Effects of diquafosol sodium in povidone iodine-induced dry eye model
Can Zhang, He Wang, Dong-Yan Chen, Kai Zhao, Wei Wang, Ming-Xin Li
1Department of Ophthalmology, the First People’s Hospital of Lianyungang, Lianyungang 222000, Jiangsu Province, China
2Department of Ophthalmology, the Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu Province, China
3Department of Ophthalmology, Zhang Ye People’s Hospital Affiliated to Hexi University, Zhangye 734000, Gansu Province, China
Abstract
● KEYWORDS: diquafosol; povidone iodine; dry eye;mucin; inflammation; tear film; rats
INTRODUCTION
Dry eye disease (DED) is a heterogeneous disorder of the chronic ocular surface encompassing a range of signs and symptoms associated with impaired ocular lubrication.The abnormality in the quality, quantity, and dynamics of tear may result in tear film instability or imbalance of the ocular surface microenvironment, which may be accompanied by inflammatory reactions, ocular surface destruction, and abnormal distribution and function of the corneal nerve[1-2].
Tear film instability is the core pathogenic mechanism of dry eye[3], the mucin layer covers the ocular surface and sustains tear film stability.The destruction of ocular surface integrity, including goblet cell loss and apoptosis, may result in abnormality of mucins, which in turn leads to tear film instability.Tear instability is related in increased tear osmolarity, which results in ocular surface inflammation[4-5].
Povidone-iodine (PI) is widely used for preoperative disinfection of ophthalmic surgery with a broad spectrum of microbicidal activity[6].Many studies established that PI was toxic to cornea, with increasing exposure time of 5% PI.Kimet al[7]observed pathophysiological ocular surface changes,such as loss of goblet cell density and damaged cornea epithelium, which were similar to those observed with dry eye disease.
In our previous studies, we observed ocular surface changes of Sprague Dawley rats after topical instillation of PI (1%,twice daily, 14d).Tear break-up time (TBUT; 0.87±0.307s,8.15±0.564s;P<0.01) and tear production (1.47±0.374,7.96±0.422 mm;P<0.01) of rats in experimental group significantly decreased compared with rats in blank control group.On day 14, we collected eyeballs for hematoxylineosin (HE) and periodic acid-Schiff (PAS) staining, we observed loss of goblet cells and inflammation of cornea and conjunctiva in rats of experimental group.Clinical parameters and histopathological changes in rats were comparable to those with DED.In consequence, we successfully induced a dry eye model by 1% PI (Figures 1A, 2).
Diquafosol, a P2Y2 receptor agonist, could activate P2Y2 receptors in ocular surface, which exists in conjunctival epithelium, lacrimal gland and meibomian gland.It had been demonstrated to improve secretion of mucin and fluid transport,eventually maintain tear film stability[8-10].Many clinical trials reported encouraging clinical improvements with diquafosol in dry eye treatment, including improvements of corneal fluorescein staining score, TBUT and tear production[11-12].
This study aimed to investigate the changes of ocular surface with PI and therapeutic effects and mechanism of diquafosol in dry eye rats.
MATERIALS AND METHODS
Ethical ApprovalAll experiment procedures abided the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were ratified by the Ethics Committee of Xuzhou Medical University (Approval ID: 202110A336, Xuzhou,China).
Experiment AnimalsForty female Sprague Dawley rats(160-180 g, eight weeks old, provided by Xuzhou Medical University Laboratory Animal Center, Xuzhou, China) were selected.All rats were free of ocular disease with examining the anterior segmentviaslit lamp microscope.Tear production,fluorescein staining score and TBUT were measured to confirm the rats were without DED.Rats lived in a standard environment (room temperature 25°C±1°C, relative humidity 60%±10%, alternating 12-hour light-dark cycles), ate and drank at ease.
