Sublethal effects of abamectin on the development and reproduction of Sogatella furcifera(Hemiptera: Delphacidae)
LIAO Qi, ZHAO Bin-Bin, FANG Ling, ZHOU Nan-Xin, HE Shu-Lin,XU Shan, YANG Hong, ZHOU Cao,*
(1. Chongqing Key Laboratory of Vector Insects, Institute of Entomology and Molecular Biology,Chongqing Normal University, Chongqing 401331, China; 2. Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Mountainous Region, Scientific Observing and Experimental Station of Crop Pests in Guiyang, Ministry of Agriculture and Rural Affairs, Institute of Entomology of Guizhou University, Guiyang 550025, China)
Abstract:【Aim】 To investigate the sublethal effects of abamectin on Sogatella furcifera. 【Methods】The 3rd instar nymphs of S. furcifera were treated with abamectin at LC10 (0.016 mg/L) and LC25 (0.031 mg/L) concentrations using rice seedling dipping method. The longevity of female and male adults and the number of eggs laid per female of F0 generation were recorded, and the developmental duration, fecundity, survival rate, female and male adult longevity, intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), and mean generation time (T) of F1 generation were recorded, and the age-stage, two-sex life table was established. 【Results】 After the 3rd instar nymphs of S. furcifera were exposed to LC10 and LC25 concentrations of abamectin, the number of eggs laid per female of the F0 generation was significantly reduced, but the hatching rate was not affected as compared to the control (distilled water). Abamectin at the LC25 concentration significantly prolonged the male adult longevity, whereas the female adult longevity was significantly shortened by the LC10 of abamectin compared with the control. In addition, LC10 and LC25 concentrations of abamectin significantly reduced the number of eggs laid per female of the F1 generation (LC10: 170.64 grains; LC25: 155.79 grains) compared with the control (193.38 grains). In the LC10 and LC25 concentration treatment groups, the intrinsic rate of increase (r), finite rate of increase (λ) and mean generation time (T) of the F1 generation were slightly lower than those in the control group, while the reproductive rate (R0) showed a decreasing trend but not significantly different from that in the control. 【Conclusion】 Abamectin stress at the sublethal concentrations could somewhat inhibit the offspring population growth of S. furcifera.
Key words:Sogatella furcifera; abamectin; sublethal effect; age-stage, two-sex life table; fecundity; growth and development
1 INTRODUCTION
The white-backed planthopper,Sogatellafurcifera(Hemiptera, Delphacidae), is a highly destructive migratory pest (Maetal., 2015).S.furciferasucks the sap from the phloem of rice and lays eggs in the stem, resulting in a slow growth, a delayed tillering stage and a reduced seed setting rate, which eventually causes plant death, called “hopperburn” (Rameshetal., 2014; Lietal., 2016). In addition,S.furciferacan transmit southern rice black-streaked dwarf virus (SRBSDV), which reduces the quality and yield of rice (Zhangetal., 2022). Currently, the main methods to controlS.furciferastill rely on insecticides. However, overuse of insecticides has led to severe resistance ofS.furciferato various insecticides (Alietal., 2016; Ruanetal., 2021).
Abamectin is a 16-membered ring macrolide compound produced byStreptomycesavermitilisand shows high-efficiency and broad-spectrum pesticidal activities against insects and nematodes. It is safe for humans and environment due to its unique mechanism of easy degradation and widely used in agriculture (Lasota and Dybas, 1990; McKellar and Benchaoui, 1996; Khalil and Darwesh, 2019). But overuse of abamectin can force insects to develop resistance (Suetal., 2013). In addition, insects may be exposed to sublethal concentrations of abamectin because it is highly unstable in light, easily degraded by microorganisms in water and soil, or diluted by rain (Lasota and Dybas, 1990; Guedes and Cutler, 2014). Studies have shown that sublethal concentrations of insecticides can affect the developmental duration, survival rate and longevity of insects (Heetal., 2013; Xiangetal., 2019). Therefore, it is very important to assess the sublethal effects of insecticides on insect physiology and behaviors (Desneuxetal., 2007). The sublethal effects of abamectin have been studied in some insects. Abamectin at the LC30concentration significantly reduced the pupal weight and female oviposition ofHelicoverpaarmigera(Vojoudietal., 2011). The sublethal concentration of abamectin significantly increased the mortality of larvae, prepupae and pupae ofGrapholitamolestaand significantly inhibited the fecundity of females (Suetal., 2021). Fecundity of short-winged females was significantly inhibited when the 3rd instar nymphs ofNilaparvatalugenswere treated with abamectin at the LC25concentration (Yangetal., 2019). Abamectin is often used to control rice pests such asCnaphalocrocismedinalis,Chilosuppressalis,Tryporyzaincertulasand rice planthoppers. Currently, there are no available reports on the sublethal effects of abamectin onS.furcifera.
