Influence of climate fluctuations on Pinus palustris growth and drought resilience
João Cmpô, Joshu Puhlick
a CENSE - Center for Environmental and Sustainability Research, CHANGE - Global Change and Sustainability Institute, Department of Environmental Sciences and Engineering, NOVA School of Science and Technology, NOVA University Lisbon, Campus de Caparica, 2829-516 Caparica, Portugal
b The Jones Center at Ichauway, 3988 Jones Center Drive, 39870, Newton, GA, USA
Keywords:Longleaf pine Slash pine Pinus elliottii Climate change Dendrochronology Dendroclimatology Forest disturbances
ABSTRACT The longleaf pine(Pinus palustris Mill.)ecosystem is an endangered ecosystem in the southeastern USA,and efforts to restore the species are ongoing.However, in recent decades, the region has experienced drastic fluctuations between wet and dry growing season conditions from year to year, and it is not fully understood how these fluctuations have influenced the growth of P.palustris.To address this topic, we cored P.palustris trees in woodlands of southwest Georgia and used dendrochronology techniques to determine how climate fluctuations have influenced the growth and drought resilience of P.palustris.We also cored slash pine(Pinus elliottii Engelm.)trees in the same woodlands to compare growth between species.While P.palustris growth was less impacted by adverse climate conditions compared to P.elliottii, the strength of correlations between P.palustris growth and temperature, precipitation, and Palmer Drought Severity Index (PDSI) changed over time.In recent decades,climate conditions during the growing season became more influential on P.palustris growth than the previous year's conditions.This is concerning given that drought severity during the growing season has been increasing.Our results also indicate that P.palustris was less resilient to droughts during the 2000s and 2010s than to those of the 1950s.Under this new climate paradigm, our results suggest that P.palustris might be more susceptible to growth reductions and less resistant to droughts than once expected.This work highlights the importance of understanding the impact of novel climate conditions on P.palustris and has implications for restoration efforts,such as using silvicultural treatments that reduce tree vulnerability to drought(e.g.,thinning)and promote other climate-adapted species in mixture with P.palustris.
1.Introduction
Forests provide numerous ecosystem services to society, such as carbon storage and clean water, and they maintain ecosystem function(Brockerhoff et al., 2017).Temperature and precipitation are principal drivers of forest ecosystem function (Menezes-Silva et al., 2019;Roces-Díaz et al., 2021), and changes in the climate regime can affect functionality by increasing the frequency and intensity of forest disturbances (Case et al., 2021).For example, changes in climate regime can predispose trees to be attacked by insects and pathogens,which can lead to tree growth reductions and mortality (Dale et al., 2001; Seidl et al.,2017).Changes in the climate regime can also alter the duration of the growing season (Nabais et al., 2014; Caminero et al., 2018), which can affect tree phenology and productivity (Case et al., 2021).Given these impacts, it is important to understand how changing climate regimes influence tree growth and mortality so that this information can inform decisions about managing forests.Our study aims to address these knowledge gaps within the context of the longleaf pine (Pinus palustris Mill.)ecosystem in the southeastern USA.This endangered ecosystem is valued for its intrinsic biological diversity including numerous species of flora and fauna of conservation concern.Prior to European settlement,the P.palustris ecosystem occupied 37 million ha (Frost, 2006).P.palustris and P.palustris/oak (Quercus spp.) forests now occupy about 2.29 million ha (Puhlick et al., 2022).Fortunately, there have been numerous actions to promote the restoration of P.palustris on public and private lands (McIntyre et al., 2018; Holland et al., 2019; Harley et al.,2023).
There has been much research on the influence of temperature and precipitation on P.palustris growth using dendrochronology methods.While some of these studies indicate that warmer temperatures are correlated with increased growth (Foster and Brooks, 2001; Pederson et al.,2008;Bhuta et al.,2009;Zampieri and Pau,2022),the majority of studies suggest that precipitation is more influential on growth than temperature, particularly summer and fall precipitation (Harley et al.,2023).Most of these findings were based on climate averages over long periods of time(often >100 years)and did not account for differences in climate regimes over shorter time spans (e.g., multiple decades).Accounting for these differences could be important for the P.palustris ecosystem given fluctuations in the regional climate record (Dai, 2013;Konrad and Fuhrmann, 2013; Peters et al., 2015).Therefore, it is important to understand how relatively short-term (decades) regional and local climate fluctuations have influenced P.palustris growth.