Experimental ProceduresTen female rats were used as blank control group, and 30 female rats were used to establish dry eye model with instillation of 20 μL 10 g/L PI solution both eyes twice daily for 14d (Yuexing, Xinghua, China).Tear production, fluorescein staining score and TBUT were measured to confirm the 30 rats were successfully induced DED.According to different treatments, the 30 rats with DED were divided into three groups: dry eye group with no treatment (DED group,n=10); phosphate buffer saline treated group (PBS group,n=10); diquafosol treated group (DQS group,n=10).The rats in DED group were not treated, both eyes of rats in PBS group were treated with 20 μL PBS solution and both eyes of rats in DQS group were treated with 20 μL diquafosol sodium eye drops (Santen, Osaka, Japan), both 6 times a day for 10d.It is necessary to be mentioned that the 3 animal groups were not installed with PI during the 10 daylong treatment period after the first 14d of PI instillations.We measured clinical parameters on days 3, 7, 10 after treatment,then overdose anesthetic was injected to all rats to collect their eye balls and adnexa for further histological analyses.
Ocular Surface Comprehensive AnalyzerOcular surface comprehensive analyzer (Kanghua, Chongqing, China) was used to detect the tear film stability and lipid film on rats by observing the flowing lipids on the eye surface and Placido ring.We recorded four to five consecutive blink cycles in all.The placido ring collected by camera was a complete concentric ring when tear film was intact, whereas the placido ring was irregular or distorted[13].Because ocular surface comprehensive analyzer examination is non-invasive, it was conducted firstly.
Measurement of Tear ProductionPhenol red cotton threads (Jingming, Tianjin, China) were used to evaluate tear production on days 3, 7, 10 after treatment.After isoflurane anesthesia, phenol cotton thread was immediately inserted under the lower conjunctival fornix of rats for 20s[14].The length of the wet red thread which observed with microscope in millimeters was measured to assess amount of tear production.The procedure was repeated at least three times,and the average values were calculated.
Corneal Fluorescein Staining and TBUTSlit lamp microscope with cobalt blue filter (Shangbang, Chongqing,China) was conducted to observe ocular surface.After dropping 2 μL of 1% fluorescein sodium into the lower conjunctival sac, the time(s) from the eyelid opening to the first dry spot turning up was recorded three times in succession.The average value was taken and recorded[15].The cornea was separated into four quadrants: supranasal, infranasal,supratemporal and infratemporal.Each quadrant was scored separately.The fluorescein staining score was calculated as below:0, non; 1, sparse staining less than 30 spots; 2, more than 30 staining spots, but not diffuse; 3, diffuse staining, but no plaque was formed; 4, positive plaques appear, such as ulcers[16].

Figure 2 The histologic changes of each group A: The representative images of HE staining of cornea of control group and model group; B: The representative images of PAS staining of conjunctiva of control group and model group.Scale bar: 100 μm.HE: Hematoxylin-eosin; PAS: Periodic acid Schiff.
In VivoConfocal Microscopy ExaminationAfter rats anesthetized, the examination of cornea was conducted by Heidelberg Retina Tomograph II (HRT).The target area and depth were adjusted manually and images (400×400 μm2in size) were captured.Images of each layer (epithelium, stroma,endothelium, and nerve fibers) were recorded for each eye.The inflammatory cells were counted on the stromal images among the four groups[17].
HistologyEyeballs and adnexa were fixed in 4%paraformaldehyde overnight, dehydrated with gradient ethanol successively, embedded with paraffin, then cut to 5-μm thick sections with microtome (Leica, Nussloch, Germany).HE staining were conducted to observe the corneal and conjunctival epithelium.PAS staining was performed to evaluate density of conjunctival goblet cells[18-19].Alcian blue staining were executed to observed the number of mucins on conjunctival sac[20].Three sections of 300 μm intervals were selected to stain.All stained sections were recorded with HD digital camera (Nikon, Tokyo, Japan).

