Multi-national observational study to assess quality of life and treatment preferences in patients with Crohn’s perianal fistulas
Chitra Karki,Amod Athavale,Vijay Abilash,Gary Hantsbarger,Parnia Geransar,Kate Lee,Slobodan Milicevic,Marko Perovic,Leanne Raven,Magdalena Sajak-Szczerba,Abigail Silber,Annabelle Yoon,Phil Tozer
Abstract BACKGROUND Patients with Crohn’s disease (CD) are at risk of developing complications such as perianal fistulas.Patients with Crohn’s perianal fistulas (CPF) are affected by fecal incontinence (FI),bleeding,pain,swelling,and purulent perianal discharge,and generally face a higher treatment burden than patients with CD without CPF.AIM To gain insights into the burden of illness/quality of life in patients with CPF and their treatment preferences and satisfaction.METHODS This cross-sectional observational study was conducted in patients with CD aged 21-90 years via a web-enabled questionnaire in seven countries (April-August 2021).Patients were recruited into three cohorts: Cohort 1 included patients without perianal fistulas;cohort 2 included patients with perianal fistulas without fistula-related surgery;and cohort 3 included patients with perianal fistulas and fistula-related surgery.Validated patient-reported outcome measures were used to assess quality of life.Drivers of treatment preferences were measured using a discrete choice experiment (DCE).RESULTS In total,929 patients were recruited (cohort 1,n=620;cohort 2,n=174;cohort 3,n=135).Short Inflammatory Bowel Disease Questionnaire scores were worse for patients with CPF (cohorts 2 and 3) than for those with CD without CPF (cohort 1): Mean score 3.8 and 3.7 vs 4.1,respectively,(P < 0.001).Similarly,mean Revised FI and FI Quality of Life scores were worse for patients with CPF than for those with CD without CPF.Quality of Life with Anal Fistula scores were similar in patients with CPF with or without CPF-related surgery (cohorts 2 and 3): Mean score 41 and 42,respectively.In the DCE,postoperative discomfort and fistula healing rate were the most important treatment attributes influencing treatment choice: Mean relative importance 35.7 and 24.7,respectively.CONCLUSION The burden of illness in CD is significantly higher for patients with CPF and patients rate lower postoperative discomfort and higher healing rates as the most desirable treatment attributes.
Key Words: Burden of illness;Crohn’s disease;Discrete choice experiment;Perianal fistulas;Patient-reported outcomes;Treatment preferences
INTRODUCTION
Crohn’s disease (CD) is a chronic progressive inflammatory disease of the gastrointestinal tract,with an annual global incidence of up to 20.2 cases per 100000 persons[1,2].Patients with CD are at risk of developing complications such as perianal fistulas (PF),which are estimated to develop in up to 50% of patients[3,4].It has been estimated that up to 73% of patients with Crohn’s perianal fistulas (CPF) are affected by fecal incontinence (FI)[5-7].Symptoms specifically related to fistulas often include bleeding,pain,swelling,and purulent perianal discharge,and patients with CPF generally face a higher treatment burden than patients with CD without PF[3,8-10].
There are many treatments utilized for the care of patients with CPF that are aimed at initial disease control,symptom reduction,or fistula healing,depending on the nature of the fistulas and surrounding perianal disease,overall luminal disease,and the personal treatment goals.Treatment options for the management of CPF include seton placement for drainage,pharmacological therapies (e.g.,antibiotics,immunomodulators,and anti-tumor necrosis factor agents),and surgical procedures (e.g.,ligation of the intersphincteric fistula tract,advancement flaps,and newer procedures including fistula plugs,fibrin glue,and fistula tract laser closure)[8,11];however,with limited evidence to support the use of these treatments,there is a lack of consensus on the standard of care for patients with CPF[3,12-15].Most treatments for CPF are associated with low rates of remission and high rates of relapse or treatment failure,leading to patients undergoing repeated cycles of treatments and surgeries[4,16-18].
Published studies on the burden of illness and quality of life for patients with CPF are limited[4].This cross-sectional multi-country observational study was conducted to gain a more in-depth understanding of the burden of illness of CPF through a comparison of the disease burden,treatment experiences,preferences and satisfaction,and health-related quality of life (HRQoL) for patients with CPF and patients with CD without PF.Furthermore,this study compared these outcomes for patients with and without PF-related surgery to assess the impact of PF-related surgery on the burden on CPF.
Assessing patient treatment preferences is key to helping healthcare professionals with clinical management and treatment decisions associated with CPF.Given the heterogeneous treatment options available to patients with CPF(pharmacological therapies,seton placement/palliative treatment,surgical options,and stem cell therapies),this study assessed patients’ treatment preferences and satisfaction using a discrete choice experiment (DCE) methodology.DCEs are designed to elicit preferences in the healthcare setting and have been utilized increasingly over the past decade[19-23].In a DCE,patients are asked to select their preferred choice from a set of hypothetical treatment profiles that describe attributes such as treatment efficacy,treatment side effects,or health states to identify the relative importance of these treatment attributes and an underlying utility function[24].To our knowledge,this study includes the first DCE conducted in a population of patients with CPF.
MATERIALS AND METHODS
Study design
This cross-sectional observational study was conductedviaa 45-min web-enabled patient questionnaire in seven countries (France,Germany,Spain,United Kingdom,Canada,Australia,and Japan) from April 2021 to August 2021.Patient recruitment was undertaken by a third-party recruitment company,Dynata LLC (New York,United States).Patients in Dynata’s online panel of patients were invited to participate based on profile data including self-reported physician-diagnosed CD.The questionnaire was pre-tested by conducting patient interviews (n=7,60 min each) across key countries to assess whether the comprehension of the questions was as intended and to identify potential sources of response error.Patients aged 21-90 years at the time of consent were eligible if they had a self-reported physician diagnosis of CD and had either been treated for CPF in the past 12 mo (CPF cohorts) or never experienced PF (non-PF CD cohort).Patients with a diagnosis of ulcerative colitis were excluded.
Based on maximum feasibility,research questions,and the objectives of the study,the global target study size wasN=855 (n=150 Canada,France,Germany,and United Kingdom;n=120 Spain;n=90 Australia;n=45 Japan).Patients were recruited into one of three cohorts based on their responses to carefully tailored screening questions prior to entering the web-enabled questionnaire: Cohort 1 included patients with CD who had never experienced perianal fistulas (non-PF CD),cohort 2 included patients with CPF who had no PF-related surgery in the past 12 mo but may have received pharmacotherapy and/or seton placement,and cohort 3 included patients with CPF who had PF-related surgery in the past 12 mo (with or without pharmacotherapy and/or seton placement).For the purposes of this study,only reparative/interventional PF-related procedures were considered as surgery (seton placement was not included in this description because almost all patients with CPF will undergo seton placement);hence patients in cohort 2 (without PF-related surgery) as well as patients in cohort 3 may have received seton placement.
Study objectives
The co-primary objectives of the study were to compare the HRQoL and treatment experiences,preferences,and satisfaction of patients with CD with and without CPF in an international study across seven countries,using standard validated general and disease-specific patient-reported outcomes measures and a DCE.
The secondary objective of the study was to compare HRQoL and treatment experiences,preferences,and satisfaction among patients with CPF who had PF-related surgery (with or without pharmacotherapy) with those patients with CPF who had no PF-related surgery in the past 12 mo.
Study measures
Patient-reported outcome measures were used to assess the HRQoL (disease specific),FI,and its impact on HRQoL,and general health status of participating patients.