Droughts also have a major impact on forest health(Dale et al.,2001;Seidl et al., 2017), and they have been increasing in intensity and frequency across the globe (Mishra and Singh, 2010; Dai, 2013).These novel conditions have contributed to a decrease in tree growth and an increase in tree mortality (Kloeppel et al., 2003; Klos et al., 2009; Case et al., 2021), and growth reductions can persist long after the drought events(Anderegg et al.,2015;Camarero et al.,2022).In the southeastern USA, there has been an increase in the occurrence of extreme droughts since the late 1980s (Konrad and Fuhrmann, 2013; Peters et al., 2015),which have altered forest composition and structure(Clark et al.,2016).Indices of relative soil moisture conditions, such as Palmer Drought Severity Index (PDSI) (Palmer, 1965), could be used to quantify the impacts of drought on P.palustris growth.Also,trees in regions with more frequent and intense droughts have been shown to have lower resilience to drought in terms of their ability to obtain growth rates similar to those before drought(Gazol et al.,2018;Bose et al.,2020).These same studies also evaluated the ability of trees to resist (i.e., no change in growth during a drought event) and recover from droughts (i.e., post-drought growth increment).Hence, there is an opportunity to use indices of tree growth resiliency following drought, in addition to PDSI, to more fully understand P.palustris growth dynamics.
When assessing the capacity of P.palustris to respond to changing climate conditions, it may also be useful to make growth comparisons with other coexistent tree species.Many pine species have different life traits, which can lead to distinct climate-growth relationships and potentially different responses to adverse climate conditions.For example, P.palustris can coexist with other southern pine species like slash pine (P.elliottii Engelm.) and loblolly pine (P.taeda L.), but P.palustris has little aboveground growth until it emerges from the grass stage.After the grass stage, diameter growth rates can equal or even surpass the rates of other southern pines on certain sites (Alavalapati et al.,2002;Kush et al.,2004).Distinct growth strategies,such as slower growth which is a conservative resource strategy, may enable trees to better cope with droughts compared with fast-growing trees (Chapin et al.,1993;Reich et al.,2003;Bose et al.,2020).Comparisons of growth between species with different life traits,and on the same site conditions,are needed to determine the ability of different tree species to adapt to and persist under changing climate regimes.
The specific objectives of our study are to determine: 1) how P.palustris growth was influenced by climate and droughts from 1930 to 2020;2)if P.palustris was better adapted to climate fluctuations than the coexistent pine species P.elliottii; and 3) the resilience of P.palustris to droughts during two time periods of severe drought.In doing so, this work aims to contribute to our understanding of the influence of novel climate conditions on P.palustris growth dynamics,which is necessary for improving forest vulnerability models and forest management practices to accomplish P.palustris restoration objectives.
2.Materials and methods
2.1.Study area
The study was located at The Jones Center at Ichauway, in the Gulf Coastal Plain of southwestern Georgia.Soils are predominately welldrained loamy sands over sandy loams.The climate features long, hot summers and cool, short winters.Beginning in 1928, Mr.Robert W.Woodruff began acquiring local farms and land parcels to form a quail(Colinus virginianus) hunting plantation that now makes up Ichauway.The 11,740-ha property contains 7,285 ha of second-growth, naturally regenerated, P.palustris-dominated stands (Holland et al., 2019).From the late 1920s until 1994, annual, early spring (March and April)low-intensity prescribed fire was used to maintain quail habitat.Since 1994, a two-year fire return interval has been utilized on most of the property to primarily increase P.palustris regeneration.Dormant season burning continues to be important(65%of burning within a given year),but seasonality and frequency of burning have been variable across the property (Rutledge and McIntyre, 2022).Because of the relative similarity of burning across the property,we did not evaluate the influence of prescribed fire use on P.palustris growth.Additionally,researchers found no statistically significant differences among the productivity of mature P.palustris trees in stands that were burned biennially but in different seasons(Willis et al., 2021).
Our study involved coring trees on a subset of plots that are part of the long-term monitoring program at Ichauway(Holland et al.,2019).At the start of the program,eight panels of 108 monitoring plots were selected using a Random Tessellation Stratified design (Stevens, 1997).Specifically,this was a grid-based design,and a proportionally greater number of plots were selected from underrepresented communities (in terms of cumulative area)to ensure adequate representation of all communities in each panel.Since 2002, two panels have been sampled every year,resulting in a complete inventory of all 864 plots every four years.For a 0.1-ha sub-plot within each plot,trees ≥10 cm diameter at breast height(DBH;stem diameter at 1.37 m)were assigned a condition code(i.e.,live,standing dead, downed dead, or harvested) and mapped (azimuth and distance from sub-plot center).DBH and species were recorded.For trees within a nested 0.025-ha sub-plot core plot (sharing the same center as the 0.1-ha sub-plot), crown class (i.e., open-grown, dominant,co-dominant, intermediate, suppressed) and defects (e.g., fire scars and broken tops)were also recorded.
