Systematic sequential therapy for ex vivo liver resection and autotransplantation: A case report and review of literature
Chen-Lu Hu,Xin Han,Zhen-Zhen Gao,Bo Zhou,Jin-Long Tang,Xiang-Ru Pei,Jie-Nan Lu,Qin Xu,Xiao-Ping Shen,Sheng Yan,Yuan Ding
Abstract BACKGROUND Perihilar cholangiocarcinoma (pCCA) is a highly malignant tumor arising from the biliary tree.Radical surgery is the only treatment offering a chance of longterm survival.However,limited by the tumor’s anatomic location and perivascular invasion,most patients lose the chance for curative treatment.Therefore,more methods to increase the resectability of tumors as well as to improve outcomes are needed.CASE SUMMARY A 68-year-old female patient had a hepatic hilar mass without obvious symptoms.Laboratory results showed hepatitis B positivity.Magnetic resonance imaging indicated that the mass (maximum diameter: 41 mm) invaded the left and right branches of the main portal vein,as well as the middle,left and right hepatic veins;enlarged lymph nodes were also detected in the hilum.The patient was diagnosed with pCCA,and the clinical stage was determined to be T4N1M0(stage IIIC).Considering the tumor’s anatomic location and vascular invasion,systematic conversion therapy followed by ex vivo liver resection and autotransplantation (ELRA) was determined as personalized treatment for this patient.Our original systemic sequential therapeutic strategy (lenvatinib and tislelizumab in combination with gemcitabine and cisplatin) was successfully adopted as conversion therapy because she achieved partial response after three cycles of treatment,without severe toxicity.ELRA,anastomotic reconstruction of the middle hepatic vein,right hepatic vein,root of portal vein,inferior vena cava and right hepatic artery,and lymph node dissection were performed at one month after systemic therapy.Pathological and immunohistochemical examination confirmed the diagnosis of pCCA with lymph node metastasis.Although the middle hepatic vein was partially obstructed four months later,hepatic vein stent implantation successfully addressed this problem.The patient has survived for 22 mo after the diagnosis,with no evidence of recurrence or metastasis.CONCLUSION An effective therapeutic strategy for conversion therapy greatly increases the feasibility and efficiency of ELRA.
Key Words: Perihilar cholangiocarcinoma;Ex vivo liver resection and autotransplantation;Systemic sequential therapy;Conversion therapy;Case report
INTRODUCTION
Originating from the biliary tree and/or within the hepatic parenchyma,cholangiocarcinoma (CCA) is a highly lethal epithelial cell malignancy.CCA predominantly arises from bile duct epithelial cells and displays features of cholangiocyte differentiation[1].These heterogeneous cancers can be classified according to anatomic subtype as intrahepatic CCA (iCCA),perihilar CCA (pCCA) or distal CCA (dCCA).pCCA is localized between the second-order bile ducts and the junction of the cystic duct into the common bile duct,dCCA is confined to the common bile duct below the cystic duct insertion,and iCCA is located within the liver parenchyma[2,3].Each of the anatomic subtypes is characterized by unique genetic aberrations,clinical presentations and management options.
As the most common subtype of CCA,pCCA accounts for more than 50% of cases,and radical surgical resection is the only treatment offering a chance of long-term survival for patients with pCCA[4].Unfortunately,due to its highly invasive biological characteristics and lack of specific symptoms,most pCCA patients are diagnosed with advanced disease,which decreases the opportunity of radical surgery[5].In addition,extensive hilar invasion,liver involvement and vascular encasement often preclude curative resection.Therefore,the traditional surgical effect is far from satisfactory;liver transplantation may be a more promising option for pCCA patients[6].
In 1988,exvivoliver resection and autotransplantation (ELRA) was first introduced by Professor Pichlmayretal[7] as an alternative to liver transplantation for unresectable hepatic tumors,and ELRA has been constantly developed to improve the resectability of hepatobiliary malignancies.In 2003,Chuietal[8] first reported a type IV pCCA patient who underwent ELRA and survived for two years without any sign of tumor recurrence.Currently,the rapid development of autologous liver transplantation surgical techniques and vascular reconstruction techniques as well as the application of novel immunosuppressive agents are greatly contributing to expanding the limits of resectability and reducing the incidence of chronic allograft rejection,which may greatly benefit select patients[9,10].However,because of the rigorous admission criteria for liver transplantation,more methods to increase the feasibility of ELRA are needed[11].