HRQoL
The HRQoL measures administered in this study included the Short Inflammatory Bowel Disease Questionnaire (SIBDQ)[25] and the Quality of Life in patients with Anal Fistula (QoLAF) questionnaire[26].SIBDQ is a 10-item questionnaire designed to assess the impact of inflammatory bowel diseases in general on HRQoL,with each item scored on a 7-point scale (1=poor health-related quality of life,7=optimum health-related quality of life).The recall period was 2 wk,and a difference of 9 points was considered a clinically significant difference based on total score (prior to dividing the total score by 10)[26].The QoLAF questionnaire,designed to specifically assess the impact of anal fistulas on HRQoL,is composed of physical impact and biopsychosocial impact domains and summed scores range from 14 to 70 (14 points=zero impact,15-28 points=limited impact,29-42 points=moderate impact,43-56 points=high impact,and 57-70 points=very high impact)[26,27].
Fecal incontinence
FI and its impact on daily life was measured using the Revised Faecal Incontinence Score (RFIS)[28] and the Fecal Incontinence Quality of Life (FIQL) questionnaire[29].The RFIS is a questionnaire with five items related to FI and leakage altering a person’s lifestyle and two additional items related to FI associated with urge and undergarment soiling.Scores range from 0 to 20 (≤ 3=none or very mild FI,4-6 mild FI,7-12 moderate FI,≥ 13 severe FI).Scores for each item were summed and the mean was taken.The recall period for the RFIS was 4 wk.The FIQL is a 29-item questionnaire composed of four domains: Lifestyle,coping/behavior,depression/self-perception,and embarrassment.Scores range from 1 to 5 for each domain (no overall score),with a lower score indicating a worse HRQoL in that domain.The minimally important difference is 1.1-1.2 points per subscale[29,30].The recall period for the FIQL was “the last month”.
Health status (EQ-5D)
The EuroQol EQ-5D-5L questionnaire was utilized to assess the overall health status of the participating patients at the time of survey completion[31].The questionnaire measures five dimensions of health including mobility,self-care,usual activities,pain/discomfort,and anxiety/depression and also includes a visual analog scale (VAS) to rate overall health.Each dimension has 5 levels: No problems,slight problems,moderate problems,severe problems,and extreme problems.The total score ranges from 0 to 1,with a higher score indicating a better HRQoL.In countries where descriptions for only 3 levels of each dimension were published (EQ-5D-3L),a crosswalk score that maps EQ-5D-3L to EQ-5D-5L (3vs5 response options) was utilized.
Drivers of treatment preferences: DCE
Patient preferences for CPF treatment attributes were assessed through a DCE in patients with CPF.The DCE for this study included six treatment attributes across 2-4 levels (Table 1).It was estimated that a sample size of 260 patients would be sufficient to analyze each attribute,based on guidance by Yangetal[32].Levels for each treatment attribute were derived from evidence in currently available literature[33-46] and used to develop hypothetical treatment profiles.The attributes included type of treatment,treatment success rate (overall success rate,potentially including radiologic healing rate),postoperative pain (pain following the treatment),rehabilitation time (time to resuming normal daily activities),recurrence rate (proportion of patients with a recurrence of CPF following treatment),and FI rate (proportion of patients with FI following treatment).In total,10 choice sets were presented to each patient with two hypothetical treatments available for each choice.Patients had the option of selecting one hypothetical treatment profile as their most preferred treatment in each choice set,or to select neither.
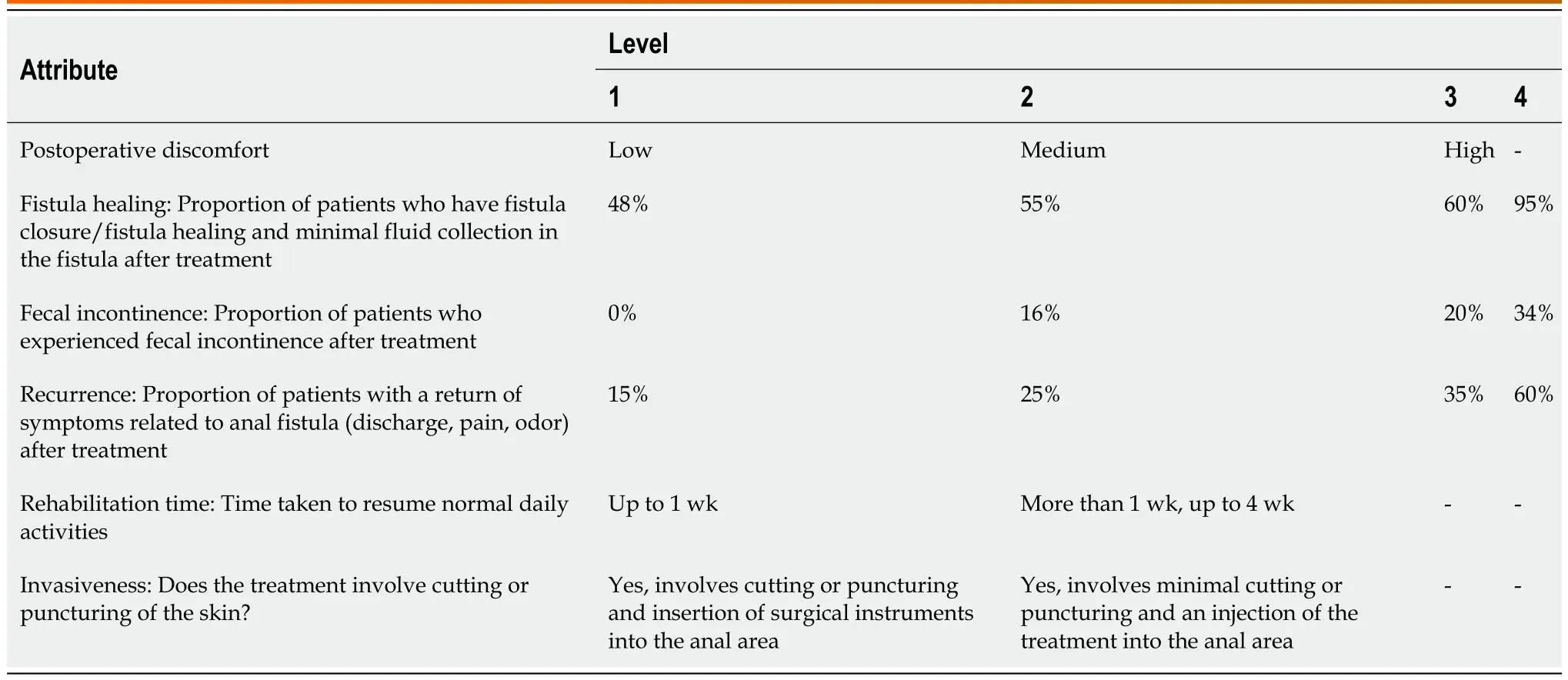
Table 1 Discrete choice experiment attributes and levels
Disease insights and experience
Patients were asked to complete a list of questions to assess their treatment experience and CD experience.Questions included a wide range of demographic and clinical characteristics including diagnosis,treatment,and disease severity and complications,with medication and surgical experience being of particular interest.Interference with patients’ lives due to CD/CPF and specific disease attributes was assessed over the past 12 mo using a score ranging from 1 to 9 (a higher number indicating more significant interference with life).The impact of CPF on activities of daily living (ADL)over the past 12 mo was assessed using a score ranging from 1 to 7 (a higher number indicating more significant interference with ADL).
Patient satisfaction with currently available treatments for CPF was measured by assessing patient satisfaction with current PF treatments and PF treatment attributes,both scored 1-9 (low score indicating low satisfaction).Patients were asked to rate their level of involvement in CD/PF treatment decision making as “not at all”,“slightly”,“moderately”,“very much”,or “I don’t feel the need to be involved”.
Statistical analysis
For all endpoints,data were analyzed using descriptive statistics,Pvalues were calculated usingt-tests and statistical significance was assessed at the 5% level.Bivariate comparisons were made between CPF cohorts (cohorts 2 and 3) and the non-PF cohort (cohort 1).Generalized linear models were used to statistically control for the effects of potential confounders in the data between patients with and without CPF.
