Short-term night lighting disrupts lipid and glucose metabolism in Zebra Finches: Implication for urban stopover birds
Na Zhu, Jing Shang, Shuping Zhang
aKey Laboratory of Ecology and Environment in Minority Areas(National Ethnic Affairs Commission),Minzu University of China,Beijing 100081,China
bCollege of Life and Environmental Sciences,Minzu University of China,Beijing 100081,China
Keywords:Birds Glucose Lipid Metabolism Night lighting Short-term
ABSTRACTNight lighting has been shown to affect wild animals.To date, the effects of night lighting on the metabolic homeostasis of birds that spend short time in urban environments remain unclear.Using model bird species Zebra Finch (Taeniopygia guttata), we investigated the effects of short-term night lighting on liver transcriptome,blood glucose, triglyceride, and thyroxine (T4 and T3) levels in birds exposed to two different night lighting duration periods (three days and six days).After three days of night lighting exposure, the expression of genes involved in fat synthesis in the liver was upregulated while the expression of genes involved in fatty acid oxidation and triglyceride decomposition was downregulated.There was also a reduction in blood triglyceride,glucose, and T3 concentrations.However, after six days of night lighting, the expression of genes associated with fatty acid decomposition and hyperglycemia in the liver was upregulated, while the expression of genes involved in fat synthesis was downregulated.Simultaneously, blood glucose levels and T3 concentration increased.These findings indicate that short-term exposure to night lighting can disrupt the lipid and glucose metabolism of small passerine birds, and longer stopovers in urban area with intense night lighting may cause birds to consume more lipid energy.
1.Introduction
Urbanization has led to an increase in environmental night lighting(Marzluff, 2001; Falchi et al., 2016; Watson et al., 2017; Raap et al.,2018).Since most animals evolved in environments where day and night alternate in a continuous cycle (Bell-Pedersen et al., 2005; Luarte et al.,2016), night lighting disrupts this natural light cycle, negatively affecting animal behavior and physiology, and ultimately survival(Navara and Nelson, 2007; Brown et al., 2008; H¨olker et al., 2010;Alaasam et al., 2021; Bumgarner and Nelson, 2021; Lynn et al., 2021).Birds are among the most common and visible wild animals living in the urban environment.Numerous studies have found that physiological processes of birds such as endocrine rhythm (Jesko et al., 2005; Singh et al., 2012; Dominoni et al., 2013; Zhang et al., 2014a; Ouyang et al.,2015; Russ et al., 2015; Alaasam et al., 2018; Taufique et al., 2018;Moaraf et al., 2019) and immunity (Raap et al., 2016; Ouyang et al.,2017; Cooper et al., 2019; Malek and Haim, 2019; Ziegler et al., 2021)are significantly affected by night lighting.
Most studies investigating the effects of night lighting on the physiological process of birds have focused on urban resident species which are exposed to long-term night lighting.How night lighting affects the birds that temporarily stop over in urban areas, such as numerous migratory species, is less well understood.Migration routes of many passerine birds pass through urbanized regions, and migrants have been shown to use urban habitats as stopover sites for three to ten days(Seewagen et al., 2010).Energy metabolism is an essential physiological process for migrating birds to meet the high energy demands of migration (Cohen et al., 2022).However, the physiological effects of short-term night light exposure on the metabolic homeostasis of birds remain poorly understood.Short-term night light exposure in mammals has been shown to increase basal plasma glucose levels (Nagai et al.,2019), due to increased glucose production by the liver and a subsequent increase in the expression of Per 1, Per 2, and phosphoenolpyruvate carboxykinase (Masís-Vargas et al., 2020).Variation in triglyceride levels was found in some migratory species at urban stopover sites (Seewagen et al., 2011), suggesting that urban environments may influence bird metabolism.Therefore, it is necessary to investigate the short-term effects of night light exposure on the metabolic homeostasis of birds.