Recently,conversion therapy using systematic therapy and/or nonsurgical local therapy to inhibit tumor progression,reduce the tumor burden,and even decrease TNM staging has provided patients with the opportunity for radical surgery and significantly improved prognosis[1,12,13].In 2021,we first reported that an original systemic sequential therapeutic strategy (gemcitabine 1000 mg/m2and cisplatin 25 mg/m2on days 1 and 8;lenvatinib 8 mg/d from days 1 to 21;tislelizumab 200 mg on day 15) showed reliable treatment effects on conversion surgery for advanced iCCA patients[14].Here,we present our new single-center experience with this conversion therapy strategy for treatment of pCCA before ELRA.The prognosis of our 68-year-old female patient was favorable,and no evidence of tumor recurrence was found until July 15,2023.
CASE PRESENTATION
Chief complaints
A 68-year-old female patient was incidentally found to have a mass in the second hepatic portal region to the caudate lobe at a local hospital and was admitted to our hospital for treatment on August 30,2021.
History of present illness
No special discomfort was reported by the patient.
History of past illness
None.
Personal and family history
None.
Physical examination
No signs were detected by physical examination.
Laboratory examinations
No abnormal laboratory results were recorded,except for alanine aminotransferase (ALT) 43 U/L and aspartate aminotransferase (AST) 46 U/L;anti-HBs (+),anti-HBe (+) and anti-HBc (+) were also obtained.We performed liver biopsy,and the results revealed moderately poorly differentiated adenocarcinoma (Figure 1A and B).Immunohistochemical staining results were as follows: CK7 (+),CK19 (+),HepPar-1 (-),arginase-1 (-) and Ki-67 labeling index 40%.
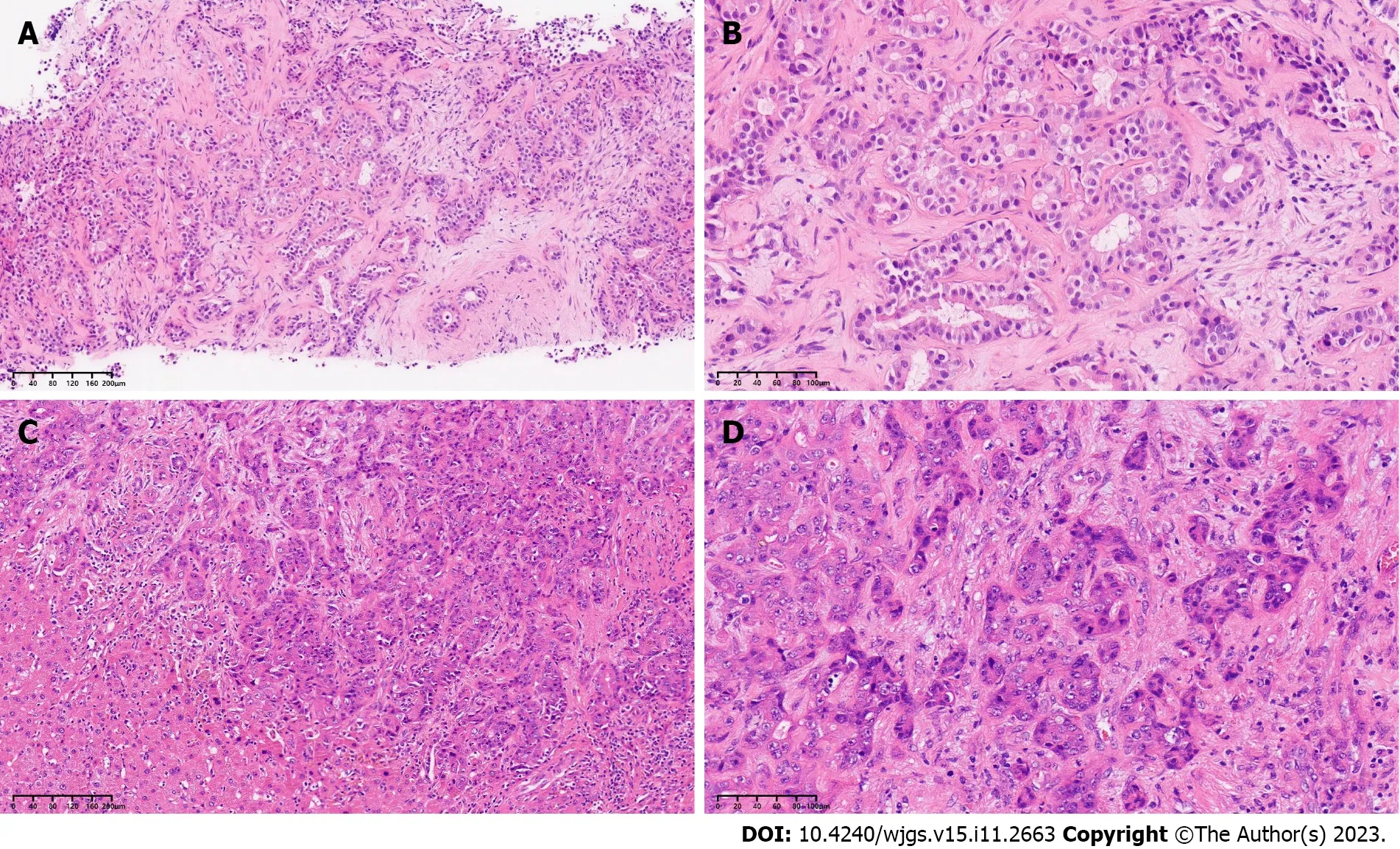
Figure 1 Pathological examination of the tumor before and after systematic sequential therapy. A and C: Hematoxylin and eosin (H&E),original magnification × 100;B and D: H&E,original magnification × 200.