2.2.Sampling and dendrochronology procedures
In ArcMap 10.8.1, we selected plots from the long-term forest monitoring program that occurred within sandridge and upland ecotypes and that were established at the start of the long-term monitoring program.Within each sub-plot of these plots, we selected the largest (in diameter) open-grown, dominant, or co-dominant P.palustris and P.elliottii trees without defect,within 9 m of sub-plot center.Because only five of the plots that met these criteria had P.elliottii, we cored an additional 21 P.elliottii trees from an area of the property that was dominated by overstory P.elliottii and had site conditions that matched our criteria (Fig.1).For these 21 trees, a wedge prism (10-basal area factor,English)was used to quantify the total basal area from a random point that was established 1 m from the cored tree.Using an increment borer with 5.15 mm diameter,two cores were taken perpendicular to one another at breast height.The cores were mounted in wooden supports,gradually sanded with 120, 220, 320, and 400 grit sandpaper, and scanned with an Epson Expression 12000XL.Tree ring boundaries were delineated and then cross dated using the dendrochronological software CooRecorder and CDendro 9.6; cross-dating was also verified with COFECHA software (Holmes, 1983).We dated a total of 42 P.palustris and 26 P.elliottii.
Detrending was done according to methods by Nabais et al.(2014)to remove the influence of age-related growth trends using the packages dplR (Bunn, 2008) and detrendeR (Campelo et al., 2012) in R freeware program.Specifically,a negative exponential or a straight line with slope≤0 was fitted to each individual ring-width series.Then, we applied a spline with a 50% frequency cutoff and a length of 67% of the mean sample length(Klesse,2021).To the detrended series,an autoregressive model was fitted to remove the influence of the previous year.Finally,we applied a biweight robust estimate of the mean to obtain a residual chronology for each species.
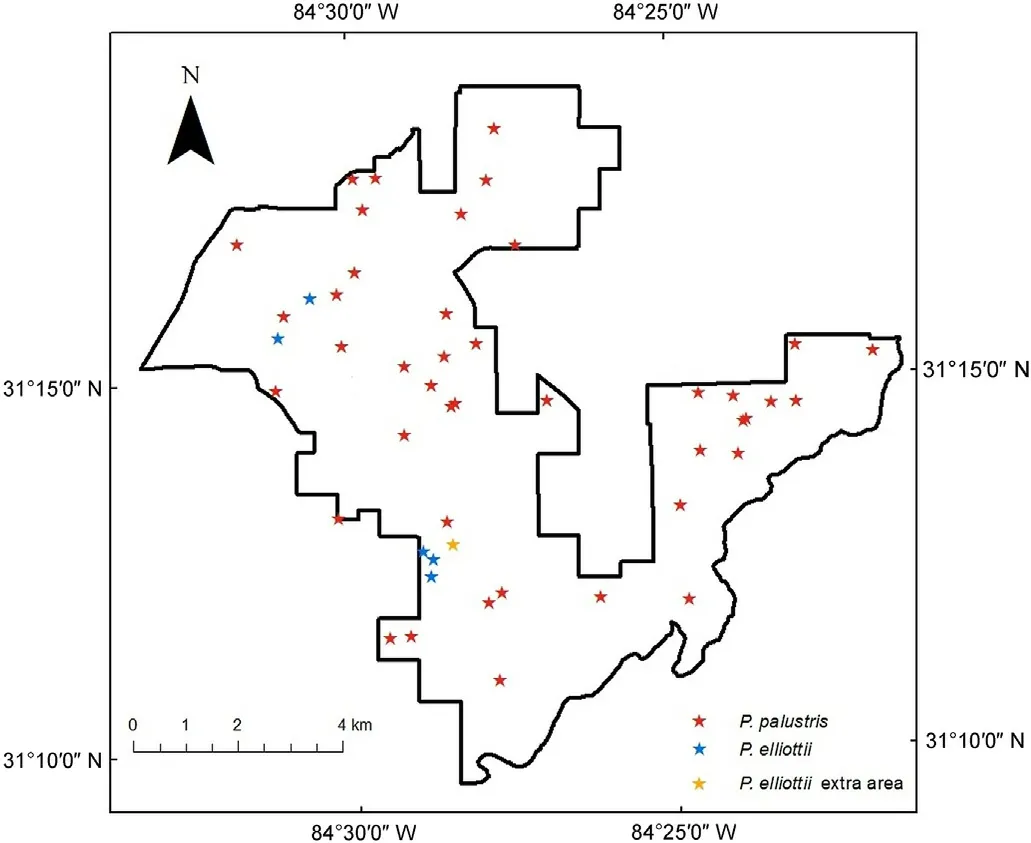
Fig.1.Distribution of sampled trees across The Jones Center at Ichauway in southwestern Georgia,USA.Red and blue stars indicate plots from the long-term monitoring program.The orange star indicates an extra area where 21 P.elliottii trees were sampled.(For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The residual chronologies were evaluated by calculating several dendrochronological statistics: the first-order autocorrelation (AC) of raw and residual series which indicates the year-to-year growth similarity;the mean correlation between trees of raw and residual series(rbt)which indicates how similar growth is among trees;signal-to-noise ratio(SNR)which indicates the strength of the common climate signal among trees; and the expressed population signal (EPS) which assures that the quality of the residual chronology for climate analysis must be at least 0.85.