Traditional life table allows analysis of the longevity and oviposition of females, but ignores the important role of males in population differentiation (Chietal., 2020). The age-stage, two-sex life table, analyzing two sexes and comprehensively describing instar differentiation, has become an important tool for population ecology and integrated pest management (Huetal., 2010; Chietal., 2020). In this study, the age-stage, two-sex life table (Chi and Liu, 1985; Chi, 1988) was used to analyze the population parameters of the F1generation ofS.furciferaand to predict the population dynamics of future generations after the 3rd nymphs ofS.furciferawere treated with abamectin at the LC10and LC25concentrations. Furthermore, the effects of abamectin on the growth and development, fecundity, survival and longevity were assessed. Our results provided important experimental evidences for understanding the toxic effects of abamectin on insects.
2 MATERIALS AND METHODS
2.1 Test insects and insecticide
S.furciferaadults were collected from rice fields in Huaxi, Guiyang, Guizhou, China in 2013 and kept in the laboratory of Guizhou University under the conditions of (25±1) ℃, 70%±10% relative humidity and a photoperiod of 16L∶8D. The 3rd instar nymphs were used for further experiments. Abamectin (96.40%) was purchased from Shandong Qifa Pharmaceutical Co., Ltd (Shandong, China).
2.2 Bioassay
The rice seedlings at the tillering stage were used for the following experiments, and randomly divided into groups. Abamectin was prepared with acetone at a concentration of 10 mg/mL, and then diluted into five gradient concentrations with distilled water. Rice seedlings were immersed in different concentrations of abamectin for 30 s, using distilled water as the control. The roots of rice seedlings were coated with absorbent cotton and then placed into a two-way glass tube. Twenty 3rd instar nymphs of similar body size were selected and placed into each glass tube covered with gauze. Each treatment was performed with 5 replicates (20 nymphs for each replicate), and a total of 600 nymphs were used for five gradient concentration treatments and the control. Nymphs were reared in an artificial climate incubator and the mortality was checked after 72 h.
2.3 Determination of the effects of abamectin on the F0 generation of S. furcifera
The seedling dipping method was applied according to a previous description (Zhouetal., 2016; Longetal., 2017). Briefly, rice seedlings were soaked in the LC10and LC25concentrations of abamectin, respectively, for 30 s, dried, wrapped with absorbent cotton and transferred to glass tubes, using distilled water as a control. Twenty 3rd instar nymphs of the similar body size were placed into each glass tube. Each treatment was performed with 5 replicates, and a total of 100 nymphs were used for each treatment. Live individuals were transferred to new tubes after 48 h treatment and fed with fresh rice seedlings without insecticide. Newly emerged female and male adults were paired for further rearing. The survival of parents and the number of newly hatched offspring were recorded daily. The hatched nymphs were collected for future studies.
2.4 Determination of the effects of abamectin on the F1 generation of S. furcifera
Eighty eggs ofS.furciferaof the F0generation from each treatment were randomly selected, and the hatching was monitored every day. After hatching, nymphs were reared individually in a glass tube with fresh rice seedlings, and the growth and developmental duration were recorded daily. Adult females and males were paired and continued to rear. The parental survival rate and oviposition were recorded daily until females died.