Imaging examinations
B-scan ultrasound showed a hilar mass that led to expansion of the intrahepatic bile duct in the left lobe of the liver.Contrast-enhanced magnetic resonance imaging (MRI) scanning found that the mass (maximum diameter: 41 mm)invaded the left and right branches of the main portal vein as well as the middle,left and right hepatic veins (Figure 2AD).Enlarged hilar lymph nodes were also detected by MRI (Figure 2E).However,there was no invasion of the abdominal aorta or its branches and no filling defect in the inferior vena cava according to computed tomography (CT) imaging of abdominal vessels.
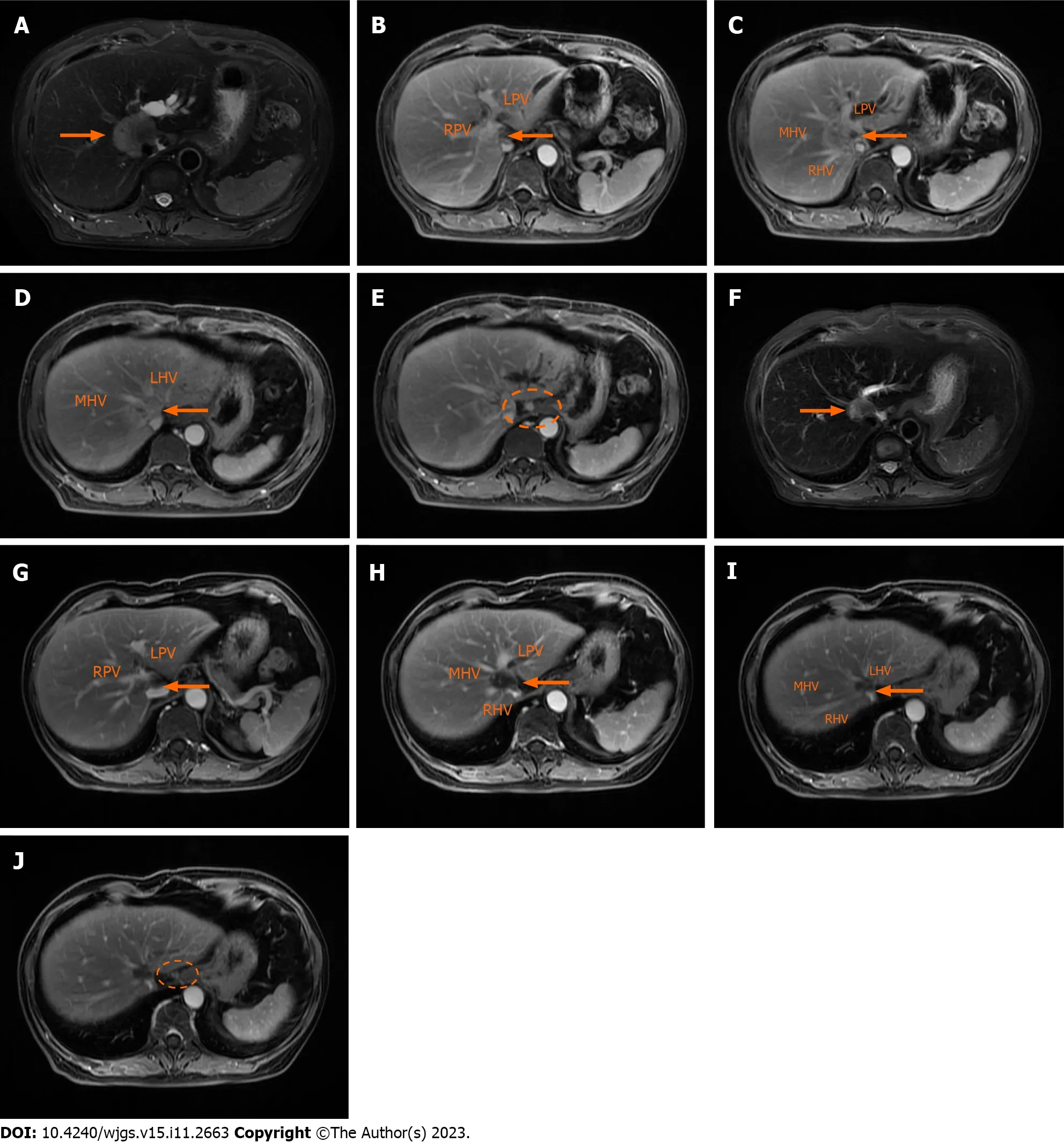
Figure 2 Contrast-enhanced magnetic resonance images of the irregular tumor or enlarged hilar lymph node before and after systematic sequential therapy. The white arrow indicates the tumor,and the white circle contains an enlarged lymph node.A: T2 phase image before therapy showing a hilar mass with a maximum diameter of 41 mm;B-D: Portal venous phase image before therapy showing that the tumor invaded the main portal vein with left and right branches,as well as the middle,left and right hepatic veins;E: Portal venous phase image before therapy showing the enlarged hilar lymph node;F: T2 phase image after therapy showing that a hilar mass shrunk to a maximum diameter of 21 mm;G-I: Portal venous phase image after therapy;J: Portal venous phase image after therapy showing that the enlarged hilar lymph nodes were smaller.RPV: Right portal vein;LPV: Left portal vein;MHV: Middle hepatic vein;RHV: Right hepatic vein;LHV: Left hepatic vein.
FINAL DIAGNOSIS
We made a diagnosis of pCCA with lymph node metastasis.The Child-Pugh score was A with 5 points.The TNM clinical stage was determined as T4N1M0 (stage IIIC) according to the American Joint Committee on Cancer staging system,8thedition.
TREATMENT
