Plumage assisted divergence in a vocally complex island endemic: The Dicrurus paradiseus species complex in Sri Lanka
Snjy Wrkkoy, Ebn Gool, Vimukthi R.Gunskr, Yng Liu,Prvn Krnth, Smpth S.Snvirtn,*
aAvian Sciences & Conservation, Department of Zoology and Environment Sciences, University of Colombo, Colombo 03, Sri Lanka
bGuangxi Key Laboratory of Forest Ecology and Conservation, College of Forestry, Guangxi University, Nanning, Guangxi, China
cDepartment of Health and Environmental Sciences, Xi'an Jiaotong-Liverpool University, Suzhou, Jiangsu, 215123, China
dState Key Laboratory of Biocontrol, School of Ecology, Sun Yat-sen University, China
eCentre for Ecological Sciences, Indian Institute of Science, Bangalore, 560012, India
Keywords:Allospecies Dicrurus lophorinus Drongo Island biogeography Speciation
ABSTRACTModels of allopatric speciation within an island biogeographic framework suggest that the division of ancestral mainland populations leads to one or more allopatric island species predominantly through natural and sexual selection or genetic drift.Here we studied phenotypic divergence in a phylogenetic framework in the Dicrurus paradiseus allospecies complex in Sri Lanka, a continental island located in the Indian plate, to understand the complexity of phenotypic divergence on an island.Members of the genus Dicrurus are known as drongos and are conserved in morphology and plumage, but highly variable in vocalization due to vocal learning and mimicry.Two closely related drongos are found in Sri Lanka:the endemic D.lophorinus(or D.paradiseus lophorinus to many authors)found in the wet zone of the island and the widespread continental species D.paradiseus,which inhabits the dry zone.Sampling from all major populations,and voucher specimens from museums across their range in Sri Lanka, we examined phenotypic and genetic variation in this group.The phenotype showed two clusters: birds with a fish-like tail and erect crest(D.lophorinus),and birds with elongated tail streamers with backwardly curved crest (D.paradiseus).There was no significant difference in the vocal traits compared.The genetic variation was examined using two nuclear(Myo 2 and c-mos)and two mitochondrial(ND2 and Cytb)loci and the phylogenetic relationship was analyzed using the Bayesian inference coalescent-based species tree estimation method.The quantitative criteria for species delimitation provided a score sufficient to consider these two taxa as distinct species by considering measurements of body and plumage,acoustics,behaviour and distribution.The phylogeny supports distinct species status for the Sri Lanka Drongo (Dicrurus lophorinus) and that the D.lophorinus and D.paradiseus sister pair diverged since 1.35 mya.The variation in the crest and the tail plumage (components of phenotype) were the main contributors of the divergence, despite the similarity in general appearance and vocalization of the allopatric species.
1.Introduction
Islands provide a unique opportunity to study the mechanisms behind incipient speciation (Mayr, 1942) by acting as barriers to gene flow,resulting in allopatric speciation, which is considered as the most predominant method of speciation(Coyne and Price,2000).Allopatric speciation usually produces two or more species that are often found adjacent to each other in geographically separate areas(Mayr,1963;Grant and Grant,1996),hence the geographic barriers that restrict dispersal and gene flow between populations are the main agents of divergence (Price, 2008).In such instances,powerful isolating mechanisms must be developed before two incipient species begin to coexist in sympatry (Mayr, 2001).The development of species recognition mechanisms through divergence in phenotype and genotype (Nosil and Schluter, 2011; Conte et al., 2012)could ensure the survival of incipient species by facilitating assortative mating(Merrill et al.,2014).Morphology(Illera et al.,2014),and display characters such as plumage patterns(Fernando et al.,2016)and vocalizations(Sturge et al.,2016),are among such(ornamental)traits that are used to assess suitable mates in birds,hence such traits are acting as prezygotic barriers preventing introgression(Clayton,1990;Price,1998).
The family Dicruridae includes the single genus Dicrurus, which contains 26 species of drongos(Rocamora et al.,2018).Dicruridae shows a very high level of morphological homogenization (Vaurie, 1949; Pasquet et al.,2007),having black,grey,or white plumage,and a forked tail.Despite this morphological homogeneity or conserveness, several significant variations exist such as: the development of spangles and hackles,the crest,and modification of the outermost tail feathers(Mayr and Vaurie, 1948; Rocamora et al., 2018).The modification in the outermost tail feathers and the forehead crest have been suggested to play an important role in the speciation process of this group (Vaurie,1949).Therefore,the study of Sri Lankan drongos,especially the Greater Racked-tailed Drongo (D.paradiseus) allospecies group would provide insights into the role of prezygotic barriers such as the plumage patterns and vocalization in allopatric speciation.
Sri Lanka has five distinct forms of drongos including four resident taxa, i.e., Greater Racked-tailed Drongo (D.paradiseus), Sri Lanka Drongo,(D.lophorinus or D.p.lophorinus)(Fig.1),White-bellied Drongo(D.caerulescens),and Black Drongo(D.macrocercus), and one migratory species,Ashy Drongo(D.leucophaeus)(Rasmussen and Anderton,2012).D.paradiseus is widely distributed in tropical Asia in both dry and wet areas alike,such as the Western Ghats of India(Ramesh et al.,2012).In Sri Lanka, however, it is mainly a lowland dry zone forest species and common only in several scattered locations in the eastern and northern dry zone, mainly associated with riverine forests (Henry, 1971; Legge,1880; Warakagoda et al., 2012; Kotagama and Ratnavira, 2017).The endemic D.lophorinus(or D.p.lophorinus),inhabits tall forests of the wet zone in the southwest part of the island (Legge, 1880; Rasmussen and Anderton, 2012; Kotagama and Ratnavira, 2017).There are specimens and sight records of intermediate phenotypes of D.lophorinus and D.paradiseus in both wet and dry zones of the island(Fig.1),hence it has been suggested that D.lophorinus is a subspecies of the latter (Whistler,1944;Ali and Ripley,1972;Phillips,1975;Ripley,1982;Kotagama et al.,2006).Those intermediate phenotypes showed alterations in the outer tail feathers.The alterations vary from having a fully unseparated lobe in the distal end of the outer tail feathers to fully separated racquets with a narrow fringe of barbs present on both sides of the bare shaft.Furthermore,unlike in D.paradiseus,the bare shaft in observed intermediates is not twisted(Warakagoda,2000).
