Retinal microvascular and microstructural alterations in the diagnosis of meibomian gland dysfunction in severely obese population: a new approach
Hai Huang, Xiao-Yu Wang, Hong Wei, Min Kang, Jie Zou, Qian Ling, San-Hua Xu,Hui Huang, Xu Chen, Yi-Xin Wang, Yi Shao, Yao Yu
1Nanchang Aier Eye Hospital, Nanchang 330006, Jiangxi Province, China
2Department of Endocrinology and Metabolism, the First Affiliated Hospital of Nanchang University, Jiangxi Clinical Research Center for Endocrine and Metabolic Disease, Jiangxi Branch of National Clinical Research Center for Metabolic Disease, Nanchang 330006, Jiangxi Province, China
3The First Clinical Medical College, Nanchang University,Nanchang 330006, Jiangxi Province, China
4Department of Ophthalmology, Eye & ENT Hospital of Fudan University, Shanghai 200433, China
5Department of Ophthalmology, the First Affiliated Hospital of Nanchang University, Jiangxi Branch of National Clinical Research Center for Ocular Disease, Nanchang 330006,Jiangxi Province, China
6Department of Ophthalmology and Visual Sciences,Maastricht University, Maastricht 6200MA, Limburg Province,Netherlands
7School of Optometry and Vision Sciences, CardiffUniversity,CardiffCF244HQ, United Kingdom
Abstract
● KEYWORDS: superficial vessel density; retinal thickness; severely obesity; meibomian gland dysfunction;optical coherence tomography angiography
INTRODUCTION
Obesity is a serious health risk[1].Body mass index(BMI) is an indicator used to evaluate obesity.BMI is calculated by dividing weight (kg) by height squared (m2),with values <25 indicating low weight or regular weight,25-30 overweight, 30-35 moderate obesity and ≥35 severe obesity[2].
There is growing evidence that obesity is strongly related to metabolic diseases such as diabetes, dyslipidemia and hyperuricemia[3].Dyslipidemia is also thought to initiate the development of meibomian gland dysfunction (MGD), leading to dry eye and loss of ocular surface homeostasis[4-5].
The meibomian gland is a modified sebaceous gland in cutaneous eyelid that generates meibum, the lipid component of tears[6].On blinking, meibum is distributed evenly across the ocular surface, protecting the water layer from evaporating and stabilizing the tear film[7].Any meibomian gland lesions will affect the formation of meibum, thus destabilizing the tear film, and ultimately affecting the corneal steady state, inducing epithelial disruption.As a result, most MGD patients have ocular irritation, such as eye pain and discomfort, and they may also experience symptoms of inflammation[8].
Optical coherence tomography angiography (OCTA) is a significant advance in ocular imaging, producing blood flow images of all retinal vascular layers with unprecedented resolution.OCTA is quick and non-invasive and can offer volumetric data.It allows localization and identification of pathology while providing information about structure and blood flow.OCTA can also be repeated in a single imaging process to obtain comprehensive, extensive microvascular information, or to assess the microvascular response to functional stimuli.In comparison with fluorescein angiography or indocyanine green angiography, OCTA images are not masked by high fluorescence due to dye leakage, so OCTA produces high-contrast, clear microvascular images.Since OCTA does not require an exogenous contrast agent, it can be performed in any patient, especially if fluorescein angiography or indocyanine green angiography are not possible.Finally,OCTA can be executed faster than these other methods,simplifying clinical workflow[9].It is currently limited,however, by a comparatively small visual field, inability to identify leaks, and image artifacts due to patient movement or blinks[10].
OCTA has provided many important clinical findings,including areas of macular telangiectasia[11], microvascular impairment[12], microaneurysms[13], capillary remodeling[14],and neovascularization[15].More importantly, OCTA provides unprecedented depth imaging information.It has been applied in assessing many kinds of diseases, including diabetic retinopathy[16], retinal vein obstruction[17], uveitis[18], and glaucoma[19].It has also been used in neurological research[20]and retinal diagnostics[21].Therefore, in the present research,we used this technology to evaluate retinal microvasculature and microstructure in MGD patients in severely obese population.