After full consideration,we determined that the patient had lost the chance for surgery and was scheduled for a personalized therapeutic scheme (intravenous gemcitabine 1000 mg/m2and cisplatin 25 mg/m2on day 1,day 8;oral lenvatinib 8 mg/d from day 1 to day 21;intravenous tislelizumab 200 mg on day 15).No grade 3/4 treatment-related adverse effects were detected after three cycles of systemic treatment.Further MRI indicated that the mass in the hilar was significantly smaller than that previously (Figure 2F-I).The diameter of the largest lesion had decreased from 41 mm to 21 mm.According to the standard RECIST 1.1 criteria,the patient successfully achieved partial response (PR),and the lymph nodes in the hilar region were also smaller than before (Figure 2J).Consequently,the preoperative multidisciplinary team carefully reviewed all preoperative results and proposed a surgical strategy for autologous liver segment autotransplantation.
The surgery was performed on December 1,2021,and lasted for 740 min.Three-dimensional reconstruction(Figure 3A) and intraoperative exploration revealed that the tumor invaded the root of the left and right hepatic ducts,left and right portal veins,and middle and right hepatic veins.First,the left hemiliver was completely removedinvivo,and the middle hepatic vein was retained in the right lobe (Figure 3B).When the right lobe including the tumor was resected (Figure 3C),the right lobe was immediately placed in an ice basin and perfused with 4°C histidine-tryptophanketoglutarate solution.Invitro,the tumor was completely resected,and we further used the allogeneic iliac vein to lengthen the middle and right hepatic veins.In addition,the allogeneic iliac vein was used as the bypass between the portal vein and inferior vena cava during the anhepatic phase.Finally,anastomotic reconstruction of the middle hepatic vein,the right hepatic vein,the root of portal vein,inferior vena cava,and right hepatic artery were performed sequentially (Figure 3D).Moreover,the hepatic portal lymph nodes and para-esophageal lymph nodes were dissected.