The liver plays an essential role in maintaining the metabolic balance of carbohydrates, proteins, and fats, and is crucial for vertebrate survival(Martin and Neuberger, 1957; Magnusson et al., 1994; Mensenkamp et al., 2001; Wang et al., 2016).Because feeding behavior is regulated by the day-night light cycle, the activity of key enzymes in glucose and triglyceride metabolism in the liver follows a circadian rhythm.During periods of sufficient food intake, vertebrates can store glucose in the liver in the form of glycogen, and when food intake is insufficient, they can use the stored energy by increasing glycogen decomposition and inhibiting glycogen synthesis (Salem et al., 2007).Previous research found that glycogen synthase, which catalyzes glycogen synthesis, peaks during the dark period (Lamia et al., 2008).In contrast, glycogen phosphatase, which catalyzes glycogen decomposition, peaks at the end of the light period (Lamia et al., 2008).The expression level of phosphoenolpyruvate carboxykinase (PEPCK), a key enzyme that catalyzes gluconeogenesis, has been shown to increase significantly during the sleep-wake transition (Zhang et al., 2010).A significant circadian rhythm has also been demonstrated in enzymes involved in fatty acid metabolism in the liver of rats, with the expression of genes involved in fat synthesis peaking during the dark period, and the expression of genes involved in fat decomposition peaking during the light period (Vollmers et al., 2009).Circadian rhythm genes regulate the expression of genes involved in carbohydrate and lipid metabolism in the liver, with BMAL1 and Rev-Erb genes regulating glucose metabolism (Lamia et al., 2008;Johnson et al., 2014), and PER genes and CRY genes indirectly regulating lipid and cholesterol metabolism (Grimaldi et al., 2010; Zhang et al., 2010; Lamia et al., 2011).Nocturnal lighting has been shown to upregulate PPAR rat liver transcription factorsαandγwhich increases fatty acid synthesis and absorption (Okuliarova et al., 2020), leading to fat deposition in the liver (Rumanova et al., 2019).Studies in rats found that night lighting exposure caused upregulation in the expression of phosphoenolpyruvate carboxylase (PEPCK) in the liver and led to an increase in blood glucose levels through increased gluconeogenesis(Cailotto et al., 2008; Opperhuizen et al., 2017; Masís-Vargas et al.,2020).
In addition to the liver, glucose and fatty acid metabolism are regulated by several hormones with thyroxine being one of the most important.Thyroid hormone (TH) exists in two forms: tetraiodothyronine (T4) and triiodothyronine (T3).T3 plays an important role in maintaining the homeostasis of material and energy metabolism primarily by regulating the enzymes involved in the fat synthesis and fatty acid decomposition pathways (Eales, 1988; Pucci et al., 2000; Yen,2001; Liu and Brent, 2010; Williams and Bassett, 2011).Thyroid hormone secretion also exhibits a circadian rhythm.Studies in Sheep (Ovis aries) found that blood T3 and T4 levels were higher during the day than at night, and changes in circadian rhythm gene expression led to thyroid hormone secretion disorders (Kupprat et al., 2021).Therefore, night lighting can also lead to material metabolism disorders by disrupting the secretion of thyroxine.
In this article, we hypothesize that short-term night lighting exposure could disrupt the metabolic homeostasis of passerine birds.To test this, we examined the effects of short-term (three days and six days)night lighting exposure on bird liver transcriptome, thyroxine (T4 and T3), plasma glucose, and triglyceride levels, and analyzed the relationship between changes in gene expression and hormone secretion and their effect on material metabolism using Zebra Finch (Taeniopygia guttata) as a model passerine bird species.
2.Materials and methods
2.1.Experiment design
We sourced the Zebra Finches (Taeniopygia guttata) from a Zebra Finch breeding company in Henan, China.For the experiment, we used climate chambers (Model RXZ-500C, Ningbo Jiangnan) measuring 73 cm wide, 77.5 cm deep, and 190 cm high, with an adjustable side panel and a cold, white light source.We only used males for the experiments to avoid potential variation between the bird sexes.Twenty-four adult male birds with an average weight of 10 g were raised in an artificial climate chamber.The cage size was 50 cm × 33 cm × 33 cm and six Zebra Finches were held in each cage.The birds were given shelled millet and water daily between 7:00 and 21:00 during the experiment period.The Zebra Finches were acclimatized to the artificial climate chamber environment for 30 days at 25◦C, 50% humidity, and 150 lux light intensity, with a daily light cycle of 16 h light (from 7:00 to 21:00)and 8 h dark (16 L:8D).After 30 days in captivity, the night lighting treatment experiment was initiated.The individuals were randomly divided into a normal photoperiod group (CTR) and a night lighting group (LAN), with 12 individuals in each group.The CTR group was maintained under a 16L:8D light cycle, while the LAN group received 50 lux light exposure during dark period of the CTR group.The night lighting intensity was determined according to the measurement of night lighting intensity in the campus of Minzu University of China located in the urban area of Beijing.The campus was divided into 20 gridles, then lighting intensity in these gridles was measured from 19:00 to 24:00 on March 20, 2021, and the average intensity is about 50 lux(Appendix Fig.S1).The feeding conditions remained unchanged.After three and six days of experimental treatment, 50 μL of blood was taken from the brachial vein of each bird at 7:00.The blood samples were collected into tubes containing EDTA heparin sodium anticoagulant reagent and immediately centrifuged for 20 min (2000–3000 rpm).The supernatant was then collected and the plasma and blood cells were stored in the refrigerator at -80◦C for later analysis of blood glucose and triglyceride concentrations.After blood collection, three individuals from each of the CTR and LAN groups were randomly selected, anesthetized and euthanized and liver tissue was collected for comparative transcriptome analysis.All these procedures complied with ARRIVE guidelines.