2.3.Climate-growth relationship
Monthly precipitation and mean temperature data were obtained from PRISM Climate Group (2023) and monthly PDSI values were obtained from the National Oceanic and Atmospheric Administration National Centers for Environmental Information(2023).Based on the span of years covered by our sample trees,we chose to evaluate the influence of climate on tree growth from 1930 to 2020.For each of these years,we calculated the deviation from the average condition(anomaly).Then, a polynomial line was fitted to each climate variable to determine the trend for the climate anomaly.The climate variables were also averaged on an annual basis,as well as for the growing season which was considered as being from May to September(Klos et al.,2009).
To identify which climate variables had the most significant influence on tree growth and to evaluate how these correlations changed over time,we used the dcc function from the package treeclim in R (Zang and Biondi, 2015).The function generates a static correlation and also performs a 35-year moving window correlation analysis between the residual chronologies and climate variables.Since climatic conditions in the year prior to the growth can affect the growth, we evaluated correlations between a given year's ring width and climatic conditions for each month from the previous September to October of the current growth year.
2.4.Drought response indices
To identify the drought years, the PDSI values for the months of the growing season were averaged because this is the period when drought most affects tree growth (Klos et al., 2009).To assess the response capacity of species to droughts between two time periods with multiple droughts, in R, a linear model was performed for P.palustris, and a hierarchical ANOVA was performed for P.elliottii.A linear model was used for P.palustris to test for an ecotype effect.A pairwise comparison with Tukey adjustment was used to assess differences between means.Some drought indices were transformed to approximate normal distributions.The first period chosen was in the 1950s, and the other was in the 2000s/2010s.In each period, drought episodes with the lowest PDSI value were chosen(PDSI1951=-2.872,PDSI1954=-3.626,PDSI2000=-4.544, PDSI2007= -4.43, PDSI2011= -4.462) so that we could examine the influence of droughts that occurred within a relatively short time period of one another.Such an analysis is important because of increasing drought frequency.Drought response indices were nested within the correspondent period.
3.Results
For the 90-year period examined in this study (1930-2020), both mean annual and growing season temperature have exhibited a nonlinear trend at Ichauway (Fig.S1).There are many values above the mean at both extremes of the study period, and a dip in the middle.However,there is much variability around this general trend.Both precipitation(Fig.S2) and PDSI values (Fig.S3) also indicate high variability during the study period.However,in the last three decades the growing season shows a lower positive anomaly for precipitation,and a higher negative anomaly for relative soil moisture conditions.
The chronologies of both tree species had a similar timespan, 126 years for P.palustris and 114 years for P.elliottii.The correlation between the raw growth series, the correlation between the residual series, and the first-order autocorrelation of the raw and residual series were higher for P.elliottii than for P.palustris.P.palustris showed a higher value for SNR and EPS.Both species had an EPS higher than 0.85 (Table 1).P.elliottii had a higher growth rate compared with that of P.palustris(Fig.2).For the most recent inventory of permanent plots on which P.palustris trees were cored,there were no noticeable differences in mean tree density and dominance between sandridge and upland ecotypes(Table 2).P.elliottii trees ≥10 cm were not detected on plots where P.palustris trees were cored.For the 21 locations that were not associated with the long-term monitoring program and where P.elliottii trees werecored, total basal area was 6.8 ± 3.1 m2·ha-1(mean ± standard deviation) and ranged from 2.3 to 16.1 m2·ha-1.All of the P.elliottii trees selected for coring occurred within the upland ecotype.The range of ages at DBH for all P.palustris and P.elliottii were 50-127 and 49-115,respectively.

Table 1Descriptive statistics for P.palustris and P.elliottii.Dendrochronological statistics are for the study period 1930-2020.rbt, mean correlation between trees; AC,first order auto-correlation; SNR, signal-to-noise ration; EPS, expressed population signal.
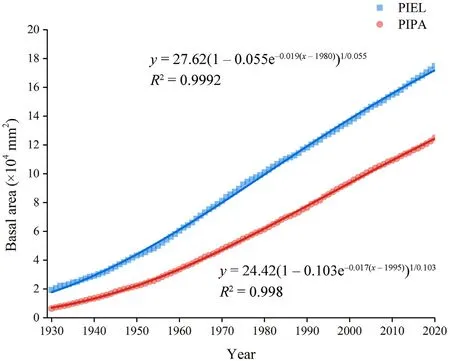
Fig.2.Average basal area increment of P.palustris(PIPA)and P.elliottii(PIEL).Squares and circles represent the average basal area.Solid lines represent the Chapman-Richards curve with the respective equation indicated beside it.