Figure 3 The changes of TBUT and tear production A: TBUT on day 10 of treatment; B: TBUT at different time points of treatment in each group; C: Tear production on day 10 of treatment; D: Tear production at different time points of treatment in each group.Each value represents the mean±SD, n=10.aP<0.001 vs control group, bP<0.001 vs DED group, cP<0.001 vs PBS group.DED: Dry eye group with no treatment; PBS:Phosphate buffer saline treated group; DQS: Diquafosol treated group; TBUT: Tear break-up time.
Ultrastructural AnalysisThe cornea tissues were fixed in 2.5% glutaraldehyde for 2h and in 10 g/L osmium acid for 2h, dehydrated with gradient ethanol, immersed with epoxy resin, then stained with lead citrate and uranium acetate[21].The ultrastructural changes of corneal epithelium were evaluated with transmission electron microscope (TEM).Images were recorded with a camera (Hitachi, Tokyo, Japan).
Immunofluorescence StainingThe 5-μm thick paraffinic sections of eyeballs and adnexa were prepared for immunofluorescence staining.The sections were incubated with 0.3% Triton X-100 for 20min, incubated with 1% bovine serum albumin (BSA) for 1h, then incubated with primary antibody mucin 1 (MUC1; 1:250, Santa, Dallas, USA)or polyrmorphonuclear (PMN; 1:1000, Fitzgerald, USA)overnight.Then sections were incubated with a secondary antibody (1:500; abbkine, Wuhan, China) for 1h.At last sections were counterstained with DAPI (Solarbio, Beijing,China).Images were captured with a fluorescence microscope(Leica, Wetzlar, Germany)[22].
Examination of Cell Apoptosis with TUNEL StainingFor evaluating apoptosis in corneal epithelium, TUNEL assay was performed in 5 μm-thick paraffinic sections of cornea using thein situcell death detection kit fluorescein system (Promega,Wisconsin, USA).Sections were counterstained with DAPI[23].Images were obtained using a fluorescence microscope (Leica,Wetzlar, Germany).
Statistical AnalysisStatistical analysis was performed using GraphPad Prism version 8.0 (GraphPad Prism, San Diego, CA,USA) and all data were expressed as mean±standard deviation(SD).Difference between groups were analyzed by one-way ANOVA, andP<0.05 was defined as statistically significant.
RESULTS
Tear ProductionIn comparison to control group, as it turned out, tear production was significantly decreased after application of PI for 14d (7.96±0.422vs1.47±0.374 mm;P<0.01).Topical application of diquafosol for 10d significantly increased tear production in rats compared with DED and PBS groups (7.26±0.440vs4.07±0.474 mm; 7.26±0.440vs3.74±0.280 mm;P<0.01).Besides, there was no significant difference between DED group and PBS group (4.07±0.474vs3.74±0.280 mm;P=0.33; Figure 3C, 3D),n=10 for each group.
Corneal Fluorescein Staining and TBUTCompared with control group (8.15±0.564s), TBUT was significantly reduced after application of PI for 14d (0.87±0.307s,P<0.01).After 10d of treatment, DQS group (7.37±0.383s) demonstrated a significant increase in tear film TBUT compared with DED group (1.49±0.260s) and PBS groups (1.42±0.437s,allP<0.01), whereas the PBS group showed no significant improvement compared with the DED group (P=0.07; Figure 3A, 3B).
On day 10, the corneal fluorescein staining scores in the DED,PBS, and DQS groups were 10.0±0.943, 9.7±0.823, and 3.1±0.738, respectively.Treatment with topical diquafosol resulted in a significant decrease in corneal staining scores compared with DED and PBS groups (allP<0.01), indicating a reduction of epithelial injury and restoration of epithelial barrier function (Figure 1B-1D).

Figure 4 Examination of corneal confocal microscopy A: Representative images of corneal epithelial cells, subepithelial nerve fibers, corneal stroma, and corneal endothelial cells in each group for day 10 of treatment.Scale bar: 100 μm.B: The number of corneal inflammatory cells of each group.Each value represents the mean±SD, n=3.aP<0.05 vs control group, bP<0.001 vs control group, cP<0.001 vs DED group, dP<0.001 vs PBS group.DED: Dry eye group with no treatment; PBS: Phosphate buffer saline treated group; DQS: Diquafosol treated group.