The DCE data were analyzed using a hierarchical Bayesian model using the attribute levels as predictor variables and choice as the outcome variable.This model generated a mean relative attribute importance score for each attribute and a mean relative preference weight (RPW) for each level within the attributes tested.
Ethics
This study was conducted in accordance with the World Medical Association Declaration of Helsinki and Guidelines for Good Pharmacoepidemiology Practices and submitted to all applicable local Institutional Review Boards and Ethics Committees to ensure compliance with all ethical standards in each country.
RESULTS
Study population
In total,929 patients were recruited;620 patients had CD without PF (non-PF CD,cohort 1) and 309 patients had CPF(cohorts 2 and 3 combined;Figure 1).From each country,except Australia and Japan,100 and 50 patients were recruited to cohort 1 and cohorts 2 and 3 combined,respectively.Australia and Japan both recruited 60 patients to cohort 1,and 29 and 30 patients to cohorts 2 and 3 combined,respectively.
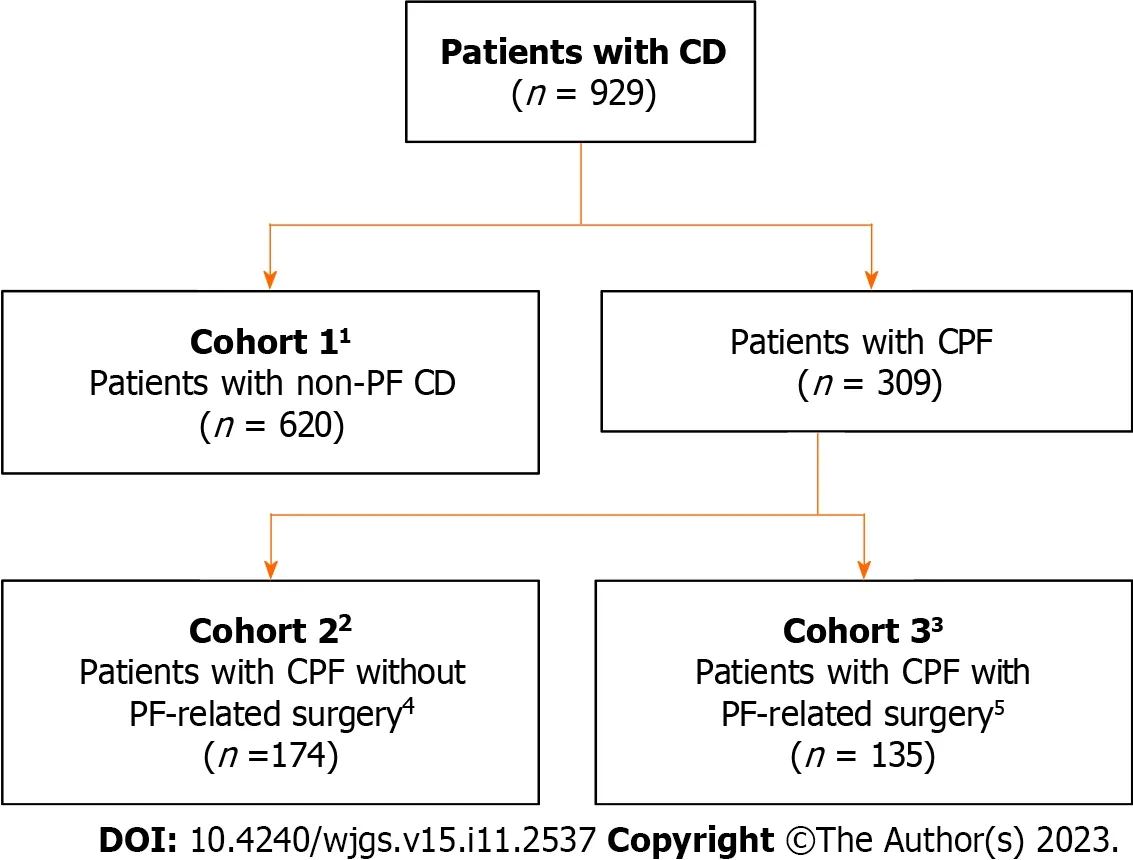
Figure 1 Patient disposition. 1Cohort 1: France,Germany,Spain,United Kingdom,and Canada: n=100;Australia and Japan: n=60;2Cohort 2: France,n=32;Germany,n=17;Spain,n=36;United Kingdom,n=33;Canada,n=24;Australia,n=12;Japan,n=20;3Cohort 3: France,n=18;Germany,n=33;Spain,n=14;United Kingdom,n=17;Canada,n=26;Australia,n=17;Japan,n=10;4Patients received pharmacotherapy and/or seton placement but no PF-related surgery in the past 12 mo;5With or without pharmacotherapy.CD: Crohn’s disease;CPF: Crohn’s perianal fistulas;PF: Perianal fistulas.
The age distribution of patients was similar across the cohorts,with the exception that the non-PF CD cohort (cohort 1)had a greater proportion of patients aged 61-80 years than cohorts 2 and 3 (Table 2).A greater proportion of patients in the CPF cohorts were male compared with the non-PF CD cohort.Further patient demographics and characteristics used in the multivariable analyses to control for potential confounders in the patient-reported outcomes (comorbidities,CD flare-up status,employment status,and marital status) are provided in Table 2 and Supplementary Table 1.
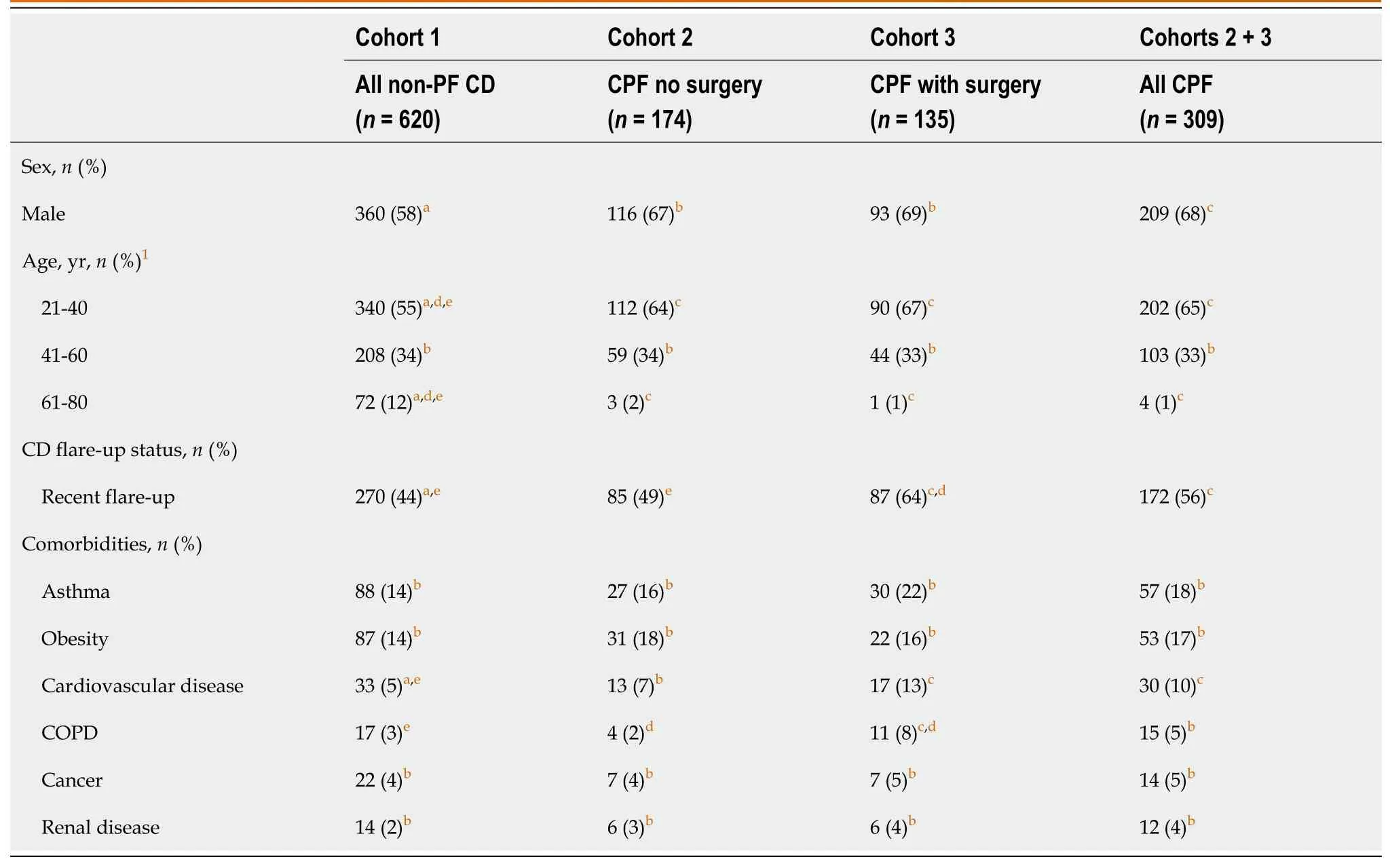
Table 2 Baseline demographics and patient characteristics
The questionnaire was generally well understood by respondents in the pre-test cognitive interviews and no major changes were required;however,in response to respondent feedback,minor modifications were made to the sentence structure and wording for further clarification.