Table 2Mean (standard deviation) and range of stand attributes by ecotype (i.e., sandridge or upland) and plot type (i.e., P.palustris chosen for coring or P.elliottii chosen for coring).Statistics were derived from the most recent inventories of trees ≥10 cm diameter at breast height(DBH)on a 0.1-ha sub-plot of long-term forest monitoring plots at Ichauway.Age was determined from coring at breast height (1.37 m)in 2023.
The correlations between ring width and the various climate variables were fairly similar for both tree species.For P.palustris, ring width was positively correlated with the previous September's temperature and was negatively correlated with the current year's August temperature(Fig.3).However, these correlations were not constant over time (Fig.S4).For example, the negative correlation between ring width and the current year's August temperature became stronger in years after the 1990s.July and September temperature also became more influential on P.palustris ring width after the 1990s.For P.elliottii, ring width was negatively correlated with July, August, and September temperature (Fig.3), and the negative correlation between ring width and July and August temperature tended to be constant over time(Fig.S5).
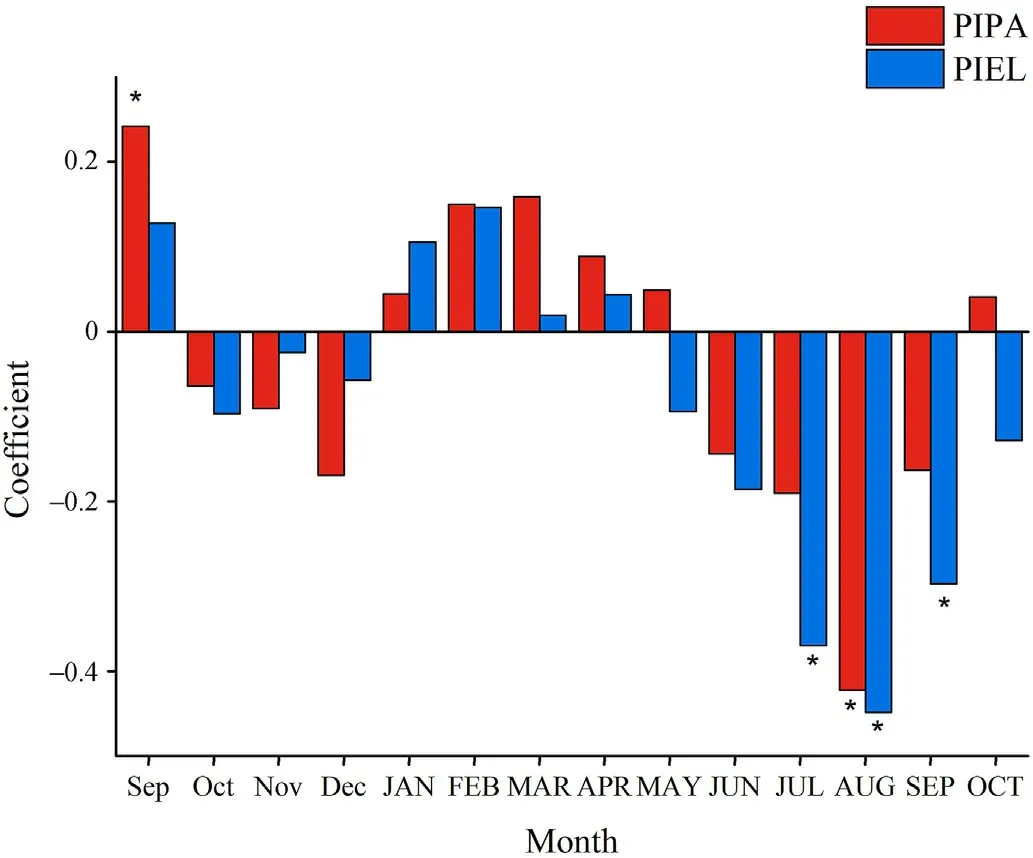
Fig.3.Correlation between tree-ring width residual chronologies and monthly temperature data for P.palustris (PIPA)and P.elliottii(PIEL).Lowercase months refer to the year previous to the current year's growth.Asterisks indicate that correlations are significant at the 95% level.
For both species, ring width was positively correlated with April,July, and September precipitation (Fig.4).Additionally, for P.elliottii,ring width was positively correlated with March, May, and June precipitation, and was negatively correlated with the previous year's December precipitation.For P.palustris,the positive correlation between ring width and April and July precipitation was more prevalent for years after the 1980s (Fig.S6).For P.elliottii, these same correlations were relatively constant and significant over time (Fig.S7).For both species,the correlation between ring width and the current year's September precipitation was only significant during the beginning of the study period,and the correlation between P.palustris ring width and previous year's September precipitation was only significant from approximately 1950-1990.

Fig.4.Correlation between tree-ring width residual chronologies and monthly precipitation data for P.palustris(PIPA)and P.elliottii(PIEL).Lowercase months refer to the year previous to the current year's growth.Asterisks indicate that correlations are significant at the 95% level.