SUBJECTS AND METHODS
Ethical ApprovalThe study methods and protocols were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (Nanchang, China;No.2021039) and followed the principles of the Declaration of Helsinki.All subjects were notified of the objectives and content of the study and latent risks, and then provided written informed consent to participate.
ParticipantsTwelve MGD patients in severely obese population (PAT group; 4 females and 8 males) were selected from the Department of Ophthalmology, the First Affiliated Hospital of Nanchang University.Recruitment criteria: 1)BMI≥30; 2) waist circumference ≥85 cm in female, waist circumference ≥90 cm in male; 3) diagnosed as MGD.There are symptoms of dry eye: dryness, foreign body sensation,signs of dry eye: narrowing or interruption of the tear river, and evidence of meibomian gland abnormalities: such as absent meibomian glands, abnormal meibomian gland openings, or abnormal meibomian gland secretions[22]; 4) able to perform OCTA.
This study recruited 12 healthy controls (HC group; 6 females and 6 males), all matched for age and gender in the PAT group.Recruitment criteria: 1) 20≤BMI≤24; 2) able to perform OCTA.
Exclusion criteria: patients in both groups were required to have no 1) mental illness, 2) drug or alcohol abuse history,3) current pregnancy, 4) history of diseases that lead to OCTA changes: macular hole, cystic macular edema, diabetic retinopathy, age-related macular degeneration, glaucoma,hypertension, diabetes, cerebrovascular disease, Alzheimer’s disease, and some intracranial pathologies.
MethodsTVue Avanti XR OCTA system (Optovue,Fremont, CA, USA) was used to image both microvascular and retinal cross sections.Scan parameters were scanning rate 70 000 A-scans per second, horizontal definition 22 μm,axial resolution 5 mm, center fold wavelength 840 nm, and bandwidth 45 nm.B-scans were conducted in 3×3 and 6×6 mm2scanning modes, five repeated images each at 216 grating positions, were focused on the central fovea, and took 3.9 seconds to complete.A 1080 B-scan (216×five positions) was acquired at 270 frames per second[23].We used two horizontal and two vertical gratings to obtain 3×3 and 6×6 mm2OCTA images through a series of four individual scans.OCTA images of 3-dimensional 3×3 and 6×6 mm2en-face were recorded from both eyes.

Figure 1 OCTA images of SVD in HC and PAT groups A: 3×3 mm2 angiography image of superficial vessel; B: 6×6 mm2 angiography image of superficial vessel; C: OCT fundus.OCTA: Optical coherence tomography angiography; SVD: Superficial vessel density; HC: Healthy control; PAT: Meibomian gland dysfunction patients in severely obese population.
After scanning, each retinal image was divided into five[superior (S), nasal (N), inferior (I), temporal (T), and central foveal (C)] or nine [inner superior (IS), outer superior (OS),inner nasal (IN), outer nasal (ON), inner inferior (II), outer inferior (OI), inner temporal (IT), outer temporal (OT), and C] subregions[24], consisting of two or three concentric circles,and their microvessels and microstructure were investigated.Each retinal layer covered (I) the inner retina (from the inner boundary membrane to the inner plexiform layer), and (II) the whole retina (from the inner boundary membrane to the retinal pigment epithelium).A two-dimensional panoramic image of the superficial retina (between vitreoretinal interface and anterior boundaries of ganglion cell layer) was formed using a threshold method to determine vascular density.According to the pixel size, we scaled the skeleton plate average of the region of interest to calculate the superficial vessel density(SVD) from the fovea to the 3×3 and 6×6 mm2brightness gradient image edges.SVD and retinal thickness (RT) were measured and compared.SVD images of each participant were divided into five and nine subregions, and RT images were divided into nine subregions (Figures 1 and 2).