D.lophorinus was first described by Vieillot (1817).Subsequently, it was subjected to many revisions such as, Dicrurus edoliiformis (Blyth,1847; Kelaart and Gardner, 1852), Dissemurus lophorhinus (Holdsworth,1872), Dissemuroides edoliiformis (Sharpe, 1877), Dissemurus lophorhinus(Legge,1880),Dissemurulus lophorhinus(Wait,1931).Although the initial reason and the literature for considering D.lophorinus as a subspecies of D.paradiseus is unclear, Whistler (1944) was the first to treat it as a subspecies(Dissemurus paradiseus lophorhinus).Later,several authors had treated the wet zone inhabitant as D.p.lophorinus(Ali and Ripley,1972;Phillips, 1975; Ripley, 1982; Goodale and Kotagama, 2006, 2008;Kotagama et al.,2006).Recently,D.lophorinus has been treated again as a distinct species due to morphological and possible vocal distinctiveness(see Fig.1)by Rasmussen and Anderton(2012).Although D.lophorinus is now considered as a distinct species (Rocamora et al., 2018), many authors including Rasmussen and Anderton (2012) emphasized that molecular analysis and other taxonomic studies are required to confirm its species status.Using field sampling across Sri Lanka,as well as studying museum collections in the region, we here conducted a comprehensive analysis of its morphometric, plumage and genetic markers, which include both mitochondrial and autosomal fragments of 2763 bases, to evaluate its species status and the role of the plumage and other key phenotypic traits in divergence.
2.Materials and methods
2.1.Sampling
2.1.1.Field sampling
Fieldwork was conducted under the permits of the Department of Wildlife Conservation (Permit no.–WL/3/2/19/13) and Forest Department (Permit no.–R&E/RES/NFSRC/2013-01-P-02) of Sri Lanka.We sampled adult birds and the age was determined by the absence of white spots in the underwing (Legge, 1880).Since drongos are sexually monomorphic, we were unable to determine the sexes of sampled individuals.We sampled 12 adult individuals of D.paradiseus, 28 individuals of D.lophorinus and two aberrant individuals (from two separate populations–Kottowa Arboretum and Deraniyagala) using mist nets from June 2017 to March 2018 (Appendix A: Table S1).The two aberrant individuals were sampled from the wet zone of the island,within the range of D.lophorinus (see Fig.2).Playback calls of the relevant species and a decoy(a life-size replica made from plastic)were used to attract birds to mist-nets.We obtained morphological measurements,a blood sample(~50 μL)from the brachial vein of the wing(Sheldon et al.,2008), and a set of photographs (to be maintained as a reference collection for later phenotypic analysis) from captured birds.We geo-referenced the capture site of each bird.All the birds were released back to the wild upon sampling after marked with darvic colour rings for later identification.
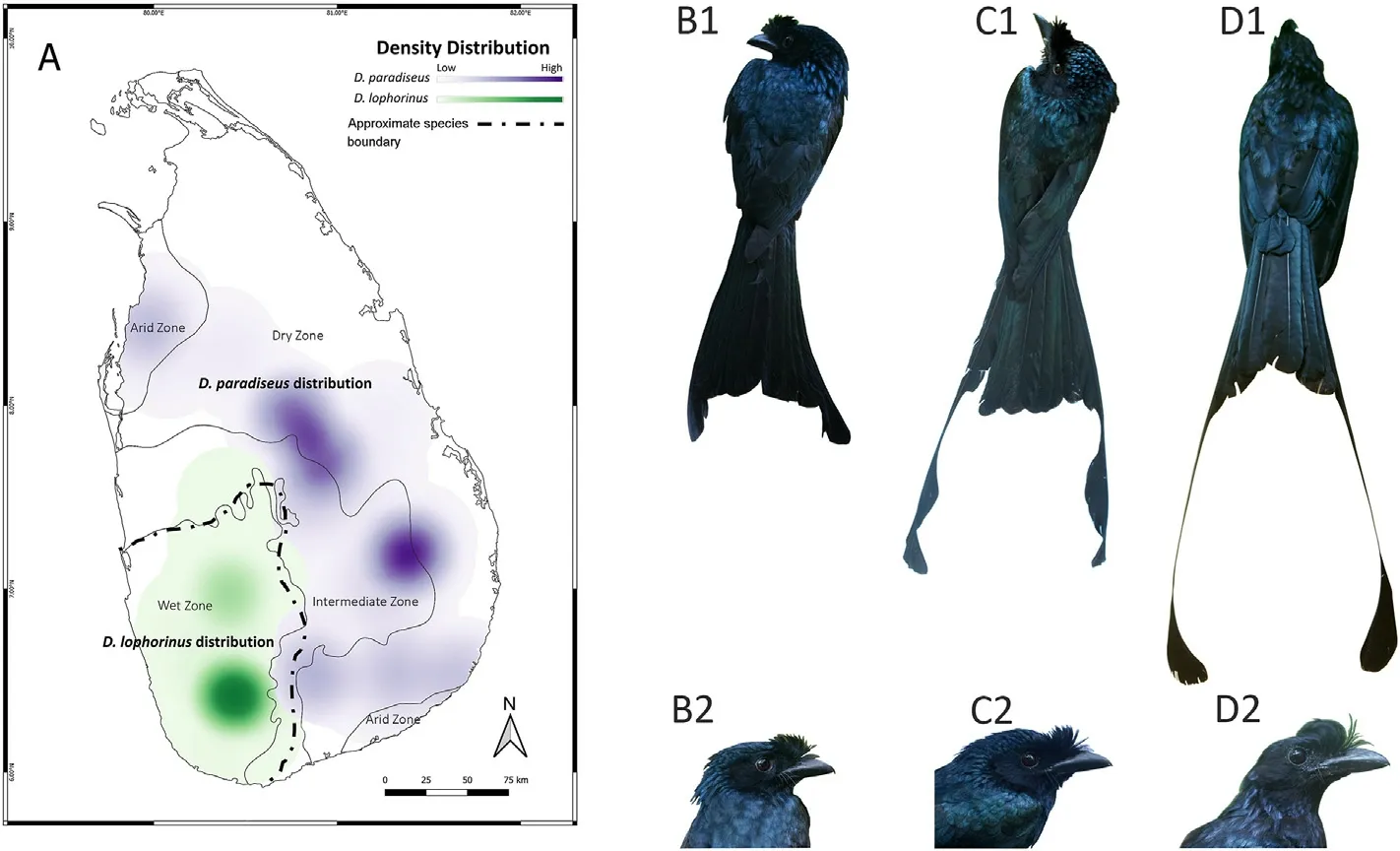
Fig.1.The distribution and the key phenotypic characters of the two species.D.lophorinus is distributed in the southwest wet zone of Sri Lanka,and D.paradiseus can be found in the intermediate,dry and arid zones,based on the eBird distribution data(https://ebird.org/;A).The images from left to right represent D.lophorinus(B1,B2), intermediate phenotype (C1, C2) and D.paradiseus (D1, D2).
