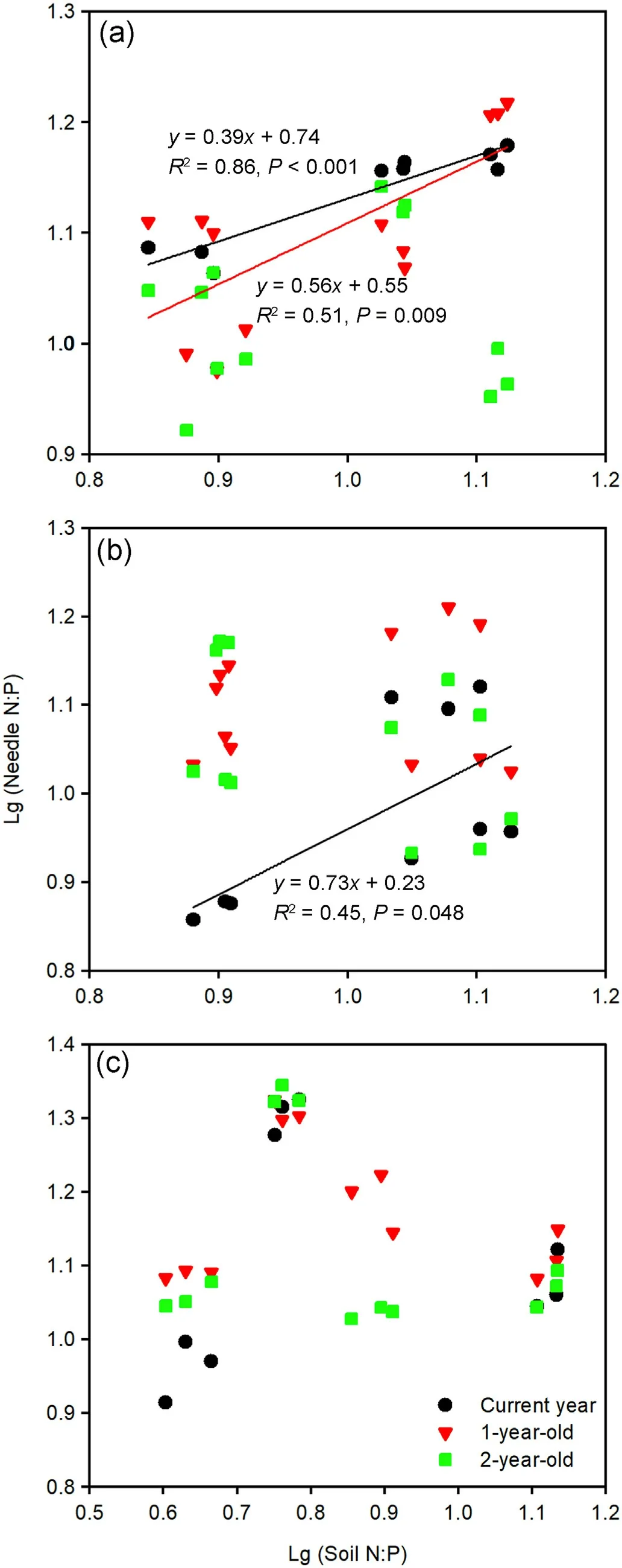
Fig.7.Relationships between needle and soil N:P ratios in Mongolian pine(a),Japanese red pine (b), and Chinese pine (c) plantations.(For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
CRediT authorship contribution statement
Chang Liu:Data curation, Writing - original draft.Kai Wang:Conceptualization, Methodology, Writing - review & editing.Hon
gzhang Kang:Methodology,Writing-review&editing.Baoming Du:Methodology,Writing-review&editing.Risheng Zhang:Investigation,Resources.Shanshan Tai:Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Hong Lei,Chengjiao Zhao and Yihang Li for assistance with the field and laboratory work.
- Forest Ecosystems的其它文章
- Divergent responses of Picea crassifolia Kom.in different forest patches to climate change in the northeastern Tibetan Plateau
- Tree-based ecosystem services supply and multifunctionality of church forests and their agricultural matrix near Lake Tana, Ethiopia
- Influence of climate fluctuations on Pinus palustris growth and drought resilience
- Book review “Continuous Cover Forestry - Theories, Concepts, and Implementation” by Arne Pommerening
- Impact of black cherry on pedunculate oak vitality in mixed forests:Balancing benefits and concerns
- Sensitivity of forest phenology in China varies with proximity to forest edges