Statistical AnalysisSPSS (version 26.0) and GraphPad Prism(version 8.0) were used to analyze data, which were recorded in the form of mean±standard deviation (SD).Independent samplest-tests were utilized to analyze data between PAT and HC groups.The relationship between visual acuity and related factors was studied by univariate and multivariate regression analyses.To examine the difference between HC and PAT groups, receiver operating characteristic curves (ROC) were plotted for SVD, RT, FAZ, and retinal volume.P<0.05 was considered statistically significant.

Figure 2 OCTA images and analysis of RT in HC and PAT groups A:Cross-sectional view of RT in the HC and PAT group under OCTA; B:The microstructure of retina in the HC and PAT group under OCTA; C,D: The results of RT in the HC group and PAT group were compared.OCTA: Optical coherence tomography angiography; RT: Retinal thickness; HC: Healthy control; PAT: Meibomian gland dysfunction patients in severely obese population.

Table 1 Characteristics of PATs and HCs mean±SD
RESULTS
Each group included 12 participants (24 eyes).The groups were statistically matched in age and gender.Visual acuity in PATs was significantly poorer than in HCs (P<0.0001;Table 1).

Figure 3 Analysis of SVD and RT results in the PAT group and control group A, B: Analysis of SVD results in the PAT group and control group.The vertical coordinate is the value of SVD, and the horizontal coordinate is the retinal subregions.C: Analysis of RT results in the PAT group and control group.The vertical coordinate is the value of RT, and the horizontal coordinate is the retinal subregions.PAT: Meibomian gland dysfunction patients in severely obese population; SVD: Superficial vessel density; RT: Retinal thickness; S: Superior; N: Nasal; I: Inferior; T:Temporal; C: Central foveal; IS: Inner superior; OS: Outer superior; IN: Inner nasal; ON: Outer nasal; II: Inner inferior; OI: Outer inferior; IT: Inner temporal; OT: Outer temporal.aP<0.0001, bP<0.001, cP<0.01, dP<0.01.Bars: Standard errors.

Table 2 Comparison of SVD at different locations between PATs and HCs mean±SD, %
Analysis of the Retinal Superficial Vessel DensitySVD of each group is shown in Table 2, Figure 3A, 3B.SVD of PATs in N (P<0.0001) and T (P<0.0001) was significantly lower than that of HCs.S, I and C showed no statistically significant difference between the groups.SVD was significantly decreased in PATs in OS, IN, OT (allP<0.0001), and ON(P=0.0068), while no difference was found in IS, II, OI, IT,or C.
Analysis of the Retinal ThicknessRT of each group is shown in Table 3, Figure 3C.Compared with HCs, RT was significantly decreased in PATs in IS, OS, OI, OT, ON(P=0.0001), IT (P=0.0002), IN (P=0.0014), and II (P=0.0108).C showed no statistically significant difference between the groups.