Vocalizations of both D.paradiseus and D.lophorhinus, both of captured and not captured birds, were recorded near capture sites at a sampling rate of 48 kHz using a portable Marantz PMD 661 digital recorder with either a Sennheiser MKH 20-P48 microphone and a Telinga parabolic reflector, or a Sennheiser ME67 shotgun microphone with a windsock.The recordings were made during the early morning(6:00–8:00) and evening (16:30–18:30), when the drongos showed the highest level of vocal activity.
2.1.2.Sampling from museum collections
We examined a total of 51 museum skins including 9 specimens of D.lophorinus, 22 specimens of D.paradiseus and 5 aberrant individuals housed at the National Museum of Sri Lanka (NMSL); again, all individuals were adults.Further,two Indian specimens of D.paradiseus and 13 Crow-billed Drongo (D.annectens) (4 from the Trivendrumpuram Museum,India and 9 from Kunming Natural History Museum of Zoology,China)were sampled(Appendix A:Table S1).D.annectens was included to our analysis because it formed an unresolved branch with D.paradiseus in the phylogenetic tree of Pasquet et al.(2007).Similar to live birds sampled in the field, we measured morphological and plumage characters (see below); we also obtained a toe pad for tissue sample (5 mg) in certain skins,and photographed each skin.
The measurements we took in both the field and the museum skins(Table 1) included: head length,head width,total culmen,exposed culmen,bill depth,bill width,eye length,tarsus length,and first claw length(all with 0.01 mm precision, as in Seneviratne et al.(2012) and flat wing length(in 0.1 mm,using a stop ruler).In addition,we measured crest length, and took four different measurements of the tail (all with1 mm precision)(see Appendix B:Fig.S2 for details).
2.2.DNA extraction, PCR amplification and sequencing
Total genomic DNA extraction was conducted using the Phenol–-Chloroform DNA extraction method with modifications(Fernando et al.,2016).We amplified two nuclear (myoglobin intron-2 and c-mos) and two mitochondrial (NADH dehydrogenase subunit 2 and cytochrome b)loci (Pasquet et al., 2007) and sequenced them using the Sanger sequencing method.The myoglobin intron-2 was amplified with the primers Myo2 and Myo3F (Slade et al., 1993; Heslewood et al., 1998),and a set of internal primers: Myo2int, Myo3int (Fuchs et al., 2004),Myo-Ma169R, Myo-Ma183F, Myo-Ma280F, Myo-Ma329R,Myo-Ma405R,and Myo-Ma440F(Fuchs et al.,2005).A 605-bp fragment of c-mos locus was amplified with the primer pair of 944 and 1550(Cooper and Penny, 1997) and following internal primer pair cmosintF and cmosintR (Fuchs et al., 2007).ND2 was amplified using primers L5219 and H6313 (Sorenson et al., 1999), and with internal primers:L-ND2Di360F, L-ND2Di250F, H5736, H-ND2Di670R (Pasquet et al.,2007).A 441-bp fragment of the cytochrome b was amplified with the primer pairs L14990 (Kocher et al., 1989) and H15509 (Pasquet et al.,2007),and with the internal primers CYTB8DIC,CYTB10DIC,CYTBFDIC and CYTBRDIC(Pasquet et al.,2007).The internal primers were used to amplify the targeted regions in museum specimens.The amplification protocol used is as below: initial denaturation at 94 C for 2 min, followed by 36 cycles of denaturation at 94 C for 40 s,annealing at 52–56 C for 45 s,extension at 72 C for 40–50 s and a final extension step at 72C for 5 min.Molecular work was done at the Laboratory for Molecular Ecology and Evolution, Department of Zoology and Environment Sciences,University of Colombo,Sri Lanka,for the majority of the samples.Samples from China were analyzed at Sun Yat-Sen University,China.
2.3.Data analysis
2.3.1.Phenotypic analysis
A multivariate analysis of variance (MANOVA) (Haase and Ellis,1987) was conducted to identify statistically significant morphological traits that differed between D.lophorinus and D.paradiseus.The R statistical platform (R Core Team, 2021) and the MVN package (Korkmaz et al., 2014) was used for the MANOVA.Two separate Principal Component Analyses (PCAs) in R were performed to summarize the morphometric patterns in field captured birds and museum skins.The phenotypic analysis of field captured birds included 28 D.lophorinus and 12 D.paradiseus.The analysis of museum skins included 9 D.lophorinus,24 D.paradiseus including 2 individuals from India,and 13 individuals of
D.annectens.
2.3.2.Phylogenetic analysis
We used Chromas(version 2.6.5,Technelysium Pte Ltd,Australia)to examine the quality and edit the chromatogram files.MEGA7(Molecular Evolutionary Genetics Analysis version 7.0;Kumar et al.,2016)was used to align the sequences using the ClustalW (Thompson et al., 2003)alignment method.The sequence data available in GenBank for Pasquet et al.(2007)(36 individuals belong to 23 species including 7 species of outgroups) was downloaded (Appendix A: Table S2) and added for the analysis.Along with the downloaded data, five D.paradiseus, eight D.lophorinus and two D.annectens individuals sampled from this study were included into the phylogenetic analysis.Further,four D.lophorinus individuals from Deraniyagala (members of the population in which an aberrant bird was sampled) were included to a separate analysis to understand the placement of that population in the phylogenetic tree.Prior to the concatenation, four separate Maximum Likelihood trees were constructed using RAxML GUI v1.5, incorporated in RAxML v8 (Randomized Axelerated Maximum Likelihood) (Stamatakis, 2014) for each marker (Cyt b, ND2, c-mos, Myo2) to examine whether the trees were congruent.The four markers were concatenated and PartitionFinder V1.1.1 (Lanfear et al., 2012) was used to determine the best nucleotide substitution model and the best partitioning scheme,using the“greedy”algorithm under the Bayesian information criterion(BIC).The Partition Finder provided specific nucleotide substitution model sets for MrBayes and set of appropriate RAxML style partitions, that we used for later analysis(Appendix A:Table S3).