2.2.RNA extraction from liver samples
We extracted total RNA from liver tissue using TRIzol (Life Technologies Invitrogen, USA).To obtain tissue homogenate, 500 mg of liver tissue was ground in a mortar frozen with liquid nitrogen, then placed in a precooled RNase free centrifuge tube containing 1 mL TRIzol thoroughly mixed and left for 10 min to allow the tissue cells to lyse completely.The sample was then centrifuged for 10 min at 4◦C and 12,000 rpm.The supernatant was transferred to a new RNase free centrifuge tube, and 200 μL chloroform was added and the sample mixed with an oscillator before centrifuging for 3 min, followed by 15 min at 4◦C and 12,000 rpm.The upper colorless water phase was transferred to a new RNase free centrifuge tube containing an equal volume of isopropyl and shaken to mix before being placed on ice for 10 min.The sample was then centrifuged for 15 min at 4◦C and 12,000 rpm.The sediment was then washed twice with 400 μL of 75% cold ethanol (DEPC water configuration) and centrifuged for 5 min at 4◦C and 12,000 rpm after which the supernatant was discarded and the remaining sediment was left to dry for 5–10 min at room temperature.Finally, 30–50 μL of double distilled H2O was added to fully dissolve the RNA which was then extracted and stored at -80◦C.
2.3.Sequencing of liver transcriptome and analysis of differentially expressed genes
Transcriptome sequencing of samples was conducted by Lianchuan Biological Co., Ltd.Using the following steps.After the total RNA sample passes the test, mRNA was isolated from total RNA using Oligo (dT)coupled to magnetic beads.Segment buffer was then used to break the mRNA into short segments and the fragmented RNA was passed through the reverse transcriptase (Invitrogen SuperScript™ II Reverse Transcriptase).DNA polymerase I (E.coliDNA polymerase I) and ribonuclease RNase H was used to synthesize the second strand of cDNA.The second strand DNA was then placed into dUTP solution to convert the ends of double stranded DNA into flat ends.Base A was added to both ends of the magnetic bead to link it to the connector with a base T at the end.The magnetic bead was then used to screen and purify the fragment size.The two strands were digested using UDG enzyme, and then amplified by PCR to form a 300 ± 50 bp library.Illumina Novaseq™6000 (LC Bio Technology CO., Ltd.Hangzhou, China) was used to conduct the double ended sequencing using the PE150 sequencing mode following the manufacturer’s instructions.To obtain valid data, we used Cutadapt to filter out unqualified sequences before proceeding with the next analysis.HISAT2 was used to compare the valid data to the reference genome and generate statistics for comparable reads.Gene expression level analysis is primarily aimed at analyzing protein coding genes (mRNA) that have been annotated by the genome, generating gene expression statistics, and evaluating the correlation of gene expression characteristics of samples within and between groups, as well as differentially expressed genes.FPKM (Fragments Per Kilobase of xon model per Million mapped reads) was used as a measure of gene expression abundance.Significant differences between samples were analyzed using the statistical software R (edgeR and DESeq2).Genes with an FC ≥2 times or FC ≤0.5 times (|log2FC| ≥1) and aP-value<0.05 were defined as differentially expressed genes.The RT-qPCR was performed on some deferential expressed genes to validate the result of transcriptome sequencing.Two micrograms of the total RNA sample was reversely transcribed to cDNA using M-MLV Reverse Transcriptase with oligo dT primers.Primers were designed based on sequences of the differential genes in NCBI using Primer 5 software (Appendix Table S1).The RT-qPCR protocol was as follows: pre-degeneration at 95◦C for 1 min, degeneration at 95◦C for 15 s, annealing at 59◦C for 15 s and extension at 72◦C for 40 s, for a total of 40 cycles.After the reaction, a melting curve analysis from 55 to 95◦C was applied to ensure the consistency and specificity of the amplified product.TheGAPDHgene was used as the reference gene to normalize mRNA levels among samples.The values of the average cycle threshold (Ct) were determined,andCtscores for gene transcripts in each sample were normalized using the ΔCtscores forGAPDHand expressed as the fold change (FC) in gene expression using equation 2-ΔΔCt.
