Effects of oxygen/nitrogen co-incorporation on regulation of growth and properties of boron-doped diamond films
Dong-Yang Liu(刘东阳), Kun Tang(汤琨), Shun-Ming Zhu(朱顺明), Rong Zhang(张荣),You-Dou Zheng(郑有炓), and Shu-Lin Gu(顾书林),‡
1School of Electronic Science and Engineering,Nanjing University,Nanjing 210093,China
2The Shanghai Huahong Grace Semiconductor Manufacturing Corporation,Shanghai 201203,China
3Collaborative Innovation Center of Solid-State Lighting and Energy-Saving Electronics,Nanjing University,Nanjing 210093,China
Keywords: boron doped diamond, nitrogen and oxygen co-doping, crystal quality, Hall effect measurement,acceptor doping concentration
1.Introduction
Diamond has excellent physical and chemical properties, such as ultra-broadband gap (5.47 eV), high hardness,thermal conductivity (14 times to silicon), electron mobility(4500 cm2/V·s), hole mobility (3800 cm2/V·s), chemical stability, and biocompatibility,[1–4]making it a best choice for preparing high power, high frequency, and high temperature electronic devices.The p/n type doping is the core and key technology to develop semiconductor device structures and to realize device applications.However, there are serious doping difficulties in diamond semiconductors,i.e.,to achieve the p/n type doping with high concentration and efficiency.Such a hurdle leads to a stagnant state for development of diamond semiconductor characteristics and applications of photoelectric devices related to diamonds.[5,6]
As is known,p-type doping of diamond films is relatively mature.Generally, the hole concentration in the films can be changed by adjusting the doping concentration of boron.Synthesized diamond films have been applied in electrochemical and other fields.[7–11]The boron acceptor level is located at 0.37 eV above the diamond valence band maximum(VBM) by analyzing the temperature dependence of the resistivity.However, this level position is still deep compared with boron acceptor(∼meV)in silicon.[12–16]For n-type doping of diamonds, both the V and VI main family elements may be the potential donors, and the representative elements are N and P.The ionization energy of the substitutional nitrogen donor is relatively large at 1.7 eV, resulting in the device based on N-doped n-type diamond not working at room temperature.[17–19]Nevertheless, the nitrogen-vacancy (NV)color center formed by N-doping is applied in quantum communication and sensing.[20]On the contrary,phosphorus has a relatively shallow donor level of∼0.6 eV.[21–24]However,the electron density is quite low because of low phosphorous content in the CVD diamond limited by chemical kinetics.[25,26]Compared with single-element doping, researchers believe that two-element co-doping could be a strategy to achieve more shallow donors.[27–32]Representative B/N and B/O codopings have been extensively studied, and theoretical calculations and experiments have demonstrated the feasibility of forming relatively shallow donors.Croot and Moussa have predicted from their calculations that the BN2complex could be a donor with an ionization energy of 0.8–1.2 eV.[33,34]In addition,previously we realized n-type diamond films at high temperature by B–N co-incorporation technology with the ionization energy of B–N complex donor at about 0.8 eV.[35]In the study of B/O co-doping,[36,37]the experimental results of some scholars have shown that the diamond samples are still ptype.However, researchers have realized the n-type diamond film by B/O co-incorporation.The activation energy of B–O complex as the donor is 18.67 meV,and the electron concentration is 0.778×1021cm-3.[38]Liuet al.have also predicted that the B3O and B4O complexes with smaller forming energy exist in diamond films as donors by using the first-principles calculation.
At present, there have been many reports on the diamond preparation and properties of single B-doping, single N-doping,B/N co-doping,and B/O co-doping.However,few studies about B/N/O co-doping in diamond are presented, in which the doping behavior will be more complicated.Therefore,in this work,we synthesize p-type diamond with B/N/O co-doping using diborane (B2H6) and laughing gas (N2O) as doping sources by micro-wave plasma chemical vapor deposition(MPCVD).Since the microwave-plasma could simply and easily generate N and O simultaneously from N2O,we use the nitrous oxide as the precursor for a convenient approach.We analyze the regulation on the growth rate,crystal quality,luminescence properties and electrical properties of boron-doped diamond film with different laughing gas concentrations.Furthermore, the compensation mechanism between donor and acceptor is also explored adequately.