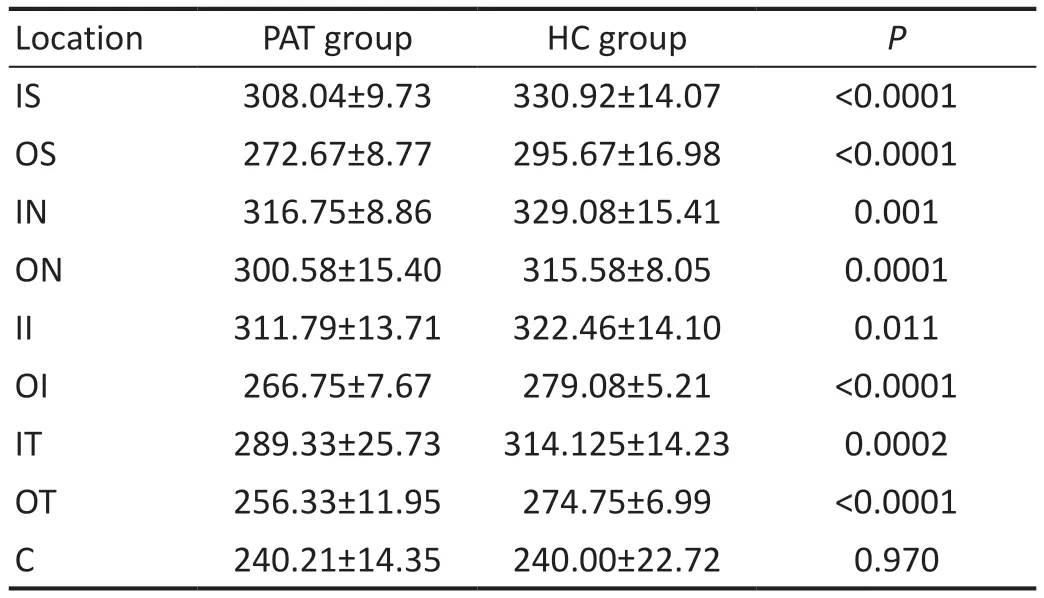
Table 3 Comparison of RT at different locations between PATs and HCs mean±SD, μm
Univariate regression analysis showed that visual acuity was negatively correlated with RT (β=-0.634,P<0.001), SVD(β=-0.486,P<0.001), retinal volume (β=-0.651,P<0.001),average volume thickness (β=-0.597,P<0.001), FAZ perimeter(β=-0.507,P<0.001), and FAZ area (β=-0326,P=0.024), but not for FAZ substantiation, blood pressure, age, or gender.Multivariate regression found a significant positive correlation between visual acuity and FAZ perimeters (β=-1.406,P<0.001;Table 4).

Figure 4 ROC curve analysis of SVD (A, B) and RT (C) ROC: Receiver operating characteristic; RT: Retinal thickness; SVD: Superficial vessel density; S: Superior; N: Nasal; I: Inferior; T: Temporal; C: Central foveal; IS: Inner superior; OS: Outer superior; IN: Inner nasal; ON: Outer nasal;II: Inner inferior; OI: Outer inferior; IT: Inner temporal; OT: Outer temporal.
ROC Analysis of Superficial Vessel Density and Retinal ThicknessSensitivity and specificity of SVD and RT as diagnostic indicators were investigated by using ROC(Figure 4).Significant between-group differences were found in SVD in all four quadrants and in the central fovea.The area under the ROC curve (AUC) in the temporal quadrant was 0.961 [95% confidence interval (CI): 0.908 to 1.000], and in the nasal quadrant 0.890 (95%CI: 0.782 to 0.998; Figure 4A), indicating that SVD has a medium to high diagnostic sensitivity in PAT[25].Significant differences were found between groups in all nine subregions.The AUC in OT was 0.935 (95%CI: 0.870 to 0.999), and 0.915 in IS (95%CI: 0.838 to 0.992), indicating that SVD has a high diagnostic sensitivity for retinal anomalies in PAT (Figure 4B).
Analysis of RT results showed significant differences between groups in all nine subregions.The AUC for OS was 0.962(95%CI: 0.915 to 1.000), and 0.885 for OT (95%CI: 0.766 to 1.000), indicating medium to high diagnostic sensitivity in PAT(Figure 4C).
Analysis of the Foveal Avascular ZoneFAZ analysis in PAT and HC is shown in Table 5 and Figure 5A.In the 3×3 and 6×6 mm2angiography results, the area (P=0.0015 andP=0.0039) and perimeter (P=0.0068 andP<0.0001) were significantly decreased in PATs.The AUC was 0.729 for area(95%CI: 0.572 to 0.887), and 0.838 for perimeter (95%CI:0.704 to 0.972), indicating medium diagnostic sensitivity in PAT.