2.4.Differential expression gene pathway analysis
Ingenuity Pathway Analysis (IPA) (www.ingenuity.com) was used to analyze the related pathways involved in the differentially expressed genes.IPA is a bioinformatics application which uses two statistical values, theP-value andZ-score.TheP-value is based on the Right Detailed Fisher’s Exact Test algorithm and reflects the association between differentially expressed genes and known pathways (P< 0.05 is significant).TheZ-score predicts the activation or inhibition of diseases,biological functions, and toxic functions.PositiveZ-scores indicate activation, and higher values indicate a more significant activation.NegativeZ-scores indicate inhibition, and smallerZ-scores indicate a more significant inhibition.
2.5.Detection of glucose,triglyceride and thyroid hormone in peripheral blood
Peripheral blood glucose and triglyceride concentrations were detected using Leagene kits (TC0711 and TC1245).The glucose detection kit works on the principle that glucose is oxidized into gluconic acid under the catalysis of glucose oxidase, while simultaneously consuming the oxygen in the solution.The generated hydrogen peroxide reacts with the oxidized chromogen to generate red quinones.The amount of hydrogen peroxide generated in the initial reaction is directly proportional to the glucose concentration.We used a 505 nm microplate meter for colorimetric determination.Triglyceride concentrations were determined using a triglyceride test kit, utilizing lipoprotein lipase (LPL)to hydrolyze triglyceride into glycerol and fatty acids.Glycerol was then phosphorylated by glycerol kinase (GK) and adenosine triphosphate(ATP) to form 3-phosphate glycerol (G-3-P).The absorbance is directly proportional to the triglyceride content in the sample, which can be determined by colorimetry at 500–520 nm with the microplate reader.Plasma T3 and T4 levels were determined using enzyme-linked immunoassay kits (T3: Andy gene, AD0500 C h; T4: Andy gene, AD0499C).The kits have been validated for Zebra Finch by serial plasma dilutions following the method described in Zhang et al.(2017b).We followed the kit protocols, which included diluting 10 μL of the plasma samples with dilution buffer (1:5) for the assays.The OD values of the reactants were determined using an enzyme-labeled instrument (Thermo, USA) set at a wavelength of 450 nm.All samples were assayed in triplicate.The mean intra-assay coefficients of variation for T3 and T4 were less than 8% and the inter-assay coefficients were less than 10%, respectively.
3.Results
3.1.Effects of night lighting on gene expression in liver
On the third day of experimental treatment, there were 480 differentially expressed genes (|log2FC| ≥1 andP<0.05) in the experimental group (LAN) compared to control group (CTR), including 278 downregulated genes and 202 up-regulated genes (Appendix Fig.S2).Compared to CTR, LAN had 592 differentially expressed genes (|log2FC| ≥1 andP<0.05) on the sixth day of treatment, including 266 down-regulated and 326 up-regulated genes (Appendix Fig.S3).We selected differentially expressed genes of the PPAR signaling pathway for RT-qPCR validation.The results are shown in the Appendix Figs.S4 and S5, and the trend of gene expression increase and decrease is consistent with the transcriptome results.
3.2.Enrichment analysis of differentially expressed genes by IPA
IPA analysis revealed that three-days of night light exposure could activate the cellular pathways of hepatic steatosis (Fig.1).The differentially expressed genes are related to fatty acid metabolism in the liver(Fig.2).Compared with control group, some of these genes are downregulated in LAN group such asPNPLA2(FC = 0.34,P< 0.001),ACSL3(FC = 0.48,P= 0.003),SREBF1(FC = 0.44,P= 0.001),IL1R1(FC = 0.43,P< 0.001),CPT1A(FC = 0.40,P< 0.001),MC5R(FC =0.28,P< 0.001), ESR2 (FC = 0.49,P= 0.01),FASN(FC = 0.39,P<0.001),NOCT(FC = 0.39,P< 0.001), while some of the genes are upregulated in LAN group such asG0S2(FC = 5.54,P< 0.001),ELOVL2(FC =2.51,P=0.02),GPAM(FC =2.69,P<0.001),PPARG(FC =7.19,P<0.001),PLIN2(FC = 3.62,P< 0.001),ABCC9(FC = 2.17,P=0.005),FABP1(FC = 2.19,P= 0.016).After six-days night light exposure treatment, IPA analysis showed that differentially expressed genes in the liver were also enriched in hepatic steatosis pathways (Fig.3).The differentially expressed genes are related to fatty acid and glucose metabolism in the liver (Fig.4).Compared with control group, some of these genes are up-regulated in LAN group, such asSIRT1(FC =2.15,P< 0.001),RBPJ(FC = 2.99,P< 0.001)GPD2(FC = 2.66,P= 0.01),ACSL6(FC = 2.43,P= 0.01),PNPLA2(FC = 2.27,P< 0.001),CEBPD(FC =2.02,P=0.01),TLR5(FC =2.95,P=0.03),CD36(FC =2.75,P<0.001),IGFBP1(FC =3.51,P=0.02) andPCK1(FC =3.38,P<0.001),while some of the genes are down-regulated in LAN group, such asG0S2(FC =0.02,P<0.001),GPAM(FC =0.27,P<0.001),PLIN2(FC =0.25,P<0.001),PPARG(FC =0.19,P<0.001),SCD(FC =0.30,P<0.001),CAV1(FC = 0.33,P= 0.03),SLC6A14(FC = 0.17,P= 0.002),ACTA1(FC = 0.08,P<0.001) andCRY1(FC =0.24,P<0.001).