For both species, ring width was negatively correlated with the previous year's September PDSI, and positively correlated with monthly PDSI from June to October(Fig.5).Additionally, P.palustris ring width was negatively correlated with previous year's November PDSI, and P.elliottii ring width was positively correlated with April and May PDSI.The negative correlation between P.palustris ring width and PDSI during the previous year's fall months was relatively constant over time.However, the positive correlation between P.palustris ring width and PDSI during the current year's summer months only became prevalent half way through the study period (Fig.S8).P.elliottii displayed more constant correlations over time,in particular,for the summer/fall months.
For P.palustris, ecotype was not a statistically significant factor in models of growth responses to drought.Additionally,we did not detect a statistically significant difference in P.palustris drought resistance between two time periods of severe drought conditions(Table 3).However,for the droughts in the 1950s,the species had a greater resistance index in 1951 compared that of 1954(Fig.6a).Furthermore,during the period of severe drought in the 2000s and 2010s, the species had a greater resistance index in 2000 in comparison to drought in 2007 (Fig.6a).Trees had a greater capacity to recover from droughts in period 1 than in period 2,with the response in each year being similar within each period(Fig.6b).Trees were also more resilient in period 1 than in period 2.During period 2, trees had a lower resilience capacity to the 2007 drought than to the others (Fig.6c).Similarly, P.elliottii was also less resilient to droughts during the 2000s and 2010s compared to those of the 1950s (Table 3, Fig.6f).However, P.elliottii was slightly more resistant in the second period (Table 3, Fig.6d).While there was no difference in P.elliottii recovery capacity between the two periods,trees had a greater ability to recover from the 1954 drought than the 1951 drought(Fig.6e).
4.Discussion

Fig.5.Correlation between tree-ring width residual chronologies and monthly PDSI data for P.palustris(PIPA)and P.elliottii(PIEL).Lowercase months refer to the year previous to the current year's growth.Asterisks indicate that correlations are significant at the 95% level.

Table 3Linear model results for drought indices for P.palustris and hierarchical ANOVA for P.elliottii.For P.palustris, resistance and resilience indies were log transformed and recovery indices were inversed transformed.For P.elliottii,recovery and resilience indices were square root transformed.The 1951 and 1954 drought years were nested in period 1,and the 2000,2007 and 2011 drought years were nested in period 2.DF,degrees of freedom;SS,sum of squares;MS,mean squares;F,F-value; P,P-value.
This work aimed to determine the long-term (1930-2020) influence of various climate variables on growth of P.palustris,which is considered to be a species that is tolerant of drought in the southeastern USA.We also evaluated how the growth of P.palustris was influenced by recent(since approximately the 1990s) climate fluctuations and increase in drought conditions.We found that P.palustris and P.elliottii were less resilient to frequent and intense droughts that have occurred since the 2000s in comparison to droughts that occurred during the 1950s.An important consideration is that our study involved examining the growth response of the same trees over time,so it is possible that as trees aged,they became less resilient to droughts.In a global analysis of drought responses by various tree species,researchers found that younger trees in upper canopy positions were more resilient to droughts than older trees in upper canopy positions(Au et al.,2022).However,even though older trees may be less resilient to droughts, they can be more resistant to drought conditions (Au et al., 2022).In the short term, this means that older trees can buffer the adverse impacts of drought on carbon stocks and accumulation in uneven-aged stands.
While we found that P.palustris and P.elliottii trees were less resilient to drought over time,there was not a statistically significant difference in P.palustris resistance to droughts that occurred during the 1950s and those that have occurred since the 2000s.In contrast,P.elliottii trees had a greater resistance to more recent droughts.Again,this could be due to tree age,and agrees with findings by Au et al.(2022)that younger trees are less resistant to drought than older trees.In a study of P.palustris growth response to a severe drought in Alabama, trees ≤55 years old were more sensitive to drought conditions than trees ≥80 years old(Goode et al.,2019).Both of these studies and ours suggest that there are benefits to having multiple cohorts of pine within the same stand.Older pines can minimize short-term impacts of drought on stand productivity and carbon sequestration, while resilient younger pines that are able to better recover from drought conditions can contribute towards long-term productivity and carbon accumulation.A final point about considering differences in growth responses between the two time periods is that extreme drought conditions (<-4 PDSI) in 2000, 2007, and 2011 occurred earlier in the growing season (May or June) than the 1950s droughts, and these extreme drought conditions extended into fall months.