Confocal MicroscopyConfocal microscope was used to observe the layers of cornea.In control group, the arrangement of corneal epithelial cells was tight, the neural stems were clear and straight, and endothelial cells were closely arranged.After application of PI, there were bending of neural stem, branches of rats and inflammatory cells infiltration (bright cells) in corneal stroma, in DED (156.3±12.50) and PBS (147.9±15.73)groups, whereas the inflammatory cells in DQS group(62.5±12.50) decreased significantly (P<0.01).The result showed that PI could cause inflammatory reaction and destroy corneal nerve fibers, whereas diquafosol could significantly inhibit inflammation, but short-term use of diquafosol had no effect on repairing corneal nerve (Figure 4).
Tear Film StabilityPlacido ring and lipid of rat ocular surface were observed by ocular surface comprehensive analyzer.Placido ring represents tear film stability.Placido rings were smooth and complete in control and DQS groups, rough and incomplete in PBS and DED groups (Figure 5B), lipid flowing of ocular surface was observed in DQS and control groups, but not in PBS and DED groups.The results showed that PI could destroy tear film stability and inhibit ocular surface lipids secretion, whereas diquafosol could repair tear film stability and increase the secretion of lipid (Figure 5A).
Histopathologic FindingsIn control group, corneal epithelium was intact and arranged orderly, with 4-6 layers, and endothelial cells arranged in a single layer.Corneal epithelial layers increased in DED and PBS groups,inflammatory cells infiltration and edema were seen in subepithelial stroma.Corneal layers in DQS group were roughly normal, there was no obvious inflammatory cells infiltration in stroma, and corneal edema was slight.It showed that PI could cause corneal epithelial injury and inflammation,whereas diquafosol could inhibit inflammation and promote corneal epithelial repair (Figure 6A).
Number of Conjunctival Goblet CellAfter PAS staining,the conjunctiva and goblet cells of rats in each group were observed by microscope, and the goblet cells were counted by Image J software.In control group, the conjunctival epithelial cells of rats arranged in order, whereas those in DED and PBS groups were irregular, disorganized, and the number of epithelium layers increased.The conjunctival epithelial cells of DQS group arranged orderly, and the number of layers was approximately normal (Figure 6B).In comparison to control group (11.74±1.346 cells/0.1 mm2), the density of conjunctival goblet cells in DED group (5.21±0.813 cells/0.1 mm2)and PBS group (5.36±0.615 cells/0.1 mm2) decreased(P<0.01).Compared with DED and PBS groups, the density of goblet cells in DQS group was significantly increased(8.45±0.718 cells/0.1 mm2,P<0.01).It showed that PI could cause goblet cells loss and epithelium destruction.Diquafosol could promote the regeneration of goblet cells and repair conjunctival epithelial cells (Figure 6D).
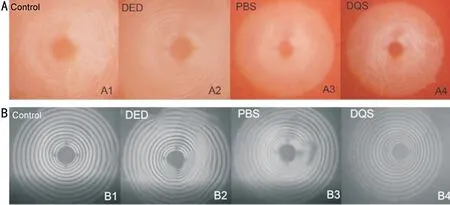
Figure 5 Examination of ocular surface comprehensive analyzer A: Lipid flowing on the ocular surface of each group; B: Placido ring of each group.DED: Dry eye group with no treatment; PBS: Phosphate buffer saline treated group; DQS: Diquafosol treated group.