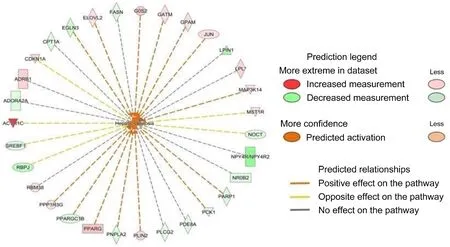
Fig.1.Differentially expressed genes involved in hepatic steatosis in the liver of Zebra Finch (Taeniopygia guttata) between the control and night lighting exposure groups after three days of treatment.

Fig.2.The functions related to differentially expressed genes in the liver of Zebra Finch (Taeniopygia guttata) in the control and night lighting exposure group after three days of treatment.One square represents one gene.The colors of the squares represent theZ-value (negative indicates inhibition effect and positive indicates activation effect).
3.3.Blood glucose and triglycerides
After three days of night lighting exposure treatment, the experimental group had significantly lower blood glucose concentrations (ttest,t=4.877,P<0.05) (Fig.5A), and blood triglyceride concentrations(t-test,t= 4.656,P< 0.05) (Fig.5B) compared to the control group.After six days of night lighting treatment, the blood glucose concentration of the LAN group was significantly higher than that of the control group (t-test,t=-7.946,P<0.001) (Fig.5A), and the blood triglyceride concentration was significantly lower (t-test,t= 3.367,P< 0.05)(Fig.5B).After six days of treatment, Zebra Finches in the experimental group had significantly higher blood glucose concentrations (t-test,t=-9.568,P<0.001) (Fig.5A), and significantly lower blood triglyceride concentrations (t-test,t= 2.685,P< 0.05) compared to three days of treatment (Fig.5B).
3.4.Blood thyroid hormone concentration
There was no significant difference in blood T4 concentration between the control group and the experimental group (t-test,t=-1.108,P>0.05) after three days of night lighting exposure treatment,whereas blood T3 concentration decreased significantly in LAN group (ttest,t=4.689,P<0.05) (Fig.6).After six days of night light exposure treatment, blood T4 concentrations were significantly higher in the control group compared to the experimental group (t-test,t=-5.604,P< 0.001) (Fig.6A).In contrast, there was no significant difference between the two groups in blood T3 concentrations (t-test,t=-0.621,P>0.05) (Fig.6B).Within the experimental group, the blood T4 (t=-3.799,P< 0.05) (Fig.6A) and T3 (t= -6.610,P< 0.001) (Fig.6B)concentrations in Zebra Finch were significantly higher on the sixth day of night lighting treatment compared to the third day.