Figure 3 The key intraoperative process. A: Three-dimensional reconstruction of the relationship between the hepatic vein and tumor after systematic sequential therapy;B: The right hemiliver retaining the middle hepatic vein;C: Removal of the right hemiliver and resection of the tumor invading the inferior vena cava;D: Reconstruction of the middle hepatic vein,inferior vena cava and right hepatic artery.IVC: Inferior vena cava;MHV: Middle hepatic vein;PV: Portal vein;RHA: Right hepatic artery;RHD: Right hepatic duct;SHVC: Superior hepatic vena cava.
OUTCOME AND FOLLOW-UP
Postoperative pathological examination (Figure 1C and D) showed the following: The tumor bed with a large necrotic area,interstitial fibrosis and tumor residue was 4.0 cm × 3.0 cm in size as well as a negative surgical margin;the hepatic portal lymph nodes were 0/3 positive,but the para-esophageal lymph nodes were 2/2 positive;no microvascular or Glisson's capsule invasion was found.Results of immunohistochemical analysis were as follows: CK7 (+),CK19 (+),HepPar-1 (-),Arginase-1(-) and Ki-67 labeling index 80% (Figure 4).The patient was sent to the intensive care unit and extubated on postoperative day 2.Tacrolimus (0.5 mg,QD7;1 mg,QD19) was administered to prevent immune rejection,and the value of FK506 was closely monitored.According to postoperative ultrasound,the maximum blood flow velocities of the portal vein,hepatic artery,right hepatic vein and middle hepatic vein were 40.64 cm/s,70.96 cm/s,69.02 cm/s and 23.22 cm/s,respectively.No severe complications occurred during hospitalization.The patient was successfully discharged on postoperative day 22 with normal liver and heart function.
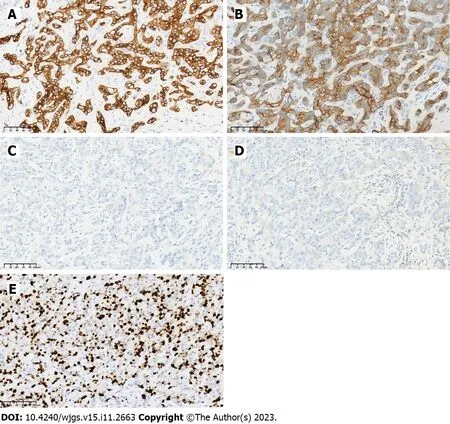
Figure 4 Immunohistochemical staining results for the tumor. A: CK7 (+);B: CK19 (+);C: HepPar-1 (-);D: Arginase-1 (-);E: Ki-67.
At 2 mo after the surgery,the patient continued to take oral capecitabine (1750 mg,twice daily) and lenvatinib (8 mg,once daily) as maintenance treatments.At a 3 mo follow-up,the patient felt subjectively well,and no obvious abnormity was found by contrast-enhanced MRI re-examination (Figure 5A and B).Unfortunately,abdominal enhanced CT indicated the existence of hepatic congestion and hepatomegaly on April 19,2022 (Figure 5C).Her liver function and coagulation function were abnormal,showing the following: ALT 52 U/L,AST 92 U/L,total bilirubin 40.3 μmol/L,direct bilirubin 17.5 μmol/L,indirect bilirubin 22.8 μmol/L,albumin 32.1 g/L,and serum D-dimer 1040 μg/L.Furthermore,the middle hepatic vein was partially obstructed,and the maximum blood flow velocities of the portal vein,hepatic artery,right hepatic vein and middle hepatic vein were significantly slower than before.Considering that a thrombus may further impair liver function,the patient underwent hepatic vein stent implantation on May 5,2022.After treatment,her liver function returned to normal levels.Postoperative ultrasonography indicated that blood flow in the stent was clear,and the maximum blood flow velocity of the stenting area was 118.8 cm/s.