Mucin Secretion in the ConjunctivaAfter alcian blue staining, the conjunctival mucins of rats in each group were observed by optical microscope, and the mucins were counted by Image J software.Compared with control group (13.45±1.18 cells/0.1 mm2), the number of mucins in DED group (7.23±0.614 cells/0.1 mm2) decreased significantly (P<0.01).Compared with DED and PBS groups(7.17±0.968 cells/0.1 mm2), the number of mucins in DQS group (11.83±0.828 cells/0.1 mm2) increased significantly(P<0.01).The results showed that PI could inhibit secretion of conjunctival mucin and diquafosol could significantly promote mucin secretion and maintain tear film stability (Figure 6C, 6E).After MUC1 immunofluorescence staining of the ocular sections of rats in each group, the positive expression in cornea and conjunctiva was observed under fluorescence microscope.Positive expression in cornea and conjunctiva could be observed in control group, and only a little positive expression could be observed in DED and PBS groups.Compared with DED and PBS group, the positive expression of DQS group increased.The results indicated that PI could inhibit MUC1 secretion in cornea and conjunctiva, while diquafosol could promote MUC1 secretion (Figure 7).

Figure 7 The MUC1 immunofluorescence staining of cornea and conjunctiva of each group A: The MUC1 immunofluorescence staining of cornea of each group; B: The MUC1 immunofluorescence staining of conjunctiva of each group.Scale bar: 100 μm.DED: Dry eye group with no treatment;PBS: Phosphate buffer saline treated group; DQS: Diquafosol treated group; HE: Hematoxylin-eosin; PAS: Periodic acid Schiff; MUC1: Mucin 1.

Figure 8 The PMN immunofluorescence staining of cornea and conjunctiva of each group A: The PMN immunofluorescence staining of cornea of each group; B: The PMN immunofluorescence staining of conjunctiva of each group.Scale bar: 100 μm.DED: Dry eye group with no treatment; PBS: Phosphate buffer saline treated group; DQS: Diquafosol treated group; PMN: Polymorphonuclear.
Inflammatory Cells InfiltrationThe positive expression of PMN (polymorphonuclear) in cornea and conjunctiva of rats in each group was observed under fluorescence microscope.No positive expression was found in control group, a large amount of expression was found in DED and PBS groups and positive expression decreased significantly in DQS group.The results indicated that PI could induce inflammation in the cornea and conjunctiva, and diquafosol could significantly inhibit inflammatory cells infiltration (Figure 8).
Apoptosis of Corneal Epithelium CellsAfter TUNEL staining, positive apoptotic cells were evaluated using fluorescence microscope.A few of apoptotic cells were observed in corneal epithelium of control and DQS groups,plenty of apoptotic cells were observed in the cornea of DED and PBS groups.The results showed that PI could induce corneal cell apoptosis, and diquafosol could effectively inhibit corneal cell apoptosis (Figure 9).
Effect of DQS in Changing Ultra-Structure in CorneaIn control group, microvillus extended in finger shape and arranged neatly on corneal epithelium, and intercellular connection was tight.In DED and PBS groups, only a few microvilli on the corneal epithelium, the desmosome structure was destroyed, and the intercellular connection was interrupted.The number of microvillus and desmosomes increased and cell junction partially recovered in DQS group.The results showed that PI could destroy the microvilli and desmosome structure, and diquafosol could repair the ultrastructure of cornea, promote formation of microvillus and desmosomes,and restore corneal intercellular connection (Figure 10).
DISCUSSION
In ophthalmic surgery, the ultimate purpose of using disinfectants is to prevent infectious endophthalmitis and PI is the most commonly used disinfectant[24].However, there is no standard on the optimal time, concentration and dose of PI solution for ophthalmic surgery disinfection.PI is used in ophthalmic surgery at a variety of concentrations (0.01%-10%),duration (30s-3min) and frequency (once or twice)[25-26].At present, exposure to 0.5% PI solution for 5min is considered to be the choice of eye disinfection before corneal transplantation[27].The ocular surface damage caused by PI infusion before cataract surgery, including goblet cell loss and corneal epithelial damage, is comparable to that of dry eye[28-29].Therefore, we hypothesized that povidone iodine solution may be the pathogenic factor of dry eye in patients undergoing ophthalmic surgery.