Disease-specific patient-reported outcome measures
HRQoL: Overall SIBDQ scores were lower (worse) for patients with CPF (cohorts 2 and 3) than those with non-PF CD(cohort 1) with significantly lower scores across all four domains of the SIBDQ (Figure 2A).Multivariable analyses to control for potential confounders (patient demographics and characteristics,identifiedviaa model building approach)showed that SIBDQ scores after adjustment were still significantly lower for patients with CPF compared with thosewithout CPF (other variables that were statistically significant are shown in Supplementary Table 2).

Figure 2 Comparison of Short Inflammatory Bowel Disease Questionnaire scores and Quality of Life in patients with Anal Fistula scores in patients with Crohn’s disease,with and without perianal fistula. A: Short Inflammatory Bowel Disease Questionnaire (SIBDQ) scores;B: Quality of Life in patients with Anal Fistula scores.Scoring key for SIBDQ (range 1-7): Poor health-related quality of life (HRQoL)=1 point and optimum HRQoL=7 points.Scoring key for QoLAF (range 14-70): Zero impact=14 points,limited impact=15-28 points,moderate impact=29-42 points,high impact=43-56 points,very high impact=57-70 points.CD: Crohn’s disease;CPF: Crohn’s perianal fistulas;HRQoL: Health-related quality of life;PF: Perianal fistulas;QoLAF: Quality of Life in patients with Anal Fistula;SE: Standard error;SIBDQ: Short Inflammatory Bowel Disease Questionnaire.
In patients with CPF,total (overall) QoLAF scores were comparable between cohorts 2 and 3.Biopsychosocial impact scores were similar,but for the physical impact domain,patients in cohort 3 (who had PF-related surgery) had a significantly higher (worse) score than those in cohort 2 (patients with no surgery,Figure 2B).
Fecal incontinence
Overall,47% of patients reported FI and completed the RFIS and FIQL questionnaires.A significantly lower proportion of patients with non-PF CD reported FI than those with CPF (40% in cohort 1vs59% and 59% in cohorts 2 and 3,respectively).Furthermore,mean RFIS scores were significantly higher (worse) in patients with CPF than in those without (Figure 3A).After using multivariable analyses to control for patient demographics (identifiedviaa model building approach),RFIS scores were still significantly higher for patients with CPF compared with those without CPF(other variables that were statistically significant are shown in Supplementary Table 3).
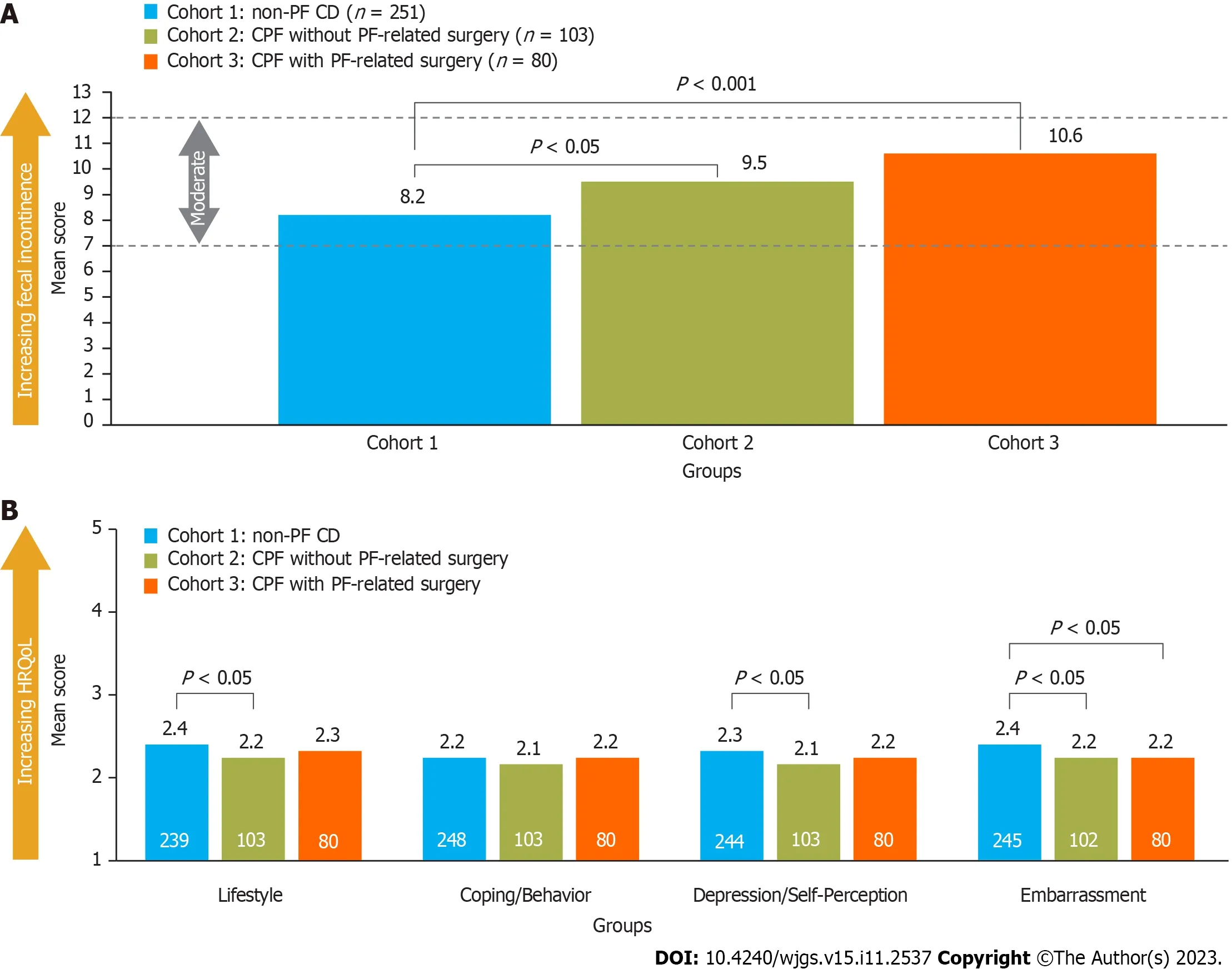
Figure 3 Comparison of Revised Faecal Incontinence Scale and Fecal Incontinence Quality of Life scores in patients with Crohn’s disease,with and without perianal fistula. A: Revised Faecal Incontinence Scale scores;B: Fecal Incontinence Quality of Life scores.Scoring key for RFIS(range 0-20): No fecal incontinence=0 points,very mild ≤ 3 points,mild=4-6 points,moderate=7-12 points,severe ≥ 13 points;scores for each item were summed and the mean taken,with lower scores indicating less fecal incontinence.Scoring key for FIQL (range 1-5): Lower scores indicating lower health-related quality of life;the minimally important difference is 1.1-1.2 points per subscale.Numbers inside the bars present the number of patients.CD: Crohn’s disease;CPF: Crohn’s perianal fistulas;FIQL: Fecal Incontinence Quality of life;HRQoL: Health-related quality of life;PF: Perianal fistulas;RFIS: Revised Faecal Incontinence Scale.