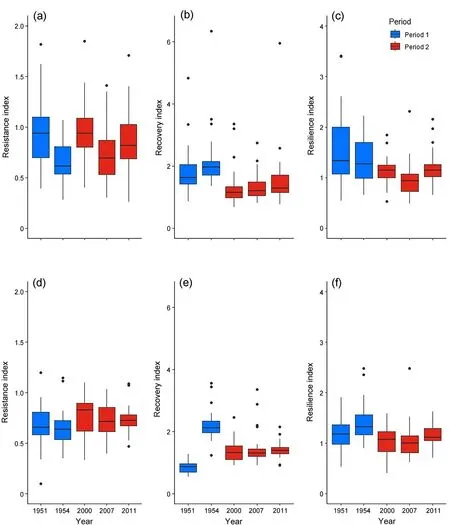
Fig.6.Drought response indices for P.palustris(a,b and c)and P.elliottii(d,e and f).(a)and(d),Resistance Index(Rt);(b)and(e),Recovery Index(Rc);(c)and(f),Resilience Index (Rs).P.palustris: Rt1954 >Rt1951, t179 = 4.18, P <0.001; Rt2000 >Rt2007, t179 = 3.73, P = 0.002; Rs2000 >Rs2007, t179 = 2.87, P = 0.037; Rs2011 >Rs2007,t179=-3.36,P=0.008.P.elliottii:Rc1954 >Rc1951,t121=-13.091,P <0.001.t indicates the degree of freedom of the shown t-statistics for the variable tested.
In addition to tree age, prescribed burning could also influence tree growth responses to drought.Goode et al.(2019) compared P.palustris growth response to drought between unmanaged stands and stands managed with both prescribed fire and tree cutting.Growth response to drought was not influenced by management but was influenced by tree age.Although, we did not include prescribed fire in our models of P.palustris growth responses to droughts in the 1950s and 2000s/2010s because of a lack of burn records before 1994,we tested for the influence of prescribed fire and tree age on growth responses to drought in 2000.Ecotype(i.e.,sandridge or upland),time since prescribed fire use before the 2000 growing season, tree age, and their interactions were not statistically significant in models of resistance, recovery, or resilience.Future research should consider the influence of other metrics for prescribed fire(e.g.,that incorporate fire intensity or the cumulative effects of multiple fires)and a wider range of tree ages on tree growth response to drought.
The negative correlation between P.palustris growth and summer temperatures is consistent with the findings of other studies(Henderson and Grissino-Mayer, 2009; Mitchell et al., 2019; Soulé et al., 2021;Stambaugh et al., 2021).This correlation may be explained by the fact that to avoid water loss,trees close their stomata,which in turn reduces their photosynthetic activity, and consequently leads to growth reductions (Granda et al., 2014).The positive correlation between the previous year's September mean temperature and P.palustris growth may indicate that higher September temperatures could promote the production of carbohydrates allocated to reserves that are used during the following year's growing season (Meldahl et al., 1999; Henderson and Grissino-Mayer,2009).
Our finding of a positive correlation between P.palustris growth and precipitation is likely related to soil moisture availability(Meldahl et al.,1999; Foster and Brooks, 2001).Water availability to trees is an important driver for cambium activity, and ample winter and early spring precipitation promotes the development of earlywood (Henderson and Grissino-Mayer, 2009).It can also be important for the development of latewood (Henderson and Grissino-Mayer, 2009), as is summer and fall precipitation (Foster and Brooks, 2001; Henderson and Grissino-Mayer,2009; Mitchell et al., 2019; Soulé et al., 2021; Stambaugh et al., 2021).Since P.palustris growth invests more in latewood(Harley et al.,2023),wet summer and fall are essential to cope with high temperatures, and thus prolong the growth season,and with an increase in growth.Ample and persistent precipitation for P.palustris growth may be even more important at our study site because stands are burned biennially.Frequent burning may reduce the soil moisture holding capacity by exposing bare mineral soil to rainfall causing aggregate clogging of soil pores and by increasing soil moisture evaporation from blackened soil surfaces.However,the low tree densities of these open stands may result in greater soil moisture availability to individual trees because of less competition among trees for soil water.This may depend on the amount of light reaching the forest floor and amount and type of understory vegetation such as perennial grasses.
Other researchers have also documented the influence of summer droughts on P.palustris growth reductions (Meldahl et al., 1999; Foster and Brooks,2001;Henderson and Grissino-Mayer,2009;Mitchell et al.,2019; Soulé et al., 2021; Stambaugh et al., 2021).Summer drought conditions can inhibit cambium activity(Deslauriers et al.,2016)and the allocation of carbohydrates to produce new tissues (Henderson and Grissino-Mayer, 2009).Our finding of a negative correlation between growth and the previous fall's PDSI values is consistent with other studies(Meldahl et al., 1999; Foster and Brooks, 2001; Henderson and Grissino-Mayer, 2009).This correlation could be related to our observation that recent drought years have been followed by excessively wet years, which would be conducive to increased tree growth.