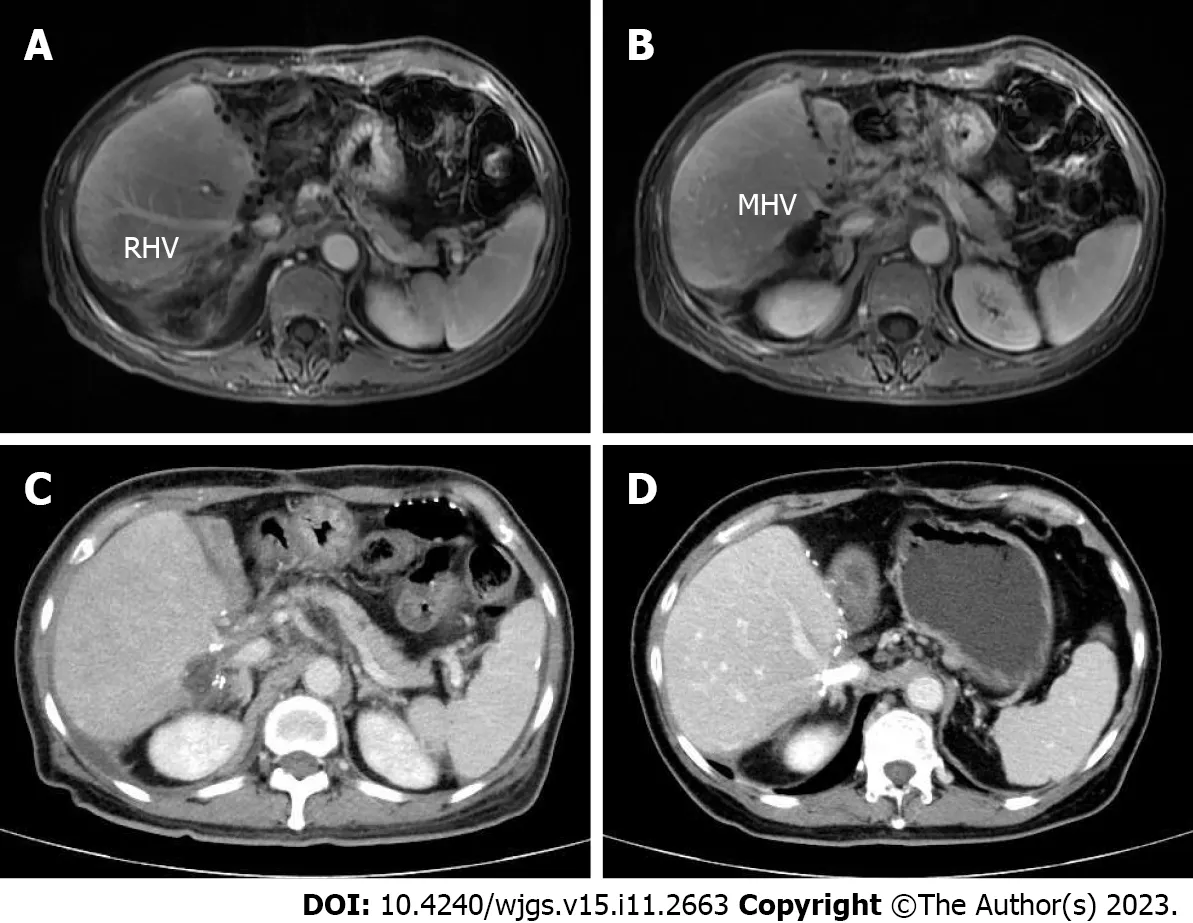
Figure 5 Contrast-enhanced magnetic resonance images and computed tomography images after surgery. A and B: Portal venous phase image after surgery clearly showing the middle hepatic vein and right portal vein,respectively;C: Portal venous phase image showing the existence of hepatic congestion and hepatomegaly;D: Portal venous phase image showing the hepatic vein stent.MHV: Middle hepatic vein;RHV: Right hepatic vein.
One month later,the patient received six cycles of targeted therapy and immunotherapy (lenvatinib 8 mg/d from day 1 to day 21;tislelizumab 200 mg on day 15).Further follow-ups showed no evidence of local recurrence or distant metastasis,and the hepatic vein stent was good (Figure 5D).To date,the patient has survived well without any severe discomfort for more than 22 mo.Figure 6 shows this patient’s timeline of initial diagnosis,systemic therapy,surgery,adjuvant therapy and follow-up.
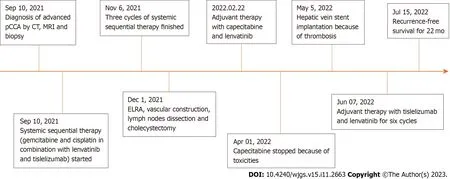
Figure 6 Timeline of the initial diagnosis,systemic therapy,surgery,adjuvant therapy and follow-up. ELRA: Ex vivo liver resection and autotransplantation;Pcca: Perihilar cholangiocarcinoma;CT: Computed tomography;MRI: Magnetic resonance imaging.