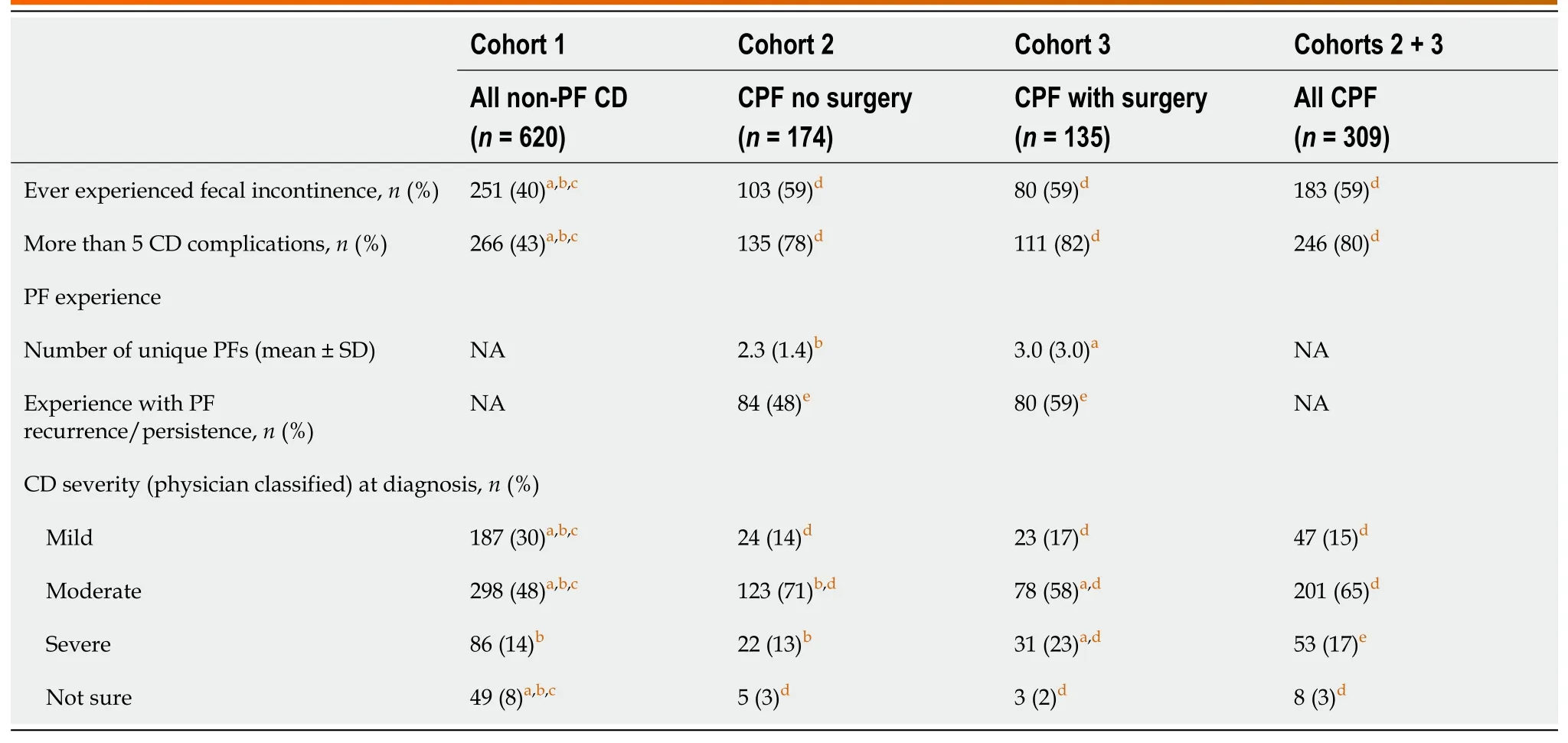
Table 3 Disease presentation and symptom severity
Significantly lower (worse) FIQL scores were noted for patients with CPF and no PF-related surgery experience than for those with non-PF CD (cohort 2vscohort 1) across all domains except coping/behavior,whereas patients with PFrelated surgery experience (cohort 3) reported significantly lower RFIS scores than cohort 1 only for the embarrassment domain (Figure 3B).
Health status (EQ-5D)
EQ-5D scores were not significantly different between cohorts,except in France where scores were significantly higher(better) for patients with non-PF CD (cohort 1) than those with CPF without PF-related surgery (cohort 2),and in Japan where scores were significantly higher for patients with non-PF CD than those with CPF,irrespective of PF-related surgery experience (Figure 4).After adjusting for confounding variables (identifiedviaa model building approach),CPF was found to have a significantly negative impact on EQ-5D-5L scores in France,Germany,and Japan,but not in the other countries.EQ-5D VAS scores for overall health were not significantly different between cohorts across all countries(Supplementary Figure 1).
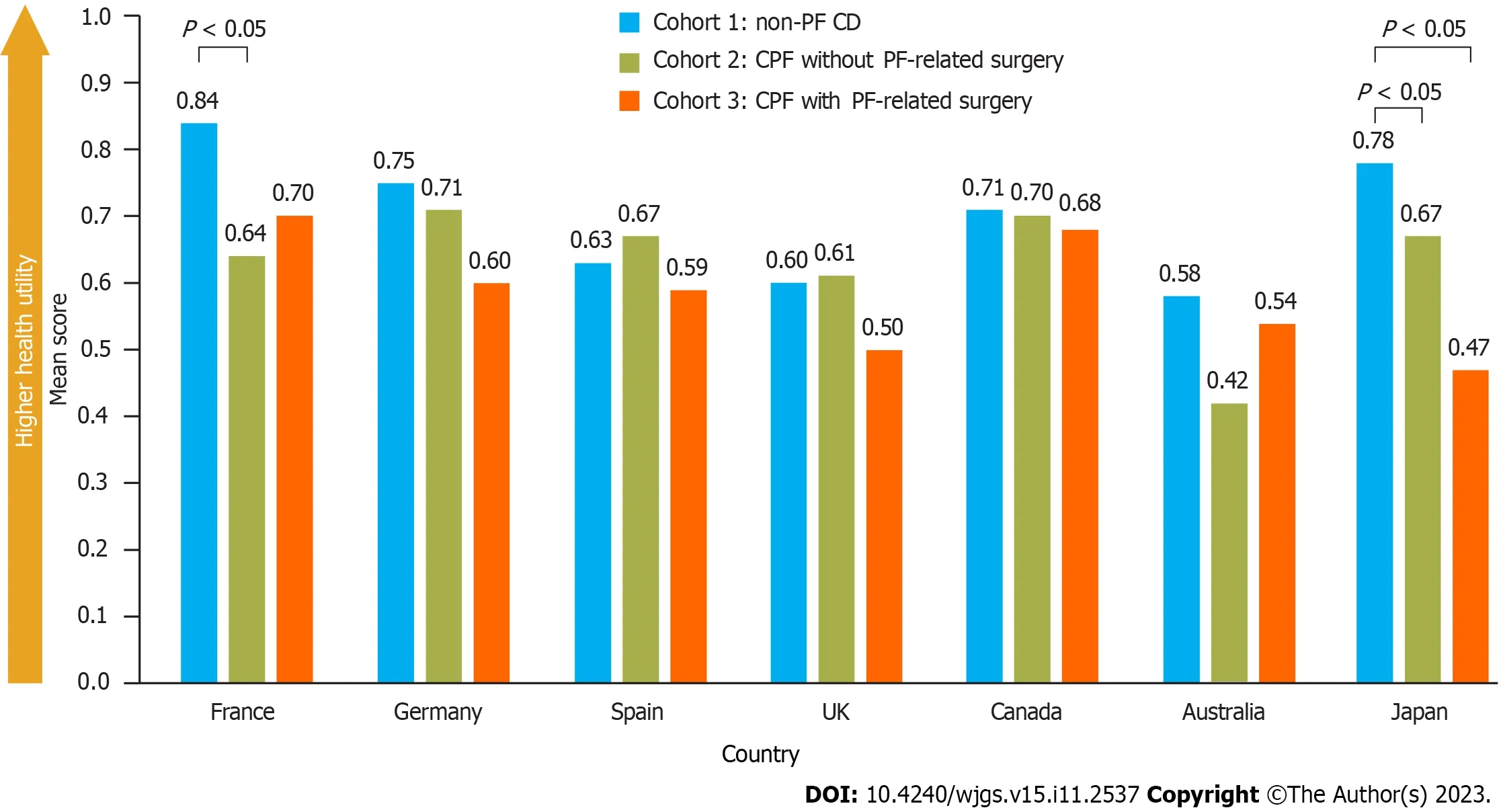
Figure 4 Comparison of EQ-5D health status scores in patients with Crohn’s disease,with and without perianal fistula. Scoring key for EQ-5D (range 0-1): Higher scores indicate better health-related quality of life.United Kingdom,Spain,and Australia used a shortened form of the EQ-5D-5L (i.e.,the EQ-5D-3L).Populations for each country for cohorts 1,2,and 3,respectively: France,n=100,n=32,and n=18;Germany,n=100,n=17,and n=33;Spain,n=100,n=36,and n=14;United Kingdom,n=100,n=33,and n=17;Canada,n=100,n=24,and n=26;Australia,n=60,n=13,and n=17;Japan,n=60,n=20,and n=10.CD: Crohn’s disease;CPF: Crohn’s perianal fistulas;PF: Perianal fistulas.