The dataset was subjected to two major categories of tree building techniques: concatenated and coalescent based analysis methods.We used both Bayesian Inferences(BI)and Maximum Likelihood(ML)based approaches for the concatenated analysis in MrBayes v3.2.6 (Ronquist et al.,2012)and RAxML,respectively.For the BI analysis,Markov-chain Monte Carlo(MCMC)chains were run for 100 million generations in two independent runs in MrBayes, trees being sampled every 1000 generations(as in Wickramasinghe et al.,2017)with the best partition scheme and substitution models obtained from Partition Finder.The average deviation of split frequency value of less than 0.01 was considered as the convergence between the two runs.The two runs were assessed in Tracer v1.7.1 (Rambaut et al., 2018) to confirm saturation.The initial 10,000 trees were discarded as“burn-in”,and the remaining trees were used to build the consensus tree.ML analysis was performed using RAxML GUI v1.5, incorporating the RAxML-style partition defined by Partition Finder,and node support was computed by rapid bootstrap method(BP)for 10,000 iterations(Wickramasinghe et al.,2017).

Fig.2.Sampling locations of D.lophorinus and D.paradiseus including intermediate (aberrant) forms in Sri Lanka.
The program BEAST V2.6.3 (Bouckaert et al., 2019) was used to perform the coalescent based analysis.We carried out a log likelihood ratio test with and without enforcing the molecular clock in MEGA 7.The molecular clock test rejected the null hypothesis at a 5%significant level and hence a relaxed clock under lognormal distribution was used as the prior for the clock model(as in Sudasinghe et al.,2020).We ran the two independent runs using the Markov Chain Monte Carlo (MCMC) algorithm for 100 million iterations, sampling every 1000 generations in a Yule Coalescent model.The effective sample size (ESS) values for the priors were checked using Tracer software v1.7.1, and a value greater than 200 was used as the threshold.The initial 10% of the trees were discarded as the burn-in,which was suggested by Tracer v1.7.1,and the consensus tree was constructed by combining the two runs using Tree Annotator (Drummond and Rambaut, 2007).This same method was adopted to construct the phylogenetic tree with the aberrant population in BEAST V2.6.3.Trees obtained from ML and BI methods were visualized using FigTree v1.4.3(http://tree.bio.ed.ac.uk/software/figtree/).
2.3.3.Divergence-time estimation
Divergence timing was estimated using BEAST 2 (Rambaut et al.,2018)using the concatenated data set of Cyt b,ND2,Myo2 and c-mos.We used two calibration points.These calibration points were: 1) The split between Platysteira cyanea and Chlorophoneus sulfureopectus was considered as 33.9±4.4 mya(Fuchs et al.,2006).An upper limit of 38.3 mya and lower limit of 29.5 mya was used as described in Pasquet et al.(2007); 2) The split of Dicrurus aldabranus from its sister taxa was considered to date back to no more than 0.125 mya.This date corresponds to the oldest age that allowed D.aldabranus to colonize Aldabra atoll since it was inundated around 0.125 mya (Thomson and Walton,1972).Prior to the analysis, a log likelihood ratio test was carried out,with and without enforcing the molecular clock in MEGA 7 under the Hasegawa-Kishino-Yano model (Hasegawa et al., 1985).Since the molecular clock test rejected the null hypothesis, the relaxed clock under lognormal distribution and Yule speciation process as the tree prior was used in the analysis.Two independent MCMC runs were implemented for 100 million generations to sample every 1000th generation.The ESS values were checked using Tracer v1.7.1 and the consensus tree was attained using Tree Annotator, with a burn-in of the first 10% of trees(Drummond et al.,2015).
2.3.4.Acoustic analysis
The acoustic analysis of the two taxa was performed by analyzing three homologous vocal types performed by both D.lophorinus and D.paradiseus.One of the selected vocal types is associated with alarm calls (‘Crack call’), and another (‘Song’) with non-alarm vocalizations(Fig.1 in Goodale and Kotagama, 2006); a single note was randomly selected to measure of each of these types.Another non-alarm vocal type,the‘Rally call’,is usually heard in the morning and consists of two notes at different frequencies,each of which we sampled(mentioned as“Rally First” and “Rally Last” in the results).The recordings were subjected to high pass filtering over 600 Hz before acoustic measurements (drongo calls had lowest frequencies starting around 800 Hz).
To make the acoustic measurements, the waveform window of the Raven Pro V1.5 software (Cornell Lab of Ornithology, Ithaca, NY) was used to determine the duration of the note (Seneviratne et al., 2009).After selecting the entire note, a “spectrogram slice” (power spectra) of the entire note was made and the peak frequency (frequency of highest amplitude)was recorded.The minimum and maximum frequencies that were within 10 dB of the peak frequency were then measured(modifying a technique of Podos,2001),and the frequency bandwidth calculated as the difference between the maximum and minimum frequencies.Further,the frequency modulation of each note was measured using the difference between the peak frequency of the first 1-ms and last 1-ms.In total,then,there were four acoustic measurements(duration,peak frequency,frequency bandwidth,frequency modulation).We used recordings from as many sites as possible: for “Rally” and “Song” calls 12 recordings for each species (representing 6 sites for D.lophorinus and 3 sites for D.paradiseus),and for“Crack”calls,7 recordings of D.paradiseus(from 3 sites) and 12 recordings from D.lophorinus(from 6 sites).
The statistical analysis of the acoustic data was performed by adopting a multivariate Kruskal-Wallis test(Puri and Sen,1969;May and Johnson, 1997) using the “UCL” package in the R platform (R version 4.2.1).This analysis was done separately for each of the vocal types.For the testing of species status (see next section), we also measured the number of notes in a 15-s section of recording as a measure of the pace of calling and calculate the difference between the means of the two species for this value as an effect size.For this analysis, we used 12 recordings representing 6 sites for D.lophorinus and 3 sites for D.paradiseus.
2.4.Testing species status using Tobias et al.(2010)

where n = number of individuals sampled from species 1 and 2, which expresses the difference in means in terms of the amount of within-group variation (Tobias et al., 2010).Of the five trait types considered:morphology,plumage and acoustics were evaluated into four magnitudes as minor, medium, major, and exceptional, based on the effect size (in morphology and acoustics) and on the level of relative divergence (in plumage).In the context of ecology and behaviour,it was classified into two magnitudes, the geographical separation was evaluated into three magnitudes, and both of these trait types were evaluated based on the level of relevant divergence (Tobias et al.,2021).
3.Results
3.1.Phenotypic analysis
A total of 42 drongos were incorporated for the analysis that were sampled in the field including 28 D.lophorinus, 12 D.paradiseus and 2 aberrant (intermediate) forms.A total of 51 museum specimens were incorporated for the analyses, which included 9 D.lophorinus, 24 D.paradiseus, 13 D.annectens and 5 aberrant forms (Appendix A:Table S1).The two aberrant individuals(MD19SS02 and RC21SW01)in the field analysis were sampled in the wet zone, which is the typical range of D.lophorinus.The locality of one of the aberrant museum specimens (labeled as a D.paradiseus: QL19SW06) was not identifiable.Two aberrant specimens were collected from the wet zone, with one labeled as D.lophorinus (RA25SW05) and the other as D.paradiseus(RA25SW04).The remaining aberrant specimens were collected from the intermediate zone (QL19SW15) and the dry zone (QL19SW14); both these specimens were labeled as D.paradiseus(Appendix A:Table S1).