DISCUSSION
Currently,pCCA is a malignancy with the most dismal prognoses,and the gold standard curative therapy is radical resection.Unfortunately,less than 50% of pCCA patients are eligible for resection[15],and the incidence of R0 resection reported in patients undergoing surgery is only 45%[16].A need for improvement in tumor resectability and resection safety therefore seems obvious.Of note,in contrast to liver resection,liver transplantation is logically an attractive alternative to address most problems,such as the high potential of residual tumor as well as unresectable disease with vascular involvement[6].ELRA,a unique type of liver transplantation,provides several benefits.It may improve tumor accessibility to achieve complete tumor resection with clear margins,achieve complex vascular reconstruction,reduce ischemic damage to the organ through use of cold preservation solution,decrease demand for organ donors and reduce immunosuppression compared with allotransplantation[17].In recent years,there have been numerous reports related to successful treatment with ELRA[9,18-21].Based on their large collective experience,Weineretal[9] reported relatively favorable outcomes after ELRA in select patients.A systematic review and meta-analysis revealed an R0 resection rate of 93.4% and a 1-year survival of 78.4% with ELRA[22].In addition,one study demonstrated that liver transplantation for pCCA led to similar or even better outcomes than with hepatocellular carcinoma or cirrhosis due to various causes[23].Therefore,ELRA may become a more widely accepted and practical treatment option for conventionally unresectable hepatobiliary tumors.
However,not all patients are eligible for ELRA.Ideal candidates are those with good functional reserve,can tolerate the procedures,and have a less aggressive malignant tumor[17].According to data from the Mayo Clinic,a mass not larger than 3 cm in diameter is the selection criterion for pCCA[24].In our case,the malignancy was 4.1 cm in diameter,and the tumor was located in the porta hepatis with involvement of the middle hepatic vein,portal vein and inferior vena cava.Although ELRA and vascular reconstruction may offer R0 resection and excellent survival,the patient did not have sufficient indications.Hence,it was crucial to reduce the tumor burden by some effective means before ELRA to allow the patient to obtain more benefits.
Based on our center’s experience,conversion therapy[25] involving multidisciplinary systematic treatment preoperatively and aiming to render the tumor more amenable to surgical removal can be regarded as a more suitable pre-ELRA treatment for this patient.At present,there are various systematic treatment strategies.Over the last decade,doublet chemotherapy with gemcitabine and cisplatin has been considered the most effective first-line treatment for pCCA,as based on the results of the ABC-02 trial (NCT00262769) reported in 2010[26].In the new era of targeted therapy and immunotherapy,systemic therapy with molecular and immune therapies has dramatically changed management of pCCA at advanced stages.Notably,the randomized,double-blind,phase 3 TOPAZ-1 trial (NCT03875235) showed that adding the PD-L1 inhibitor durvalumab to gemcitabine and cisplatin significantly improved overall survival (OS) for advanced biliary tract cancer (BTC) compared with gemcitabine and cisplatin alone: Median OS: 12.8 mo [95% confidence interval (95%CI): 11.1-14.0]vs.11.5 mo (95%CI: 10.1-12.5);hazard ratio (HR) 0.80;two-sidedP=0.021[27].Another recent phase 3 clinical trial (KEYNOTE-966;NCT04003636) found that adding pembrolizumab to gemcitabine and cisplatin remarkably ameliorated OS for advanced BTC [median OS: 12.7 mo (95%CI: 11.5-13.6) in the pembrolizumab groupvs.10.9 mo (95%CI: 9.9-11.6) in the placebo group;HR 0.83;one-sidedP=0.0034][28].Thus,combination chemotherapy and immunotherapy are safe and effective for patients with pCCA.