In our study,P.elliottii had greater growth rates than P.palustris,but the growth of P.elliottii was more limited by high temperatures during the summer and early fall and was more dependent on precipitation than P.palustris.P.elliottii has a greater mean daily transpiration rate compared to P.palustris(Gonzalez-Benecke et al.,2011),which partially explains why the growth of P.elliottii would be more limited by high temperatures.P.elliottii also has a greater net carbon exchange during spring (Clark et al., 1999), and is perhaps why it depends more on increased levels of soil moisture than P.palustris (Foster and Brooks,2001).Another difference between these species was the lower mean correlation among tree ring series for P.palustris in comparison to the mean correlation among series for P.elliottii, which could indicate that P.palustris is more sensitive to climate variations and drought across individual stems.
Although it is commonly suggested that P.palustris is able to cope with adverse climate conditions,our analysis showed that the correlation between growth and climate has changed over time.During the first half of our study period,the principal drivers of P.palustris growth were the previous year's fall and winter precipitation, and precipitation during February and September of the current year.In contrast, elevated temperatures during the previous year's December and current year's month of August, as well as drier conditions during the fall of the current year were negatively with tree growth.Also, the negative influence of the current year's June to September temperatures on growth may be a concern for future resiliency of P.palustris if the current climate trends continue or intensify.We acknowledge that as the trees of this study aged, they may have become more sensitive to weather extremes.This lends support to promoting multiple age classes of P.palustris within stands to buffer the uncertainties of future climate conditions and to anticipate potentially different growth responses based on tree age.
Over the course of the study period,the correlation between growth and relative soil moisture conditions also changed and low PDSI levels started to limit the growth of P.palustris from late spring onwards.Our results indicate that this novel reality of drier and warmer conditions led to a change in the climate-growth relationship from one of importance on previous years climate conditions on growth, to one of dependence on climate conditions during the growing season, especially since summer.These new climate conditions might favor greater investment in latewood development as opposed to earlywood,which agrees with findings by Stambaugh et al.(2021).However, this greater dependence on the growing season climate can be a disadvantageous situation.As fall warmer and drier conditions are outweighing the positive influence of precipitation, along with a potential shortening of the growing season under future climatic conditions, future growth reductions can be expected.Consequently,this can aggravate the capacity for growth during and after drought periods,which might affect the resiliency of P.palustris.Although we did not detect differences in the capacity of P.palustris to resist drought between the two time periods (i.e., the 1950s and the 2000s/2010s), for years within each time period the lower capacity to resist subsequent droughts may have been due to low water availability.This is because these years were followed by years with some of the lowest PDSI values.Future scenarios with more prolonged droughts could also lead to a decrease in resilience.Finally,these results highlight the utility of using PDSI instead of only temperature and precipitation.
5.Conclusion
The recent climate regime of our study area is one of more drastic fluctuations between wet and dry periods, which have contributed to corresponding fluctuations in the growth of P.palustris trees.In particular, our findings suggest that growth will be inhibited by greater midsummer to early fall temperatures.Although P.palustris is widely perceived as a drought-resistant species,our study also suggests that its resilience to drought can decrease under multiple droughts within the same decade.Since drought episodes have not been as frequent and intense until within the past few decades, former dendroclimatological studies may have led to a misperception about the future capacity of P.palustris to withstand adverse climate conditions.The present work does not intend to consider the P.palustris a drought susceptible species,but rather to alert researchers to the need to consider the impact of novel climate conditions on this species.A better understanding about how climate will influence the growth and persistence of P.palustris will contribute to how we think about restoration of the P.palustris ecosystem.
Availability of date and materials
The data collected and datasets used for this study will be made available upon request.
CRediT authorship contribution statement
Jo~ao Camp^oa:Conceptualization, Data curation, Formal analysis,Investigation,Methodology,Validation,Visualization,Writing-original draft, Writing - review & editing.Joshua Puhlick:Conceptualization,Funding acquisition,Investigation,Methodology,Project administration,Resources, Supervision, Validation, Writing - original draft, Writing -review& editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are thankful to Avery Holbrook,Ian Goldberg,Jacob Mobley,and Graham Edwards for assistance with fieldwork.We acknowledge Jean Brock for the help with GIS analysis and Kier Klepzig and Nicole Zampieri for their discussions about the research.J.Campôa was funded through a PhD scholarship(2021.05104.BD)funded by the Portuguese Foundation for Science and Technology(FCT)and a Fulbright grant with the support of FCT.This research was also supported by The Jones Center at Ichauway.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.i.org/10.1016/j.fecs.2023.100151.
- Forest Ecosystems的其它文章
- Divergent responses of Picea crassifolia Kom.in different forest patches to climate change in the northeastern Tibetan Plateau
- Tree-based ecosystem services supply and multifunctionality of church forests and their agricultural matrix near Lake Tana, Ethiopia
- Nutrient retranslocation strategies associated with dieback of Pinus species in a semiarid sandy region of Northeast China
- Book review “Continuous Cover Forestry - Theories, Concepts, and Implementation” by Arne Pommerening
- Impact of black cherry on pedunculate oak vitality in mixed forests:Balancing benefits and concerns
- Sensitivity of forest phenology in China varies with proximity to forest edges