Analysis of Superficial Vessel Density at Different Retinal LayersThe SVDs at different retinal layers in PATs and HCs are shown in Table 5 and Figure 5B.In the 3×3 mm2angiography result, the SVD of the retinal inner layer(P<0.0001) was significantly lower in PATs than in HCs, while the central foveal and the full layer showed no difference between groups.In the 6×6 mm2angiography result, the SVDs at the retinal inner layer (P<0.0001) and the full layer(P=0.0194) were significantly lower in PATs than HCs.The AUC for 3×3 and 6×6 mm2angiography in the retinal inner layer were 0.858 (95%CI: 0.739 to 0.977) and 0.828 (95%CI:0.712 to 0.944) respectively, indicating medium diagnostic sensitivity in PAT.

Figure 5 ROC curve analysis of FAZ, inner SVD, and volume A: The area under the ROC curve was 0.729 for area (95%CI: 0.572 to 0.887), and 0.838 for perimeter (95%CI: 0.704 to 0.972); B: The area under the ROC curve was 0.858 for 3×3 mm2 angiography of inner SVD (95%CI: 0.739 to 0.977), and 0.828 for 6×6 mm2 angiography of inner SVD (95%CI: 0.712 to 0.944); C: The area under the ROC curve was 0.897 for retinal volume (95%CI: 0.803 to 0.990), and 0.872 for average volume thickness (95%CI: 0.765 to 0.980).ROC: Receiver operating characteristic; FAZ:Foveal avascular zone; SVD: Superficial vessel density.

Table 5 Analysis of FAZ results and SVD results in different retinal layers mean±SD

Table 6 Analysis of retinal volume and average volume thickness results mean±SD
Analysis of Retinal Volume and Average Volume ThicknessThe retinal volume and average volume thickness of the PAT and HC groups are shown in Table 6 and Figure 5C.Compared with HCs, the retinal volume (P=0.0001) and average volume thickness (P=0.0001) in PATs were both significantly decreased.The AUC for the retinal volume was 0.897(95%CI: 0.803 to 0.990) and for average volume thickness 0.872 (95%CI: 0.765 to 0.980), indicating moderate to high diagnostic sensitivity in PATs.
DISCUSSION
Our study found significant decrease in visual acuity, retinal SVD, RT, FAZ area and perimeter, retinal volume and average volume thickness in MGD patients in severely obese population than in controls.
MGD is the major causal factor of dry eye and loss of ocular surface homeostasis.More and more research have indicated that dyslipidemia can promote the development of MGD, which is highly associated with severe obesity[26].Previous studies have shown that moderate to severe MGD is significantly related to high BMI in Chinese adults[27].
The periphery of the retina is a pathological site for several eye diseases.Changes in the retina can provide markers to diagnose and monitor these and to evaluate their therapeutic response.Retinal changes have also been reported in neurodegenerative diseases such as Parkinson’s disease[28]and Alzheimer’s disease[29].The retina and the cerebrum develop from the same embryonic tissue, so retinal research may offer a new way to understand systematic diseases that are related to the central nervous system.Since retinal vessels with tight junctions between endothelial cells are similar to cerebrovascular vessels, it is assumed that imaging of the former may be helpful in research on the latter.OCTA has been effectively applied in predicting retinal vascular anomalies in neurological diseases[30].Schӧnfeldt-Lecuonaet al[31]have provided further evidence that in schizophrenia spectrum disorder patients structural changes observed in the brain are also observable in the retina.Retinal research may help to further understand a nervous system that changes in highly complex diseases.
Decreased SVD has been detected in many diseases.OCTA in glaucomatous eyes is characterized by reduced density of superficial blood vessels at the optic disk and in the macular region, and complete loss of choroidal capillaries in localized areas of peripapillary atrophy[19].Previous research revealed that superficial parapapillary microvascular density is reduced in high myopia[32].Our study found decreased SVD in MGD patients in severely obese population, and we speculate that those patients may be at risk of the eye diseases mentioned above.Recent research has reported that decreased RT in patients with Alzheimer’s disease is correlated with disease severity[33]and outer peripapillary total retinal ring volumes reportedly differentiate papilledema from pseudopapilledema[34].As these subclinical changes also existed in the PATs group in the present study, we speculate that retinal microvascular and microstructural alterations may facilitate understanding of the pathogenesis of MGD in severely obese population and can be used for early diagnosis and staging of MGD in this population.