2.5 Statistical analysis
Probit analysis was performed by using SPSS 22.0 to determine the LC10and LC25of abamectin against the 3rd instar nymphs ofS.furcifera. The differences among various parameters of the F0generation were compared using one-way analysis of variance and the least significant difference (LSD) method for multiple comparisons. The original data of the developmental duration, survival rate and oviposition were used to calculate the age-stage specific survival rate (sxj), age-specific survival rate (lx), age-specific fecundity of female (fx7), age-specific fecundity of the total population (mx), age-specific net maternity (lxmx), age-stage specific life expectancy (exj), age-stage reproductive value (vxj), intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), mean generation time (T), adult preoviposition period (APOP), and total preoviposition period (TPOP) with TWOSEX-MS Chart and the age-stage, two-sex life table theory (Leeetal., 2022). Variances and standard errors were obtained by bootstrapping with 100 000 random samplings (Huangetal., 2018). The differences in each parameter among different treatments were assessed with the paired bootstrap test (Weietal., 2020).
The data obtained from TWOSEX-MS Chart were imported into TIMING-MS Chart to predict the population dynamics of the 3rd instar nymphs ofS.furciferain the next 60 d under the three treatments (Xuetal., 2022). Plots ofsxj,lx,fx7,mx,lxmx,exj,vxjand population prediction were drawn by using SigmaPlot 14.0 and Adobe Illustrator 2020.
3 RESULTS
3.1 Toxicity of abamectin to S. furcifera nymphs
The results showed that the LC10and LC25values of abamectin against the 3rd instar nymphs ofS.furciferawere 0.016 and 0.031 mg/L, respectively.
3.2 Sublethal effects of abamectin on the longevity and fecundity of the F0 generation of S. furcifera
After the 3rd instar nymphs ofS.furciferawere exposed to LC10and LC25concentrations of abamectin, the numbers of eggs laid per female were significantly reduced compared with the control (distilled water)(P<0.05). In addition, male adult in the LC25treatment group had significantly longer longevity than that in the control group (P<0.05), while the female adult longevity in LC10concentration treatment group was significantly shorter that in the control group (P<0.05)(Table 1).
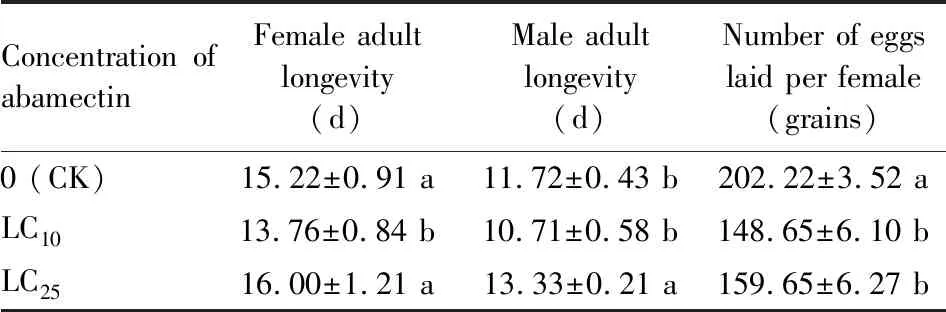
Table 1 Sublethal effects of abamectin on the adult longevity and fecundity of the F0 generation of Sogatella furcifera
3.3 Sublethal effects of abamectin on the growth and development of the F1 generation of S. furcifera
As shown in Table 2, compared to the control (distilled water), LC10of abamectin significantly shorten the 2nd instar female nymphal duration of the F1generation ofS.furcifera(P<0.05). The duration of the 2nd, 4th and 5th instar female nymphs was significantly reduced and the egg duration was significantly prolonged (P<0.05), after being treated with abamectin at the LC25concentration. However, there were no significant differences in female adult duration, female pre-adult duration and total developmental duration among various groups.
After the 3rd instar nymphs ofS.furciferawere exposed to LC10of abamectin, the duration of the 2nd instar nymph of F1males was significantly reduced as compared to the control (P<0.05) (Table 3). In the LC25concentration treatment group, the 1st instar nymphal duration, adult duration and total developmental duration of F1males were significantly shortened as compared to those in the control group (P<0.05), the 4th instar nymphal duration of F1males was significantly shortened as compared with that in the LC10concentration treatment group (P<0.05). There were no significant differences in egg duration, 3rd and 5th instar nymphal duration, and pre-adult duration of F1males among various groups (P>0.05).
3.4 Sublethal effects of abamectin on the fecundity of the F1 generation of S. furcifera
Fecundity analysis showed that the APOP and TPOP of F1females ofS.furciferain the LC10concentration treatment group were prolonged as compared with those in the control group, but the differences were not significant (P>0.05)(Table 4).