4.Discussion
IPA analysis revealed that after three days of night lighting exposure,LPL,CPT1A, and other genes related to fatty acid oxidation, lipid oxidation, long-chain fatty acid oxidation, adipose tissue mass, and the hepatic steatosis pathway differed significantly between the LAN and control groups.These genes are divided into two main categories.The first is associated with fat decomposition and includesLPL(lipoprotein lipase) which can decompose triglycerides in adipose tissue into free fatty acids and glycerol (Weinstock et al., 1995),CPT1A(carnitine palmitoyltransfer 1 A) which promotes the oxidation of long-chain fatty acids (Sando and Knight, 2015),G0S2(G0/G1 switch 2) which can bind triglyceride lipase and reduce the activity of triglyceride hydrolase(Yang et al., 2010; Wang et al., 2013; Zhang et al., 2014b; Zhang et al.,2017a), andPNPLA2(patatin like phospholipase domain containing 2)which can convert triglycerides into diglycerides (Villena et al., 2004;Zimmermann et al., 2004).The second involves genes that catalyze fat synthesis and includesELOVEL2(elongation of very long chain fatty acids like 2) which participates in the biosynthesis of polyunsaturated fatty acids, andGPAM(glycol-3-phosphate acyltransferase mitochondrial) which catalyzes the synthesis of triglycerides and phospholipids(Cao et al., 2006; Chen et al., 2008; Brockm¨oller et al., 2012).The expression ofLPL,CPT1A, andPNPLA2decreased significantly in the LAN group, whereasG0S2,PLIN2, andPPARGexpression increased significantly, indicating reduced fat decomposition by the liver.Simultaneously, the expression ofELOVEL2,GPAM, and other genes involved in fat synthesis in the LAN group increased significantly, indicating increased fat synthesis in the liver.However, after six days of night lighting exposure, the genes regulating fat synthesis, such asPLIN2,PPARG,G0S2,FASN(fat acid synthesis),SCD(stearoyl CoA desaturase),LPIN(lipin 1), andGPAM, were down-regulated, while the genes regulating fatty acid decomposition, such asPNPLA2,LPL, andSIRT1(acetylase 1, sirtuin 1), were up-regulated.This suggests that the fatty acid decomposition process was enhanced after six days of night lighting.Additionally, after the sixth day, our results showed up-regulated expression of genes associated with glucose metabolism disorders and hyperglycemia, includingCEBPD,GPD2,TLR5,CD36, andPCK1, in the liver of individuals in the experimental group, indicating increased gluconeogenesis in the liver which can lead to increased blood glucose levels.The results of our liver transcriptome study showed that when birds were abruptly switched from a normal light cycle to night lighting exposure, the expression of genes regulating the synthesis and decomposition of lipids and sugars in the liver was disrupted.Three days of night lighting exposure increased fat accumulation in the liver, but this was reversed after six days of exposure.Moreover, six days of night lighting exposure increased the blood glucose.These results indicate that the metabolic homeostasis of birds can be disrupted during short-term stopovers in urban environments.
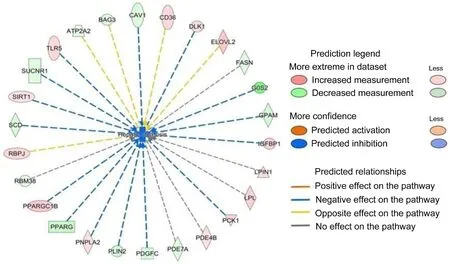
Fig.3.The differentially expressed genes associated with hepatic steatosis in the liver of Zebra Finch (Taeniopygia guttata) between control and night lighting exposure experiment group after six days of treatment.
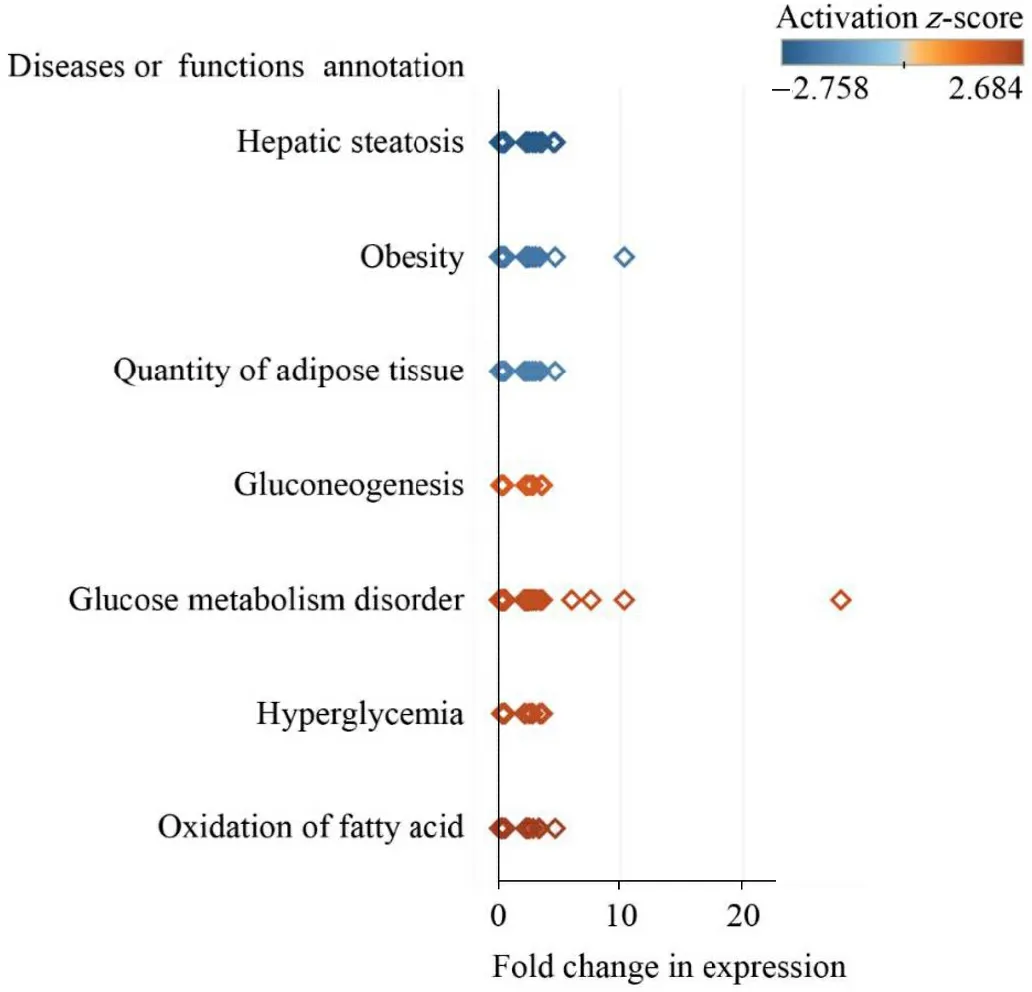
Fig.4.The functions related to differentially expressed genes in the liver of Zebra Finch (Taeniopygia guttata) in the control and night lighting exposure group after six days of treatment.One square represents one gene.The colors of the squares represent theZ-value (negative indicates an inhibition effect and positive indicates an activation effect).