Our regression analysis demonstrated that impaired visual acuity was highly correlated with retinal thinning, SVD decrease and FAZ reduction, and a number of previous studies are consistent with these findings.A study on Sjӧgren’s syndrome patients showed that the thinning of the retina affects visual acuity[35]and Islam[36]reported that vision was highly related to RT in diabetic macular edema.Nakajimaet al[37]found that deep vessel density was highly related to loss of visual field in patients with retinitis pigmentosa.Roeselet al[38]reported that foveal thickness was related to vision.
Our ROC curve analysis of SVD, RT, FAZ, and retinal volume illustrates possible approaches for early discovery of retinal variations in MGD patients in severely obese population.Early diagnosis is key to more effective treatment and better prognosis.OCTA and functional magnetic resonance imaging are revolutionary methods for visualization of blood vessels at different layers of the retina and brain[39-42].Being noninvasive and time-efficient, it has been widely used in retinal diagnostics.Microvascular and microstructural changes in MGD patients in severely obese population may precede clinically distinguishable retinopathy.The SVD, FAZ, RT, and retinal volume detected by OCTA may provide new avenues for the diagnosis of PAT.However, more studies are needed to provide evidence for future application in clinic.Therefore, in view of the important results of our study, more participants need to be enrolled in future research.
Our research has some limitations.The study included a relatively small sample, but despite this, SVD and RT were significantly different between the two groups.These results may contribute to future progress toward more precise methods to test retinal microvascular and microstructural alterations at an early stage.In addition, we found no correlation between SVD and RT in the PATs, which may need to be verified by more large-scale studies in the future.
In conclusion, we used OCTA to detect the retinal microvascular and microstructural alterations in MGD patients in a severely obese population.Our results showed reduced SVD in N, T, OS, IN, ON, OT; thinning of the RT in all subregions except central foveal; reduction in FAZ area and perimeter; significantly reduced SVD in the inner retina; and reduced retinal volume and average volume thickness.In addition, perimeter thinning at the FAZ was found to affect vision.OCTA may provide a new approach for early diagnosis of MGD in severely obese population.
ACKNOWLEDGEMENTS
Foundations:Supported by NationalNatural Science Foundation of China (No.82160195); Science and Technology Project of Jiangxi Provincial Department of Education (No.GJJ200169); Science and Technology Project of Jiangxi Province Health Commission of Traditional Chinese Medicine(No.2020A0087); Science and Technology Project of Jiangxi Health Commission (No.202130210).
Conflicts of Interest:Huang H,None;Wang XY,None;Wei H,None;Kang M,None;Zou J,None;Ling Q,None;Xu SH,None;Huang H,None;Chen X,None;Wang YX,None;Shao Y,None;Yu Y,None.
 International Journal of Ophthalmology2023年12期
International Journal of Ophthalmology2023年12期
- International Journal of Ophthalmology的其它文章
- Dynamic tear meniscus parameters in complete blinking:insights into dry eye assessment
- Effects of diquafosol sodium in povidone iodine-induced dry eye model
- Morroniside ameliorates lipopolysaccharide-induced inflammatory damage in iris pigment epithelial cells through inhibition of TLR4/JAK2/STAT3 pathway
- Role of reactive oxygen species in epithelial-mesenchymal transition and apoptosis of human lens epithelial cells
- Electroacupuncture alleviates ciliary muscle cell apoptosis in lens-induced myopic guinea pigs through inhibiting the mitochondrial signaling pathway
- De novel heterozygous copy number deletion on 7q31.31-7q31.32 involving TSPAN12 gene with familial exudative vitreoretinopathy in a Chinese family