Table 2 Sublethal effects of abamectin on the growth and development of females of the F1 generation of Sogatella furcifera
However, the TPOP of females in the LC25concentration treatment group was significantly shortened as compared to that in the control (P<0.05). In addition, abamectin significantly inhibited the number of eggs laid per female of the F1generation (LC10: 170.64 grains; LC25: 155.79 grains; CK: 193.38 grains). In conclusion, abamectin had a significant inhibitory effect on the reproductive ability ofS.furcifera.
3.5 Sublethal effects of abamectin on the population parameters of the F1 generation of S. furcifera
Effects of abamectin on the population parameters of the F1generation ofS.furciferawere not significant at the LC10and LC25concentrations (P>0.05)(Table 5). The intrinsic rate of increase (r), finite rate of increase (λ), and mean generation time (T) in the LC10and LC25concentration treatment groups were slightly lower than those in the control group. In addition, the net reproductive rates (R0) in the LC10and LC25concentration treatment groups were reduced by 19.33% and 22.4%, respectively, as compared to that in the control group.

Table 4 Sublethal effects of abamectin on the adult fecundity of females of the F1 generation of Sogatella furcifera
3.6 Sublethal effects of abamectin on the survival and fecundity of the F1 generation of S. furcifera
Age-stage specific survival rate (Sxj) analysis showed that abamectin at the LC10and LC25concentrations significantly reduced the survival rate ofS.furcifera(Fig. 1: B, C). Peak survival rate and maintenance time in treatment groups were lower for both male and female adults in treatment groups than those in the control group. Moreover, the survival rate of males was lower than that of females in the LC10concentration treatment group. However, the survival rate of females was slightly reduced in the LC25concentration treatment group, but LC25of abamectin did not affect the male survival. In addition, the longevity of females (50 d) and males (44 d) in the control group was longer than that of both sexes in the LC10and LC25concentration treatment groups.
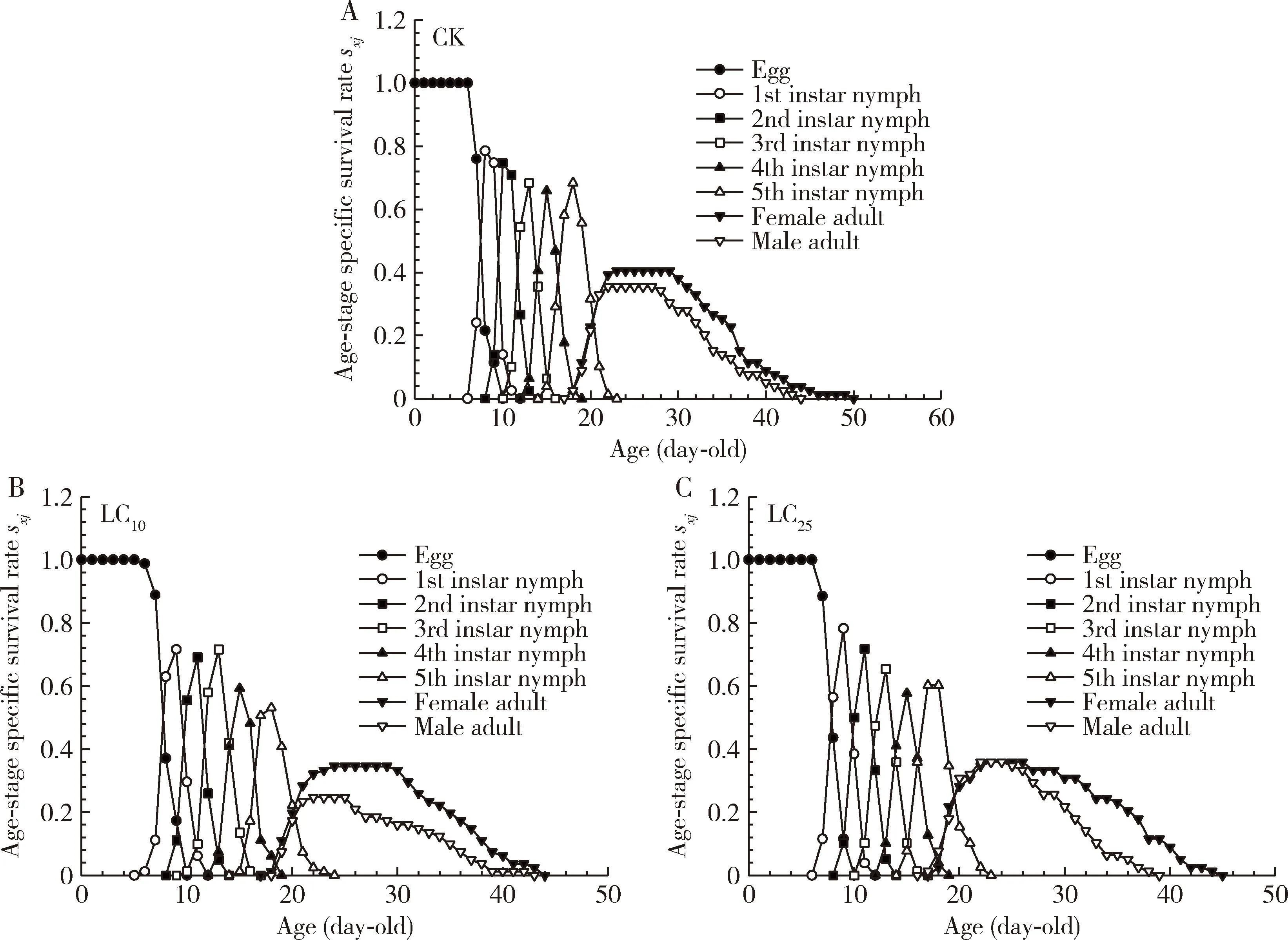