The key morphometric measurements of the two taxa were tested using MANOVA (F16,n = 40 = 66.778, P < 0.001) and summarized in Table 1;this includes field samples of 28 individuals of D.lophorinus and 12 individuals of D.paradiseus,with the 2 aberrant forms excluded.There is a marked difference in crest and tail variables between D.paradiseus and D.lophorinus.The average length of the total tail length of D.lophorinus was 176.18±2.85 mm,while the value of D.paradiseus was 294.48 ± 7.51 mm which included the bare shaft and the racket.The other important measurement was the curvature of the crest, which varied from 62.0◦to 124.0◦(average 85.59◦± 3.08◦) in D.lophorinus,and from 111.0◦to 164.0◦(average of 145.25◦±4.26◦)in D.paradiseus.D.paradiseus has a longer crest than D.lophorinus,which averaged 22.18±0.87 mm and 18.67±0.46 mm,respectively(Table 1).Except for the crest and tail,the length of the culmen and the length of the tarsus werethe two morphometric traits that exhibited significant variation between the species.The average culmen length of D.paradiseus was larger than that of D.lophorinus,which averaged 35.53±0.50 mm and 33.70±0.33 mm,respectively and D.lophorinus(25.72±1.03 mm)showed a longer tarsus than D.paradiseus(24.94±1.05 mm).
Separate PCA analyses were performed for field and museum samples, which showed a similar pattern of results.For both field and museum samples, three different PCAs were carried out: (a) using all morphological variables;(b)using variables except crest and tail;and(c)using only the crest and tail (Fig.3).There were 12 individuals of D.paradiseus,28 individuals of D.lophorinus and 2 individuals of aberrant forms used in PCAs relevant to the field samples.Two separate clusters were found in the PCA conducted using all 17 morphological variables(Fig.3A1).The first two components explained 54.57%of the variation.The morphometric data excluding the tail and crest variables did not show a clear separation between the species (Fig.3B1; Appendix A:Table S4B).The first two components explained 42.35%of the variation of the data.This included head,culmen,wing and tarsus measurements.The PCA constructed using only crest and tail parameters yielded clearly resolved clusters in multivariate space (Fig.3C1) and the first two components explained 84.48%of the variation(Appendix A:Table S4C).
The PCAs performed with the museum specimens included 24 individuals of D.paradiseus,9 individuals of D.lophorinus,13 individuals of D.annectens and 5 individuals of aberrant forms.Like the field samples,the PCA carried out with all the morphometric variables showed separate clusters for each species(Fig.3A2).The first three components explained 69.16% of the variation (Appendix A: Table S4A).The morphometric data except the tail and crest variables did not show a clear separation except for D.annectens (Fig.3B2).The first two components explained 66.24%of the variation.The first two components explained 88.44%of the variation in the data when the PCA was carried out with crest and tail variables only(Fig.3C2;Appendix A:Table S4C).
3.2.Phylogenetic analysis
The phylogenetic analysis based on the concatenated data set in ML and BI (RAxML and MrBayes) methods yielded a similar topology with low resolution in the D.paradiseus cluster(Appendix B:Fig.S3).Different nucleotide substitution models and the partitions used in RAxML and MrBayes phylogenetic analyses were provided in Appendix A:Table S3.The coalescent based analysis performed in BEAST 2 yielded a more resolved phylogenetic tree (Fig.4).The clade A consists of seven taxa which are mostly confined to South and South-East Asia except D.bracteatus, which has distribution expanded to northern Australia.Clade A splits into two clearly distinct clades, one of which, clade B,consists of three taxa including D.annectens, D.lophorinus and
D.paradiseus.D.lophorinus is sister to D.paradiseus, and the posterior probability(pp) value of the split is 1.0.The BI analysis which included individuals from Deraniyagala yielded a tree where three individuals(including the aberrant individual) out of four clustered within D.paradiseus clade.The remaining individual was clustered with D.lophorinus(Appendix B: Fig.S4).
3.3.Divergence-time estimation
The time calibrated tree (Fig.5) constructed using the coalescent based method showed very recent divergence between the two taxa as D.lophorinus and D.paradiseus have diverged around 1.35 mya, with a 95%highest posterior density (HPD)of 0.65–2.40 mya.
3.4.Acoustic analysis
The analysis of acoustic data showed that there is no significant difference between the two taxa in any of the vocal types(Crack:χ2(n=20,df=6)=3.09,P=0.80;Song:χ2(n=24,df=6)=9.40,P=0.15;Rally First:χ2(n=24,df=6)=6.08,P=0.41;Rally Last:χ2(n=24,df=6)=10.41,P=0.11).The acoustic properties of D.lophorinus and D.paradiseus are summarized in Appendix A: Table S5.
3.5.Evaluating the species status using Tobias et al.(2010) criteria
The overall score between D.paradiseus and D.lophorinus was calculated as 13(Table 2),providing a strong support for treating D.lophorinus as a distinct species according to the quantitative criteria for species delimitation (Tobias et al., 2010, 2021).The two morphological characters that showed strongest difference in the effect sizes calculation were tarsus length(effect size=0.82)and the total culmen length(effect size = -0.99).Plumage characters in the shape of tail and crest accounted for the scoring, as well.The vocal properties of D.paradiseus and D.lophorinus did not show significant variation between the two taxa but the effect size was within the minor category.Although there were a few (7% of the total sample in both field and museums) phenotypically‘intermediate’individuals reported from scattered and isolated locations,there is not enough evidence to consider that these two taxa maintain a permanent hybrid zone(see Discussion).
4.Discussion
Using an array of morphometric, plumage, vocal and genetic traits(Table 3) on wild birds and museum specimens, here we studied the contribution of different phenotypic traits towards the species status of the enigmatic Sri Lanka Drongo(D.lophorinus).To summarize our findings, the PCAs for morphometric traits showed two distinct clusters for D.lophorinus and D.paradiseus (Fig.3).Variations in the tail and crest morphology are markedly different between the two taxa and accounted for most of the separation in the PCA (Appendix A: Table S4).In the coalescent based phylogenetic analysis, D.lophorinus and D.paradiseus are reciprocally monophyletic(with the posterior probability of 1.0).The split between the two taxa is ~ 1.35 mya (Fig.5).Accounting highest possible value as the posterior probability (1.0) in multi locus phylogenetic analysis (de Queiroz, 2005, 2007) and high value in species delimitation index of Tobias et al.(2010) strongly supported the species status for D.lophorinus.Moreover, the distinct difference in appearance(Kotagama and Ratnavira, 2017; Rocamora et al., 2018) and the allopatric range within the island (Rasmussen and Anderton, 2012) further support D.lophorinus as a distinct species, even though the vocal behaviour of the two species seems quite similar(Table 3).