Treatment experience
A higher proportion of patients with CPF had moderate or severe disease,CD-related complications,and had experienced reported FI,compared with patients with non-PF CD (Table 3).CD-related complications included fatigue,abdominal pain/cramping,gastrointestinal pain,pain/difficulty with bowel movements,and pain when sitting(Supplementary Table 4).At the time of enrollment,a higher proportion of patients with CPF were currently taking or had previously taken CD-related medication than those with non-PF CD (98%vs94%,respectively,P< 0.05;Table 4).Also,a higher proportion of patients with CPF had CD-related surgeries than those without CPF (cohort 1) and the proportion was greatest in those who had PF-related surgery (cohort 3).This likely accounts for the higher proportion of patients in cohort 3 with surgical failures compared with cohorts 1 and 2.
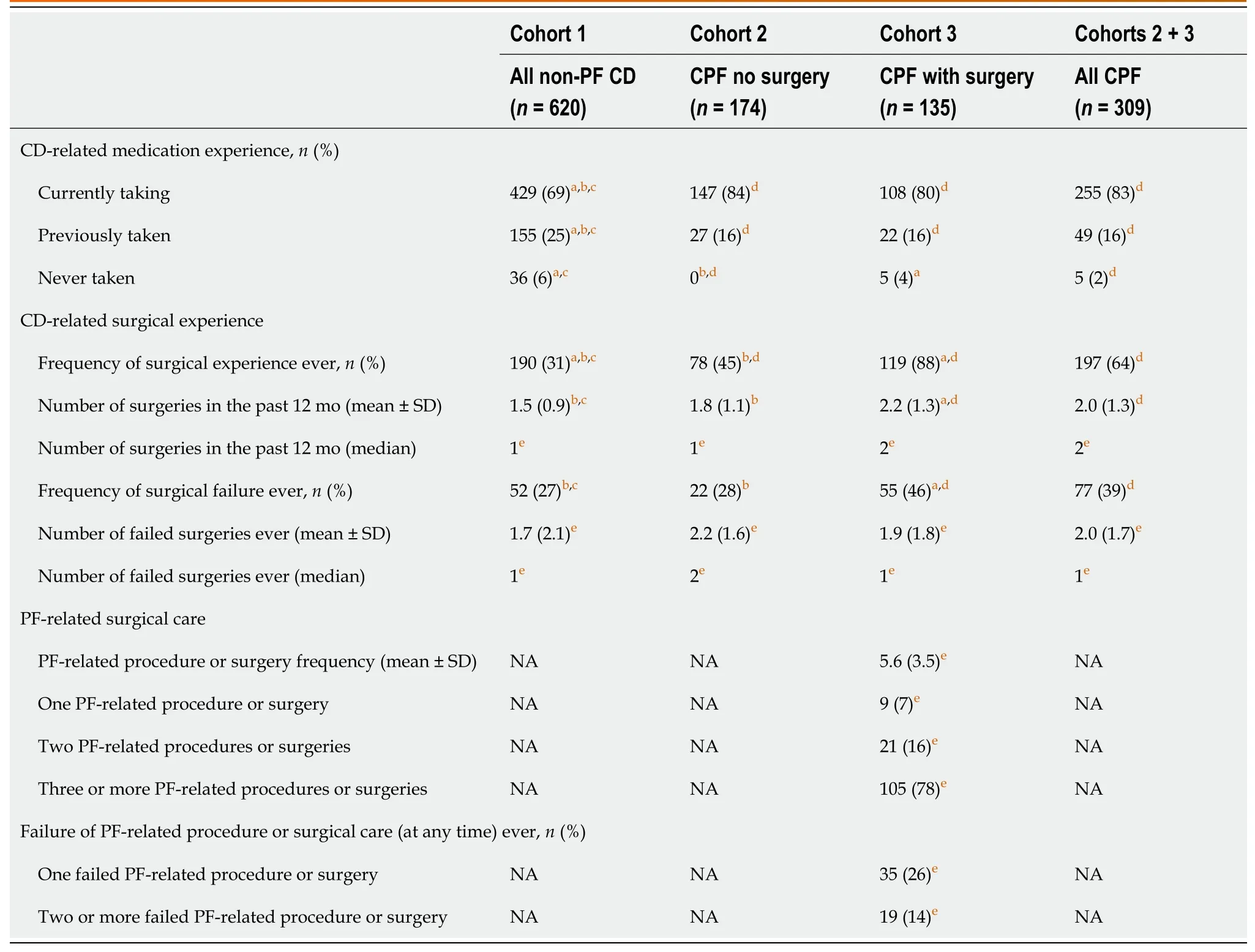
Table 4 Medication and surgical experience
In patients with CPF and PF-related surgery experience (cohort 3),78% had three or more such procedures or surgeries related to their PF and 87% of patients experienced ≥ 1 complication after surgery or seton placement.The most frequent complications after PF-related surgery or seton placement included fever/infection,worsening of pain/swelling around the anus,and worsening of bloody or foul-smelling discharge from an opening around the anus (Supplementary Table 5).
Drivers of treatment preferences: DCE
The mean RPW provided an estimation of the strength of preference for each level within the attributes tested (higher RPWs indicated a higher preference and lower RPWs indicated a lower preference).Patient preferences were driven by levels of postoperative discomfort [mean RPW (standard error,SE) of 0.20 (0.03) for low levels of discomfortvs-0.28 (0.03)for high levels of discomfort].Patients also preferred treatments that result in high rates of fistula healing with minimal fluid collection [mean RPW (SE) of 0.24 (0.04) for treatments with approximately 95% fistula healing ratevs-0.09 (0.04) for treatments with approximately 48% or approximately 55% fistula healing rate].Levels of FI after treatment were also a driving factor in patient treatment preferences [mean RPW (SE) of 0.13 (0.04) for no FIvs-0.10 (0.04) for approximately 34% rate of FI after treatment].Overall,of the tested attributes,postoperative discomfort and fistula healing rate were the most important attributes influencing patient choice in the treatment of CPF (Figure 5).