Fig. 1 Age-stage specific survival rates (sxj) of the F1 generation of Sogatella furcifera after exposure of the 3rd instar nymphs to the LC10 and LC25 concentrations of abamectin
Age specific survival rate (lx) dropped sharply from 0.83 (14th day) to 0.60 (18th day) after treatment with LC10of abamectin, with a large number ofS.furciferadeath (Fig. 2: B). In addition, the population age-specific survival rate dropped to 0 much earlier after treatments with LC10and LC25of abamectin. Age-specific fecundity of female (fx7)(LC10>CK>LC25), age-specific fecundity of the total population (mx)(LC10>CK>LC25), and age-specific net maternity (lxmx)(CK>LC10>LC25) showed an increasing and then decreasing trend for all three treatments.
The age-stage specific life expectancy (exj) of the F1generation decreased with age (Fig. 3). Under the stress of LC10(Fig. 3: B)and LC25(Fig. 3: C) of abamectin, the age-stage specific life expectancy ofS.furciferawas lower than that of the control group, indicating that abamectin had an impact on the life expectancy ofS.furcifera. The age-stage reproductive value (vxj) revealed that female adults contributed the most to population growth (Fig. 4). However, abamectin remarkably inhibited the reproductive capacity of female adults. The peak reproductive values of females in the LC10concentration treatment group (93.97 eggs on the 23rd day)(Fig.4: B) and the LC25concentration treatment group (78.37 eggs on the 23rd day)(Fig. 4: C) were lower than that in the control group (95.25 eggs on the 24th day)(Fig. 4: A). Abamectin stress caused earlier reproductive peak of female adults and shortened the total reproductive time.
3.7 Population projections
The TIMING-MS Chart was used to predict the population dynamics of the F1generation ofS.furciferafor the next 60 d (Fig. 5). Abamectin caused a reduction of the number ofS.furciferaat different developmental stages compared with the control. To be specific, abamectin at the LC10and LC25concentrations significantly reduced the predicted number of the 5th instar nymphs and egg numbers, respectively (Fig. 5: B, C). The predicted population size was roughly consistent among the three treatment groups during the early stage, but it was slightly lower in the LC10and LC25concentration treatment groups during the later stages compared with the control group (Fig. 5: D).