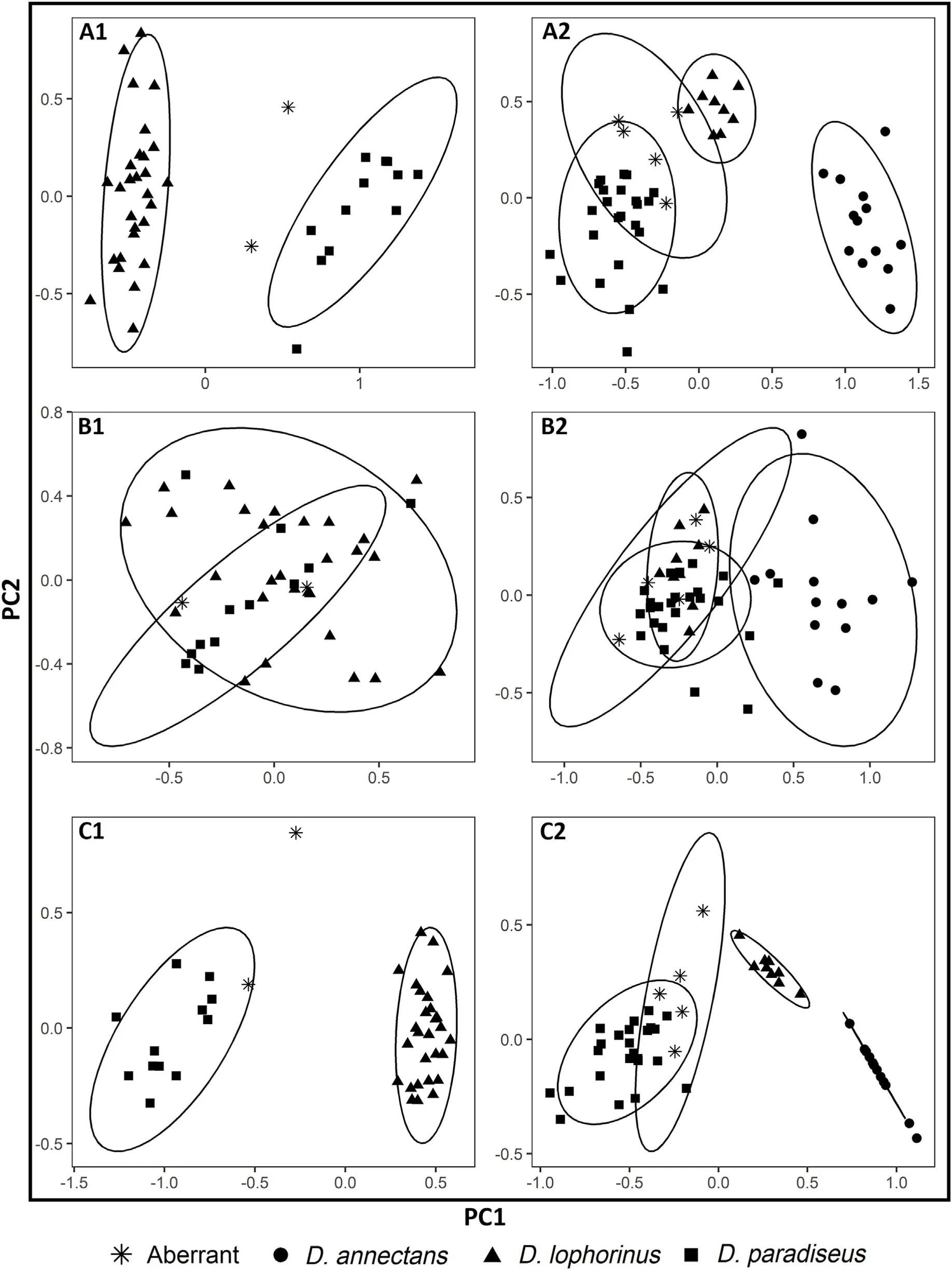
Fig.3.A1, B1 and C1 represent PCA analysis of birds sampled in the field, while A2, B2 and C2 represent museum skins.The triangles in the PCA represent D.lophorinus, squares represent D.paradiseus, stars stand for aberrant individuals and circles represent D.annectans.(A1) Ordination of 14 morphological characters showed variation across D.lophorinus and D.paradiseus sampled from the field.(B1) Distribution of seven morphological characters except the tail and crest measurements in field samples.(C1) Distribution of the tail and crest measurements in field samples.(A2) Ordination of 12 morphological characters in museum skins.(B2) Distribution of five morphological characters except the tail and crest measurements in museum skins.(C2) Distribution of the tail and crest measurements in museum skins.The ellipse was made using the default 95% confidence interval of its multivariate t-distribution in the ggplot2 package.

Fig.4.The phylogenetic relationship using BEAST 2 of the D.paradiseus cluster.The posterior probability values > 0.90 and bootstrap values > 80 are labeled.
4.1.Difference in plumage accounts for the external variation
Mayr and Vaurie(1948)described the key morphological characters that contribute to the differentiation in drongos as the body size, tail length, depth of the tail furcation, amount of gloss, development of spangles and hackles, presence or absence of a crest, and crest modifications.Here we found those same features differentiating the two drongo taxa in Sri Lanka except for the amount of gloss (something we did not take quantitative data on),and body size.Although the longer tail and crest contributed to the appearance of larger size in D.paradiseus,D.lophorinus and D.paradiseus do not exhibit significant differences in most measurements(with the tarsus being larger in D.lophorinus,and the culmen larger in D.paradiseus).
4.2.Sexual selection as a driver of divergence of plumage
The modification of outer tail feathers and the crest are the main ornamentation(showy plumage patterns)that has been developed in the D.paradiseus cluster(Mayr and Vaurie,1948).Such,relatively less-costly ornamental plumage usually play a significant role in divergence in recently diverged clades such as drongos (Price, 2008) and white-eyes(Moyle et al., 2009).The socially monogamous mating system of drongos might have facilitated ornamentation in both sexes (Jones and Hunter,1993).The cost of production,maintenance and the exact use of these plumage traits in mate selection is yet to be understood.
4.3.Vocalization as a driver of divergence

Fig.5.The time calibrated tree of drongos using secondary calibrating points (see Materials and methods for details).