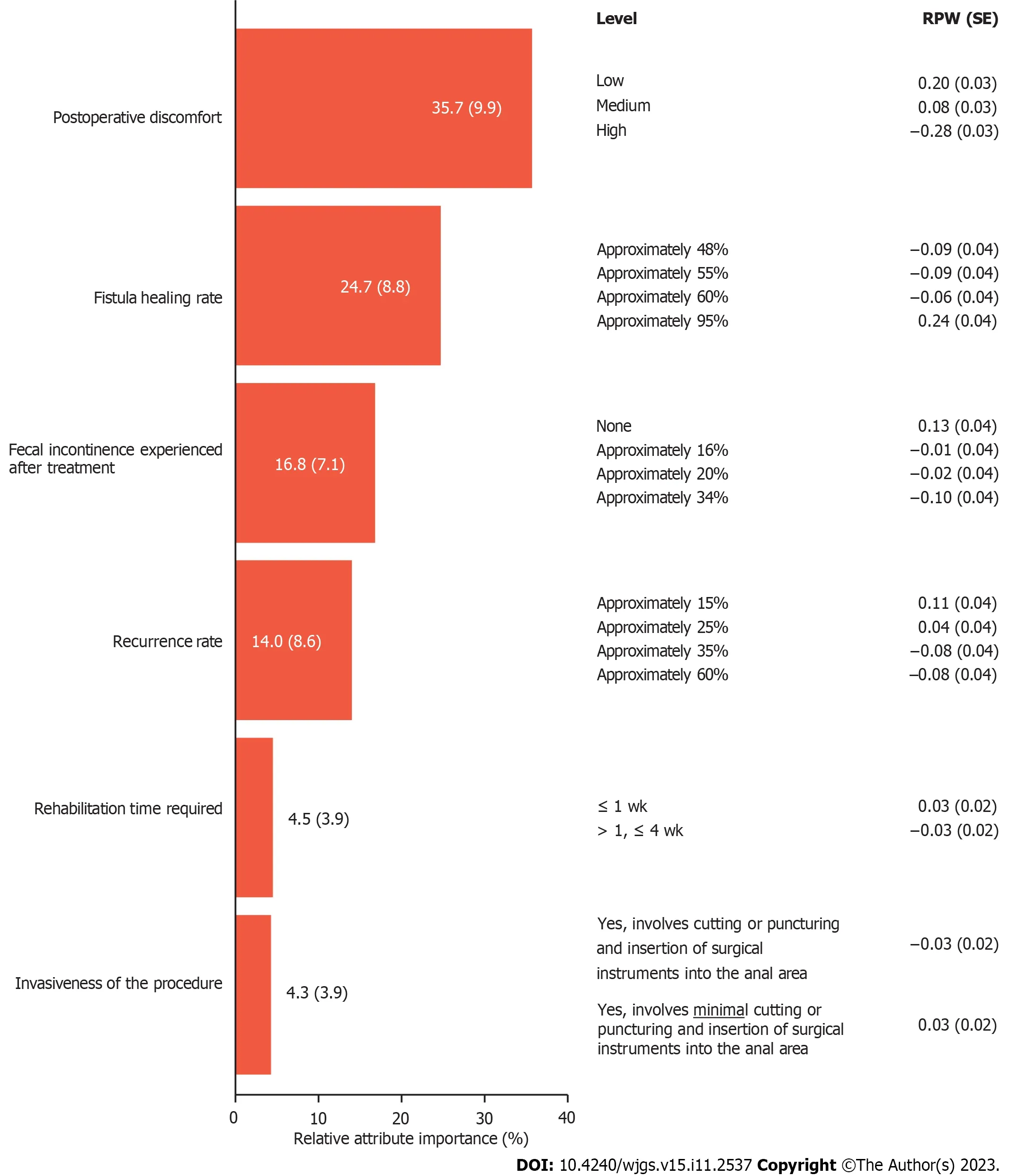
Figure 5 Patient-rated importance of Crohn’s perianal fistula treatment attribute options in a discrete choice experiment. Data inside/beside bars represent relative attribute importance ± SD.Cohorts 2+3: n=309.RPW: Relative preference weight;SE: Standard error.
Disease insights and experiences
Overall impact of CD/CPF on life:Disease impact (in terms of interference with a patient’s life) was significantly greater in patients with CPF than those without,with worse impact scores for all cohorts during flare-up (Supplementary Table 6).
Impact of CD/CPF disease attributes on HRQoL:Patients with CPF experienced a significantly higher impact of disease attributes on their HRQoL than patients with non-PF CD (Supplementary Table 7).The most impactful disease attributes were diarrhea (cohorts 1 and 2) and anorectal stricture (patients with PF-related surgery,cohort 3).
Impact of CD/CPF disease attributes on activities of daily living:Overall,significantly higher scores (higher impact)across all activities were recorded for patients with CPFvsthose without.For patients without CPF,the most affected activities were exercising [mean ± SD 4.0 (1.6)],being satisfied with life [4.0 (1.7)],and ability to go to school [including any level of education;4.0 (1.6)].For patients with CPF,the most affected activities were exercising [4.6 (1.5)],being satisfied with life [4.6 (1.5)],and ability to work outside home [4.6 (1.5)].
Treatment satisfaction:Mean satisfaction scores were moderate (6.2-6.9) for all PF treatment options and similar in both cohorts of patients with CPF;however,patients with PF-related surgery (cohort 3) had significantly less satisfaction with long-term seton placement than those without PF-related surgery (cohort 2): 6.2vs6.7,respectively;P< 0.05 (Table 5).
Involvement in CD/PF treatment decision making:The majority of patients across all cohorts in all countries were either moderately or very much involved in their CD/CPF treatment decision making (78%-81%);1%-3% indicated no involvement and 1%-2% indicated they did not feel the need to be involved.
DISCUSSION
Patients with CPF are a subset of patients with CD that experience a more complex clinical disease course and may require unique treatment considerations.This large multi-country study used validated patient-reported outcome measures and general questionnaires to assess the burden of illness for patients with CPF compared with patients with non-PF CD.For patients with CPF,these outcomes were also compared between those who had PF-related surgery and those who did not.A DCE was also conducted to assess the treatment preferences of patients with CPF.
As shown in this study,patients with CPF have an incrementally higher symptom burden due to both CD and PF than patients with non-PF CD.Severity of CD is higher in patients with CPF than in those with non-PF CD,with the greatest severity observed in those with PF-related surgery: A higher proportion of patients with CPF experience FI and CDrelated complications such as fatigue,abdominal and gastrointestinal pain,and difficulty with bowel movements.In addition,patients with CPF can experience symptoms directly related to their fistulas such as purulent discharge,perianal pain,and FI.The greater CD severity in patients with CPF is reflected in the higher proportion of patients with CPF who received CD-related medications and surgery than in patients with non-PF CD.Furthermore,patients with CPFwere shown to have a significant impact on their overall HRQoL.This finding is in line with a 2023 study by Spinellietal[47],where patients with CPF reported a greater impact on overall quality of life,well-being,relationships,social life,and work life than those with CD without CPF[47].In the current study,there was no significant difference in reported HRQoL between patients who had PF-related surgery and those who had not.Patients with CPF reported a greater impact of CD/CPF disease attributes on HRQoL,irrespective of PF-related surgery,than patients with non-PF CD.
A high proportion of patients in this study reported being actively involved in their treatment decision making,and for patients with CPF,satisfaction with PF treatment options was only moderate,regardless of whether they had experienced surgical intervention or not.The DCE performed in this study showed that patients with CPF prioritize postoperative discomfort and healing rate as the primary attributes when selecting a hypothetical treatment choice.To the best of the authors’ knowledge,this is the first time a DCE has been performed in this patient population,offering a unique perspective on patient preferences for CPF treatments.