Lenvatinib,a multikinase inhibitor that targets vascular endothelial growth factor (VEGF) receptor 1-3,fibroblast growth factor receptor 1-4,platelet-derived growth factor receptor-α,RET,and KIT,is widely used for many solid tumors,especially hepatobiliary malignancies[29-33].More than 50% VEGF overexpression is detected in CCA[34].Based on this evidence,we deeply considered whether systematic therapy,including chemotherapy,targeted therapy and immunotherapy,is sensible for CCA patients.We first reported based on our previous clinical practice that systemic sequential therapy with gemcitabine,cisplatin,lenvatinib and tislelizumab offers great therapeutic effects for preoperative advanced iCCA conversion therapy[14].Zhangetal[35] reported a patient with iCCA who first received six cycles of conversion therapy (gemcitabine 1000 mg/m2and cisplatin 25 mg/m2on day 1 and day 8;pembrolizumab,200 mg every 3 wk;lenvatinib 8 mg from day 1 to day 21) and achieved pathologic PR without severe toxicity,followed by radical liver resection,cholecystectomy and hilar lymph node dissection.A single-arm phase 2 study (NCT03951597) to explore the efficacy and safety of a similar systemic sequential therapy for iCCA was completed in 2022[36].The objective response rate was 80% and the median OS 22.5 mo (95%CI: 15.6-29.3) after combination therapy of toripalimab,lenvatinib,and gemcitabine plus oxaliplatin for advanced iCCA when the median follow-up time was 23.5 mo.In our case,our previous systemic sequential therapy (gemcitabine 1000 mg/m2and cisplatin 25 mg/m2on day 1 and day 8;lenvatinib 8 mg from day 1 to day 21;tislelizumab 200 mg on day 15) was successfully administered to a pCCA patient as conversion therapy,without any grade 3/4 adverse effects,before ELRA and allogeneic vascular reconstruction.The old patients ultimately achieved excellent outcomes.
CONCLUSION
In conclusion,this is the first case report of a novel systemic sequential therapeutic strategy followed by ELRA and vascular reconstruction for pCCA.The female patient achieved PR after the scheduled therapy,and no obvious overlapping toxicities occurred during that period.Considering the tumor’s anatomic location and vascular invasion,ELRA and vascular reconstruction was considered to be a better treatment option.The tumor was successfully resectedex vivowith a negative surgical margin;the hepatic vein and inferior vena cava were smoothly reconstructed with the allogeneic iliac vein.This case indicates that an effective therapeutic strategy for conversion therapy can greatly increase the feasibility and efficiency of ELRA and vascular reconstruction.Currently,our phase 2 clinical trial (NCT05532059),designed to explore the efficacy and safety of this systemic therapy for CCA patients,is ongoing,and we hope to provide more beneficial treatment options for advanced CCA.
FOOTNOTES
Co-first authors:Chen-Lu Hu and Xin Han.
Co-corresponding authors:Sheng Yan and Yuan Ding.
Author contributions:Yan S and Ding Y conceived of,designed and refined the study protocol;Hu CL,Han X,Gao ZZ,Zhou B,Tang JL,Fei XR,Lu JN,Xu Q,and Shen XP were involved in the data collection and analysis;Hu CL and Han X drafted the manuscript;all authors were involved in the critical review of the results and contributed to,read,and approved the final manuscript.Hu CL and Han X equally contributed to this work as co-first authors;Yan S and Ding Y contributed equally to this work as co-corresponding authors.There are two reasons for this designation.First,the research was performed as a collaborative effort,and the designation of cocorresponding authorship accurately reflects the distribution of responsibilities and burdens associated with the time and effort required to complete the study and the resulting paper.This also ensures effective communication and management of postsubmission matters,ultimately enhancing the paper's quality and reliability.Second,Hu CL and Han X contributed equally to the research process.The choice of these researchers as co-first authors acknowledges and respects this equal contribution while recognizing the spirit of teamwork and collaboration of this study.In summary,we believe that designating Hu CL and Han X as co-first authors/Yan S and Ding Y as co-corresponding authors is appropriate for our manuscript,as it accurately reflects our team's collaborative spirit,equal contributions,and diversity.
Informed consent statement:All study participants,or their legal guardian,provided informed written consent prior to study enrollment.
Conflict-of-interest statement:All authors declare that they have no competing interests.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016),and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Xin Han 0000-0001-5282-9927;Bo Zhou 0000-0002-4139-5462;Sheng Yan 0000-0002-4153-3546;Yuan Ding 0000-0002-3840-9886.
S-Editor:Lin C
L-Editor:A
P-Editor:Lin C
 World Journal of Gastrointestinal Surgery2023年11期
World Journal of Gastrointestinal Surgery2023年11期
- World Journal of Gastrointestinal Surgery的其它文章
- Gastric inflammatory myofibroblastic tumor,a rare mesenchymal neoplasm: A case report
- Comprehensive treatment and a rare presentation of Cronkhite-Canada syndrome: Two case reports and review of literature
- Isolated traumatic gallbladder injury: A case report
- Metachronous primary esophageal squamous cell carcinoma and duodenal adenocarcinoma: A case report and review of literature
- Organ sparing to cure stage IV rectal cancer: A case report and review of literature
- Effect of perioperative branched chain amino acids supplementation in liver cancer patients undergoing surgical intervention: A systematic review