The acoustic variables of D.lophorinus and D.paradiseus did not show significant difference between the two taxa(Appendix A:Table S5).This result congruence with the results of a co-occurring study of playback experiment conducted by same authors(Weerakkody et al.,2020,2022).There, a series of calls and songs were broadcasts to drongo of both species to test how these two taxa respond to conspecific and heterospecific calls (Weerakkody et al., 2020).Conflicting with the view of Rasmussen and Anderton (2005), who suggested that the species could be distinguished by their vocalizations, these two drongos did not distinguish between each other’s vocalizations in the wild.This leads to a rare situation where the vocal divergence is low in substantially diverged, vocally complex, allopatric sister taxa (Weerakkody et al.,2022).
Plumage and vocalizations are premating barriers that promote speciation in songbirds (Baptista and Trail, 1992; Edwards et al., 2005;Price, 2008).Vocal learning plays a major role in avian vocalization by allowing birds to learn conspecific vocalization (Nottebohm, 1972) anddiscriminate congeners, hence promoting assortative mating (Nowicki and Searcy, 2014).The similarity of the vocalizations in these drongos(Table 3) could be due to the recent divergence between the two taxa(1.35 mya), with both taxa expressing the ancestral patterns of vocalization (as suggested in Least Bittern Ixobrychus exilis (McCracken and Sheldon, 1997), and auklets (Seneviratne and Jones, 2010)).Vocal plasticity and the complexity of the repertoire, which includes vocal,mimicry might also have contributed to the lack of differences between the two species(Weerakkody et al.,2022).
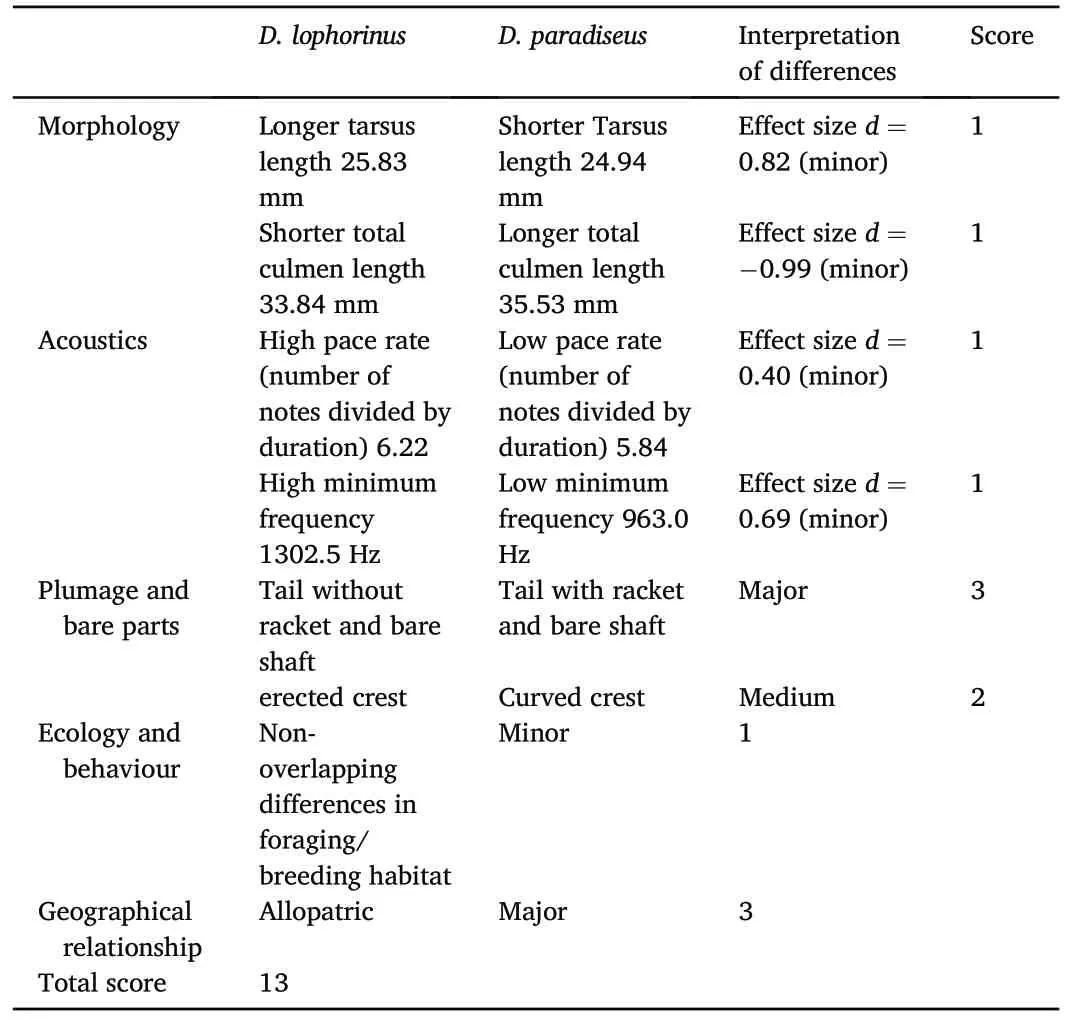
Table 2Application of Tobias et al.(2010) criteria for evaluating the species status ofD.lophorinus.
4.4.Phylogenetic divergence as evidence for species status
Our study based on mitochondrial and nuclear DNA combined with dense sampling across the range of the D.paradiseus complex provides a well resolved tree to support a distinct species status for D.lophorinus.The coalescent model-based analysis yielded reciprocally monophyletic relationship between D.lophorinus and D.paradiseus, along with the divergence dating of 1.35 mya.A similar pattern has been shown in the Madagascar island where a recently diverged island taxon D.aldabranus is nested within a widespread D.forficatus(Fuchs et al.,2013;Rocamora et al.,2018).
The ML method yielded concurrent results (Appendix B: Fig.S3).However,there was a lack of clear sorting in the D.paradiseus complex in concatenated runs in RAxML and MrBays.Therefore, we applied more reliable coalescent based method (Liu et al., 2015) in BEAST to resolve the D.paradisus clade (Xi et al., 2014).For example, coalescent-based genome analyses resolved the phylogenetic relationship of a mammalian superorder Euarchontoglires, where there was some incomplete lineage sorting (Kumar et al., 2013).Further, we have observed some discordances in the branches where the posterior probability values were equal or lesser than 0.95 with the phylogenetic tree of Pasquet et al.(2007).The phylogenetic placement of D.annectens was not resolved in Pasquet et al.(2007),since D.paradiseus and D.annectens were in single clade but did not separate out as two distinct clusters.Although we checked and confirmed the identity of the voucher of D.annectens used in Pasquet et al.(2007), we excluded it from our analysis and two new D.annectens samples were added from Kunming Natural History Museum of Zoology (Appendix A: Table S1).The new samples increased the resolution and resolved the node(Fig.4).