The key findings from this study are in keeping with the core outcomes identified by Sahnanetal[48] and are comparable with the findings of a recent study in a similar patient population conducted in the United States[48,49].Further research on the potential impact of age,sex,and disease severity on patients’ treatment preferences could support healthcare professionals in the clinical management and treatment decisions for CPF.
There are some limitations that should be acknowledged with studies of this type.Patient responses to questionnaires can be subject to recall,selection,and/or social desirability bias,and inaccuracies owing to self-reported diagnosis and the use of complex medical terminology.The risks of such effects were partly mitigated by limiting the recall period to 12 mo or less and using pre-test telephone interviews and a web-enabled questionnaire.There was no validation sample of patients in relation to self-reported diagnosis (for cohort categorization) because it was assumed that patients would know whether or not they have CPF.Finally,the sample population may not have been representative of the widerpopulation of patients with CD,and any country/regional differences need to be further evaluated.
CONCLUSION
This is the largest known observational study to quantify the burden of illness associated with CPF across multiple countries utilizing a comprehensive set of outcomes including symptom burden and impacts,and treatment experience,satisfaction,and preferences.This study confirmed that the burden of illness for patients with CD is significantly higher for those with CPF than those without.CPF management should aim to reduce the overall disease burden,including treatment-related burden or complications,such as FI,to improve HRQoL for these patients.
ARTICLE HIGHLIGHTS
Research background
The burden of illness in patients with Crohn’s disease (CD) is perceived to be greater in those with perianal fistulasvsthose without.However,there is limited literature directly comparing the symptom burden,impact on quality of life and the treatment experiences,and preferences in patients with CD with and without perianal fistula.
Research motivation
A more in-depth understanding of disease burden and treatment preferences of patients with Crohn’s perianal fistula will be key in raising disease awareness and helping healthcare professionals with the clinical management of these patients.
Research objectives
To examine the symptom burden,health-related quality of life,and treatment experiences,satisfaction,and preferences for patients with CD with and without perianal fistula,and to further assess the incremental burden of these measures for patients who have and have not received perianal fistula-related surgery.
Research methods
A large cross-sectional,multi-country observational study was conductedviaa pre-tested web-enabled questionnaire in seven countries.Data on disease insights and experiences were collected,and validated patient-reported outcome measures were used to assess the disease-specific health-related quality of life,fecal incontinence,and general health status of participating patients.All participating patients had CD and comparisons were made between patients without perianal fistula and those with perianal fistula (with further comparisons between those with and without perianal fistula-related surgery).Patient preferences for perianal fistula treatments were also assessed using a discrete choice experiment.
Research results
This study demonstrated that symptom burden,severity of disease,CD-related medication/surgical interventions,and impact on health-related quality of life in patients with CD are significantly higher for those with perianal fistula than those without.Patients with Crohn’s perianal fistula were found to prioritize postoperative discomfort and healing rate as the primary attributes when selecting a hypothetical surgical treatment choice.
Research conclusions
For patients with CD,the symptom and treatment burden and impact on health-related quality of life are significantly higher for those with perianal fistula than those without.Future Crohn’s perianal fistula management should aim to reduce the treatment-related burden or complications,in order to improve health-related quality of life for these patients.
Research perspectives
The patient satisfaction rates and surgical treatment preferences highlighted in this study should be considered by healthcare professionals when making decisions regarding the clinical management of patients with Crohn’s perianal fistula.
ACKNOWLEDGEMENTS
The authors would like to thank Emily Sharpe,PhD,for her contributions to this study;Sally McTaggart,PhD,of Oxford PharmaGenesis,Oxford,UK for the Medical writing support;and Takeda Pharmaceuticals for supporting this study.
FOOTNOTES
Author contributions:Karki C,Athavale A,Abilash V,Hantsbarger G,Geransar P,Lee K,Milicevic S,Perovic M,Raven L,Sajak-Szczerba M,Silber A,Yoon A,and Tozer P contributed to the conceptualization of the study;Athavale A,Abilash V,and Silber A contributed to the data curation;Athavale A,Abilash V,and Silber A contributed to the formal analysis;Karki C contributed to the funding acquisition;Karki C,Athavale A,Abilash V,and Silber A contributed to the investigation;Karki C,Athavale A,Abilash V,Hantsbarger G,and Tozer P performed the methodology;Athavale A,Abilash V,and Silber A contributed to the project administration;Karki C and Athavale A contributed to the resourcing;Athavale A provided software expertise;Karki C and Athavale A contributed to the supervision of the study;Athavale A,Abilash V,Hantsbarger G,Geransar P,Lee K,Milicevic S,Perovic M,Raven L,Sajak-Szczerba M,Silber A,Yoon A,and Tozer P contributed to the validation;Athavale A,Abilash V,and Silber A contributed to the visualization;Karki C,Athavale A,Abilash V,Hantsbarger G,Geransar P,Lee K,Milicevic S,Perovic M,Raven L,Sajak-Szczerba M,Silber A,Yoon A,and Tozer P contributed to the writing,review,and editing of the manuscript.
Institutional review board statement:This study was conducted in accordance with the World Medical Association Declaration of Helsinki and Guidelines for Good Pharmacoepidemiology Practices (GPP) and submitted to all applicable local Institutional Review Boards and Ethics Committees to ensure compliance with all ethical standards in each country.
Informed consent statement:Personally identifiable data were not collected in this study.As this was an observational study,consent to any interventional procedure or treatment was not applicable.Consent for participation in the study was solicited by requesting participants to agree to a statement indicating the purpose of the study and a brief summary of the information to be collected.This was carried out prior to entry into the web-enabled questionnaire with a description of the study and its purpose,and responses.
Conflict-of-interest statement:CK is an employee and shareholder of Takeda Pharmaceuticals.AA is an employee of Trinity Life Sciences,commissioned by Takeda Pharmaceuticals to conduct this study.VA is an employee of Trinity Life Sciences,commissioned by Takeda Pharmaceuticals to conduct this study.GH is an employee and shareholder of Takeda Pharmaceuticals.PG is an employee and shareholder of Takeda Pharmaceuticals.KL has served on advisory boards for Takeda Pharmaceuticals.SM is an employee and shareholder of Takeda Pharmaceuticals.MP has no conflicts of interest to disclose.LR has served on advisory boards for Roche and Takeda Pharmaceuticals.MSS has nothing to disclose.AS is an employee of Trinity Life Sciences,commissioned by Takeda Pharmaceuticals to conduct this study.AY is an employee of Takeda Pharmaceuticals.PT has received speaker’s fees from Ferring and Takeda Pharmaceuticals and served on advisory boards for Takeda Pharmaceuticals.
Data sharing statement:Data sets supporting the results from this study are available from the corresponding author upon reasonable request.The data sets will be provided after deidentification,in compliance with applicable privacy laws,data protection,and requirements for consent and anonymization.
STROBE statement:The authors have read the STROBE Statement—checklist of items,and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Chitra Karki 0000-0001-9336-7589.
S-Editor:Fan JR
L-Editor:A
P-Editor:Zhao S
 World Journal of Gastrointestinal Surgery2023年11期
World Journal of Gastrointestinal Surgery2023年11期
- World Journal of Gastrointestinal Surgery的其它文章
- Systematic sequential therapy for ex vivo liver resection and autotransplantation: A case report and review of literature
- Gastric inflammatory myofibroblastic tumor,a rare mesenchymal neoplasm: A case report
- Comprehensive treatment and a rare presentation of Cronkhite-Canada syndrome: Two case reports and review of literature
- Isolated traumatic gallbladder injury: A case report
- Metachronous primary esophageal squamous cell carcinoma and duodenal adenocarcinoma: A case report and review of literature
- Organ sparing to cure stage IV rectal cancer: A case report and review of literature