Figure 9 The TUNEL staining of cornea of each group Scale bra: 100 μm.DED: Dry eye group with no treatment; PBS: Phosphate buffer saline treated group; DQS: Diquafosol treated group.
We successfully induced the rat dry eye model in this study by dropping 10 g/L PI solution twice a day for 14d.The DED group had shorter tear film TBUT, higher fluorescein staining score and less tear production.The rougher Placido ring indicated the PI could disturb tear film stability.Based on pathological findings, we demonstrated that PI could damage corneal and conjunctival epithelium, induce inflammation reaction in cornea and conjunctiva, inhibit mucin secretion,destroy corneal ultrastructure and increase curvature and branching of corneal nerve fibers.
All of these alterations showed that changes of rat’s ocular surface were similar to symptoms of human dry eye syndrome.
The inflammation, conjunctival mucin loss and epithelial cell apoptosis caused by PI solution are very important in the pathogenesis of DED.Our dry eye model provided a wealth of information for investigating inflammatory, apoptosis, and mucin reduction mechanisms of dry eye.
The major function of goblet cells is to generate and secrete mucins.Mucins are linked to the microvillus of the epithelial cells[30], playing a critical function in maintaining hydrophilicity of tear film.It maintains the integrity of the tear film and decreases the surface tension, allowing a welldistributed distribution of the aqueous layer over the ocular surface[31].The loss of mucin could accelerate tear evaporation,contribute to tear hyperosmolarity, eventually lead to inflammation.Thus, microvilli degradation and mucin loss generated by PI could interfere the formation of mucosal layer and increase tear evaporation.
By acting on P2Y2 receptors on ocular surface and increasing intracellular Ca2+concentrations, diquafosol promotes tear and mucin production[31].It also improves fluid transport,eventually improve quantity and quality of tear film.In DQS group, TBUT and tear production increased and corneal fluorescein staining score decreased.We observed lipid flowing on ocular surface, indicating that diquafosol could promote lipid secretion and stabilize tear film.Meanwhile,corneal and conjunctival inflammation cells infiltration significantly decresed, indicating that diquafosol could inhibit stromal inflammatory infiltration.Last but not least, diquafoslo could inhibit corneal epithelium apoptosis and goblet cells loss,promote mucin secretion, repair damaged corneal epithelium and maintain tear film stability.
In conclusion, our study demonstrated PI could induce clinical symptoms and histopathological changes of dry eye.In our previous study, 10% PI solution instillation of Sprague Dawley rats once could cause corneal edema and exfoliation of the corneal epithelium right away.The toxicity of PI in cornea should be attach impotrance by ophthalmologists.The results showed that diquafosol could alleviate symptoms in the PIinduced rat dry eye model.Furthermore, diquafosol has the potential to reduce inflammation, inhibit corneal epithelial apoptosis, increase goblet cell density, and restore mucin secretion.
ACKNOWLEDGEMENTS
Conflicts of Interest: Zhang C,None;Wang H,None;Chen DY,None;Zhao K,None;Wang W,None;Li MX,None.
 International Journal of Ophthalmology2023年12期
International Journal of Ophthalmology2023年12期
- International Journal of Ophthalmology的其它文章
- Dynamic tear meniscus parameters in complete blinking:insights into dry eye assessment
- Morroniside ameliorates lipopolysaccharide-induced inflammatory damage in iris pigment epithelial cells through inhibition of TLR4/JAK2/STAT3 pathway
- Role of reactive oxygen species in epithelial-mesenchymal transition and apoptosis of human lens epithelial cells
- Electroacupuncture alleviates ciliary muscle cell apoptosis in lens-induced myopic guinea pigs through inhibiting the mitochondrial signaling pathway
- De novel heterozygous copy number deletion on 7q31.31-7q31.32 involving TSPAN12 gene with familial exudative vitreoretinopathy in a Chinese family
- A pedigree with retinitis pigmentosa and its concomitant ophthalmic diseases