4.5.The issue of intermediate phenotypes
Although the exact reason behind the historical consideration of D.lophorinus as a subspecies of D.paradiseus is not clear, it may be because of the occurrence of intermediate phenotypes (Warakagoda,2000; Rasmussen and Anderton, 2005, 2012; Kotagama and Ratnavira,2017).Based on our analysis,three out of four individuals in a population of‘intermediate phenotypes’placed in the D.paradiseus clade(Appendix B:Fig.S4).This intermediate population sampled near Deraniyagala(wet zone of Sri Lanka) is an isolated pocket surrounded by two mountain ridges in the heart of D.lophorinus’range(Fig.1).
Hybridization is widespread in birds (Gill, 1998) and particularly occurs on islands (Grant and Grant, 1992; Ryan et al., 1994).Hybridization in drongos is also reported in several species (McCarthy, 2006;Fuchs et al.,2017,2018);however,as yet there is no direct evidence of hybridization between D.lophorinus and D.paradiseus.The lack of current connectivity between dry zone and wet zone forest in the island(MoMDE, 2019) hinders significant movement of individuals between populations of the two species.However, it is difficult to rule out the possibility that these intermediates are descendants of historical hybridization events that occurred when there was substantial connectivity of these forests up until the mid-20th century(MoMDE,2019).
The percentage of the intermediate phenotypes accounted in total for 7.4% of all the Sri Lankan samples.But this percentage might not represent the actual occurrence of intermediates, since odd phenotypes
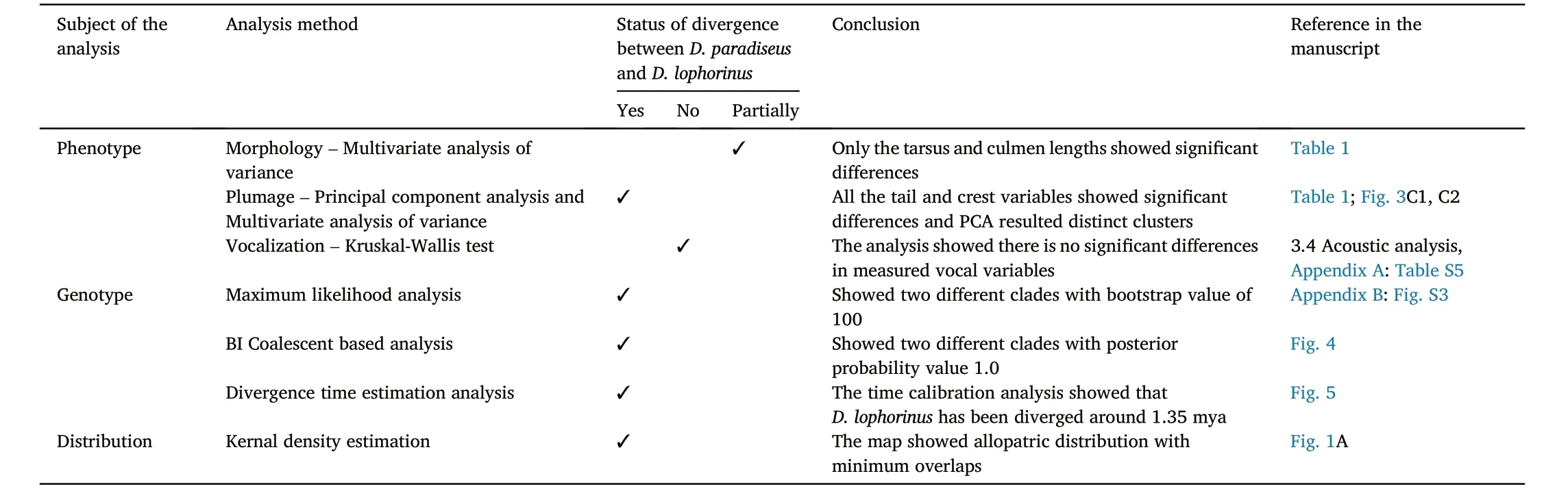
Table 3Summary of different analysis methods used in this study to test the degree of divergence between D.paradiseus and D.lophorinus.
5.Conclusions
Models of allopatric speciation within an island biogeographic framework suggest that the division of ancestral mainland populations leads to one or more allopatric island species.Our phenotypic and genetic evidence support distinct species status for the Sri Lanka Drongo(D.lophorinus) and the D.lophorinus and D.paradiseus sister pair have diverged since 1.35 Mya.The variation in the crest and the tail plumage seems the main contributor for the divergence despite the similarity in body size and vocalization.Even though the proportional contribution can be determined,the exact role of each of these phenotypic traits in the differentiation process is difficult to assess.The role of climate and drift are two other important variables that need to be further explored for this group.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge Jude Janith and Lahiru Rajapakhe for their help in the field.Kamal Raj Gosai helped with measuring the Crow-Billed Drongo skins.Members of the Avian Sciences and Conservation assisted in the fieldwork, lab work and analysis.We are grateful to Lankani Somaratne (NMSL) and Kunming Museum of Natural History, for providing details from their respective collections.Macaulay Library of the Cornell Lab of Ornithology provided the recording equipment.Uvini Senanayake helped with creating maps.We thank the editor and two reviewers for their constructive feedback on an earlier version of this manuscript.This study was funded by the Collaborative Research Grants of the University of Colombo(to SS)and the Special Talent Recruitment grant from Guangxi University (to EG).The fieldwork was conducted with the permission of WL/3/2/19/13 (Department of Wildlife Conservation, Sri Lanka) and R&E/RES/NFSRC/2013-01-P-02 (Forest Department, Sri Lanka).All authors declare that they have no conflicts of interest.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.avrs.2023.100132.
- Avian Research的其它文章
- Selecting the best: Interspecific and age-related diet differences among sympatric steppe passerines
- Morphology and morphometry of two hybridizing buntings at their hybrid zone in northern Iran reveal intermediate and transgressive morphotypes
- Quiet in the nest: The nest environment attenuates song in a grassland songbird
- Characteristics of cross transmission of gut fungal pathogens between wintering Hooded Cranes and sympatric Domestic Geese
- Fecal DNA metabarcoding reveals the dietary composition of wintering Red-crowned Cranes (Grus japonensis)
- Short-term night lighting disrupts lipid and glucose metabolism in Zebra Finches: Implication for urban stopover birds