4 DISCUSSION
Exposure to insecticides affects insect development, reproductive ability and population dynamics (Desneuxetal., 2007). In this study, abamectin at the LC25concentration significantly shortened the total developmental duration of males of the F1generation (Table 3). A study showed that the LC30concentration of sulfoxaflor significantly inhibited the female adult duration ofN.lugens(Liaoetal., 2019). However, buprofezin remarkably extended the total developmental duration of the F1generation ofS.furcifera(Wangetal., 2018). In addition, the low concentration (LC10) of sulfoxaflor also significantly prolonged the adult duration ofS.furcifera(Xiangetal., 2019). These results suggest that different effects of insecticides on the growth and development of the planthoppers depend on their specific functional mechanisms. For example, buprofezin is an insect growth inhibitor that inhibits the biosynthesis of chitin, thereby retarding the molting process and prolonging the developmental duration of insects (Uchidaetal., 1985; Wangetal., 2018). Therefore, further investigation is needed to determine how abamectin affects the developmental duration ofS.furcifera.
Thesxjcurve analysis showed that the survival rates of female adults were higher than those of male adults under all three treatments (Fig. 1). However, the survival rates of females and males in the LC25concentration treatment group were significantly lower than those in the control group and LC10concentration treatment group, indicating that the inhibitory effects of abamectin on the population growth of offspring were much more effective with concentration increasing. These results were consistent with the predicted population dynamics over the following 60 d. It was shown that thiamethoxam at the LC5and LC20concentrations reduced the survival rates of male and female adults ofBradysiaodoriphaga, and eventually inhibited the population growth (Zhangetal., 2014). These results suggest that sublethal concentrations of insecticides could affect the offspring population by reducing the survival rate.
Insect fecundity is a key factor in population dynamics. In this study, abamectin at the LC10and LC25concentrations significantly reduced the number of laid eggs in the F0and F1generations (Tables 1, 4). Similar results were also found inN.lugens(Yangetal., 2019),Tutaabsoluta(Nozad bonabetal., 2017),Habrobraconhebetor(Mahdavietal., 2011),Helicoverpaarmigera(Vojoudietal., 2011), andDeraeocorisbrevis(Kimetal., 2006), indicating that abamectin does not stimulate insect reproduction and is an ideal candidate for pest control. It is worth noting that the inhibitory effect of abamectin on the female fecundity of the F0generation ofS.furciferawas higher than that of the F1generation, suggesting that the sublethal effects onS.furciferais decreasing in the next generations. Thevxjcurves showed that the fecundity of the F1generation ofS.furciferawas decreased after exposure to abamectin. The higher the concentration of abamectin, the stronger the inhibitory effect (Fig. 4). Previous studies have shown that insecticides affected the fecundity ofS.furciferaby regulating the expression of genes related to TOR and JH signaling pathways, which subsequently modulated the expression ofSfVgandSfVgRand affected the population dynamics ofS.furcifera(Zhouetal., 2020, 2021, 2022). It was found that sublethal concentrations of spinosad reduced the synthesis of carbohydrates, proteins and lipids inGlyphodespyloalislarvae, resulting in reduction of the growth rate, fecundity, body size, pupation rate and enzyme functions (Pirietal., 2014). Whether abamectin affects the reproduction ofS.furciferaand its life table parameters in a similar way requires further investigations.
In recent years, growing evidences have shown that low concentrations of insecticides can regulate insect population dynamics. Our results showed that low concentrations of abamectin have regulatory effects onS.furciferapopulation, providing important experimental evidence for better understanding the toxic effects of abamectin. Further studies will be needed to understand the regulatory mechanisms of biochemical responses ofS.furciferaafter exposure to abamectin.
- 昆虫学报的其它文章
- 基于转录组数据的中华按蚊细胞色素P450超家族基因不同发育阶段、成蚊不同组织和吸血前后不同时期雌成蚊的表达模式分析
- 狄斯瓦螨VdesNPC2b蛋白的基因克隆、原核表达及其与寄主幼虫信息素结合机制研究
- 亚洲玉米螟对球孢白僵菌分生孢子和芽生孢子-玉米共生体的取食选择和嗅觉反应
- 紫外线辐照被寄生的黑腹果蝇蛹对果蝇锤角细蜂生长发育的影响
- Response of heat inducible heat shock protein 90 gene of Aphis gossypii (Hemiptera: Aphididae) to gossypol and flupyradifurone stresses and mutual effect on transcription factor HSF
- 蜜蜂衰老与寿命调控研究进展