Fig.5.The blood glucose (A) and triglyceride (B) concentrations of Zebra Finches (Taeniopygia guttata) in the control group (CTR) and night lighting exposure group (LAN) (mean ±standard deviation,n=24 for 3-day treatment,n= 18 for 6-day treatment).*P< 0.05.

Fig.6.The plasma T4 (A), T3 (B) concentrations of Zebra Finches (Taeniopygia guttata) in the control group (CTR) and night lighting exposure group (LAN)(mean ± standard deviation,n= 24 for 3-day treatment,n= 18 for 6-day treatment).*P< 0.05.
Field studies have shown that birds’ morning activities begin earlier,and their night activities end later when night lighting is present (Taufique et al., 2018; Aulsebrook et al., 2020).The increase in time for activity increases muscle tissue glucose consumption (Opperhuizen et al., 2017).When the amount of glucose stored in the body is insufficient, the liver activates the gluconeogenesis and lipolysis pathways to maintain a supply of glucose.The significant increase inPCK-1expression in the experimental group after six days of night lighting exposure may be linked to an increase in gluconeogenesis.Previous studies on mice have also shown that night lighting can cause an up-regulation ofPEPCKexpression in the liver, leading to increased blood glucose concentrations (Cailotto et al., 2008; Fonken et al., 2010; Coomans et al.,2013).In our study, fat decomposition gene expression increased significantly in the liver of individuals in the LAN group after six days of night lighting exposure, while fat synthesis gene expression decreased significantly, which may be related to the use of fatty acids as a source of energy.
Blood triglyceride concentrations in the experimental group were significantly lower compared to the control group after three days of night lighting exposure.This finding, combined with the significantly up-regulated expression ofPPARG,PLIN2, and other genes involved in fat synthesis in the liver of the experimental group on the third day,suggests that three days of night lighting causes triglycerides to enter the liver and promote liver fat synthesis, resulting in a reduction in blood triglyceride concentrations.This suggestion is further supported by a significant decrease in blood glucose concentrations in the experimental group after three days of night lighting exposure.Compared to the control group and three days of night lighting exposure, blood triglyceride concentrations of the experimental group were significantly lower after six days of night lighting exposure, whereas blood glucose concentrations were significantly higher.The expression ofLPLandPNPLA2associated with fatty acid decomposition, andPCK-1associated with gluconeogenesis, were significantly up-regulated in the liver of the experimental group.These findings, together with the liver transcriptome analysis results, suggest that increased nocturnal activity may lead to increased fatty acid utilization and the maintenance of a high glucose state.Elevated blood glucose levels have also been observed in mice following night light exposure, with bright light pulses causing an increase in blood glucose levels and weak night lighting having no effect on blood glucose (Opperhuizen et al., 2017).Furthermore, studies have shown that night lighting can reduce the insulin sensitivity of cells in the muscles, fat, and liver of humans and mice (Fonken and Nelson, 2014;Ursino and Coppari, 2020), reducing the rate at which blood sugar enters cells.Thus, longer stopover in urban area may lead to increased fatty acid utilization and a glucose metabolism disorder.
Compared with the control group, the LAN group showed a significant decrease in blood T3 concentration on the third day of the treatment, while there was no significant change in T4 concentration.T3 is an important hormone that regulates the oxidative catabolism of fatty acids (Pucci et al., 2000).These results indicate that three-day night lighting exposure can inhibit the oxidative catabolism of fatty acids in birds.T3 in peripheral blood mainly comes from conversion of T4 in the liver by deiodinase (Nassar et al., 2015); thus, the decrease in T3 levels in birds caused by three-days night lighting exposure may be due to a decrease in the conversion rate of T4 in the liver.Studies on the House Sparrows (Passer dominicus) and the White Crowned Sparrows (Zonotrichia leuchophrys) showed that a decrease in T3 levels can lead to a decrease in the basal metabolic rate of the birds (Chastel et al., 2003;Pérez et al., 2018).The result of plasma T3, combined with the results of a decrease in blood triglyceride levels and an upregulation of liver fat synthesis gene expression, collectively indicate that three-day lighting exposure can reduce the oxidative catabolism of fatty acids and increase liver fat synthesis.After six days of night lighting exposure, the T3 concentration in the LAN group returned to a level without significant difference from the control group.Studies on mice have shown that six weeks of 20L:4D illumination can increase the secretion of T4 in mice(Vinogradova et al., 2009), while studies on House Sparrows have shown that T3 levels increase when the energy demand of the birds increases (Chastel et al., 2003).The increase in T3 and T4 concentrations in the LAN group on the sixth day may be another factor promoting the expressions of fatty acid decomposition genes, leading to increased fatty acid utilization.
With growing urbanization, many migratory birds must stop over in urban environments for up to ten days (Seewagen et al., 2010).In contrast to urban resident birds that have adapted to urban environments, night lighting is an abrupt environmental interruption for migratory birds.Many birds have to stop at several urban sites during their migration (La Sorte et al., 2017; McLaren et al., 2018; Horton et al.,2019); thus, their normal circadian rhythm may be frequently disrupted.Most studies have focused on the effects of night lighting on the flight orientation of nocturnally migrating birds (e.g., La Sorte et al., 2017;Van Doren et al., 2017).However, the physiological effects of night lighting on migratory birds are less well understood.It has been known that some migratory species stop in urban area for up to ten days(Seewagen et al., 2010), and our surveys in Beijing also show that some species stop in urban sites for seven days in spring (unpublished data).Our findings in the six-day experiment implicate that longer urban stopover could cause birds to consume more lipid energy during stopovers at urban sites with intense night lighting.Migration is a high energy consuming process and lipids are the main source of energy for muscles during flight (Bairlein et al., 2015; Cohen et al., 2022); therefore, an increase in fatty acid consumption at stopovers would be detrimental to the body condition of birds.Further field studies are required to gain a better understanding of the effect of urban stopover on the body condition of migratory birds when they arrived at their destination.
Funding
This work was supported by grants from Key laboratory of Ecology and Environment in Minority Area, National Ethnic Affairs Commission(KLEEMA202207) and the Graduate Research and Practice Projects of Minzu University of China (BZKY2022042).
Authors’contributions
SZ conceived and designed the study.NZ and JS conducted the experiments.NZ and JS analyzed the data.NZ and SZ wrote the manuscript.All authors read and approved the final manuscript.
Ethics statement
Permission to handle our study animals was given by Animal Research Ethics Committee of Minzu university of China (permit no.20200121 A A).Experimental procedures comply with the ARRIVE guidelines and were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No.8023, revised 1978).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2023.100138.
- Avian Research的其它文章
- Selecting the best: Interspecific and age-related diet differences among sympatric steppe passerines
- Morphology and morphometry of two hybridizing buntings at their hybrid zone in northern Iran reveal intermediate and transgressive morphotypes
- Quiet in the nest: The nest environment attenuates song in a grassland songbird
- Characteristics of cross transmission of gut fungal pathogens between wintering Hooded Cranes and sympatric Domestic Geese
- Fecal DNA metabarcoding reveals the dietary composition of wintering Red-crowned Cranes (Grus japonensis)
- Variaiton in the composition of small molecule compounds in the egg yolks of Asian Short-toed Larks between early and late broods