2.Experimental details
Diamond films were grown on (100)-oriented type Ib CVD single-crystalline chemical vapor deposited diamond substrates (3.0×3.0×1.0 mm3) by the MPCVD technique.Before deposition, in order to remove organic and metallic contaminants from the substrate surface, the substrates were cleaned in aqua regia (HNO3/HCl 1:3) at 60◦C for 30 min.The flow rates of CH4and H2were kept at 20 sccm and 500 sccm, respectively.The B/N/O-dopings were carried out using hydrogen-diluted diborane and laughing gas.The ratio of B2H6to CH4(B2/C) in the gas phase is fixed at 50 ppm for all the samples.The laughing gas concentrations,defined as the N2O/CH4(N2O/C) gas flow ratio, are kept at 0%, 2.5%, 5.0%, 7.5%, and 10%.The chamber pressure and the microwave power for all depositions are fixed at 135 torr and 3.8 kW.The substrate temperature is set to 800–850◦C,as determined by an IR pyrometer.After 8 h of growth, the as-grown samples were cleaned with H2SO4and HNO3(1:1)mixed solutions at 250◦C for 0.5 h to remove the produced graphite on the surface.After chemical treatment, the samples were rinsed with acetone, alcohol and deionized water in ultrasonic cleaning machine.The samples were subsequently blow-dried with nitrogen gas, and the thicknesses of all diamond films were measured by employing a micrometer with a resolution below 1 µm.The thicknesses of the diamonds are 24 µm (N2O/C=0%), 20 µm (N2O/C=2.5%),12 µm (N2O/C=5.0%), 4 µm (N2O/C=7.5%), and 4µm(N2O/C=10.0%),respectively.
The surface morphologies of the diamonds was observed with an AFM (NTEGRA) in the tapping mode.Micro-Raman scattering spectra and photoluminescence spectra were recorded by using a 514 nm laser as an excitation with a confocal microscope (Horiba Jobin Yvon) at 100 magnifications(numerical aperture 0.9).An optical emission spectroscope(OES)was employed to monitor the composition of the plasma in the reaction chamber.The Hall effect measurement was carried out at both room temperature (300 K) and variable temperatures(300–900 K)to study the electrical properties of the dopants and impurities(specific process of Hall electrodes fabrication and Hall measurement in the supplementary material).Ti/Au in Hall electrodes was provided by ZhongNuo Advanced Material(Beijing)Technology Co.,Ltd.
3.Results and discussion
Figure 1 shows the growth rate of the diamonds as a function of the N2O/C ratio.As can be seen,the growth rate of the film decreases monotonically from 3 µm/h to 0.5 µm/h with the incorporation of laughing gas.The oxygen in the laughing gas has a significant inhibitory effect on the growth of the diamond films,while the normally observed nitrogen improving effect on the growth rate was not found.In order to elucidate the reason why the growth rate decreases with laughing gas addition in reaction gas, we performed a plasma OES measurement.
Figure 2(a) shows the OES of boron-laughing gas codoped diamond films with N2O/C ratio of 10.0%.The CH4–H2–B2H6–N2O mixture contains several optical emission lines, among which the ones located at 387.1 nm and 388.3 nm originate from the carbon-nitrogen radicals.[20,21]Furthermore, the lines located at 512.8 nm and 516.4 nm are due to the existence of the C2radicals.[21]To analyze the changes of nitrogen concentration in the reaction gas with different N2O/C ratios, we performed the OES spectrum under all growth conditions, and the results are shown in Fig.2(b).The intensity of the C2(516.4 nm) luminescence peak was extracted as shown in the inset of Fig.2(c).The intensity of the C2(516.4 nm) peak has an negligible change (1.13 to 1.20) with laughing gas incorporation, indicating that the decreased growth rate is independent of the change with carbon precursors in the reaction chamber.Figure 2(c)shows the ratio ofI(CN)(388.3 nm)/I(C2)(516.4 nm)at different laughing gas concentrations in the reaction gas.As the N2O/C ratio increases in the gas phase,the relative luminescence intensity of the CN radicals increases linearly in the reaction gas,suggesting an increase with the nitrogen content in the reaction gas.It is generally believed that nitrogen will improve the growth rate of the diamond film by enhancing the detachment of surface hydrogen.[39,40]However, with the increase of laughing gas concentration,the monotonous decreasing of film growth rate should be less related to the behavior of nitrogen and the change of C2precursor,but mainly depends on the etching and inhibition effect of oxygen in laughing gas during diamond growth.Therefore, we believe that the continuous reduction of the growth rate is due to the fact that the etching and inhibiting effect with oxygen in laughing gas on film growth is greater than the promoting effect of nitrogen on film growth.
The Raman measurement is a simple and effective method to characterizing diamond quality, and the Raman scattering spectra of boron-doped diamond films with different laughing gas concentrations by 514.5 nm laser excitation are shown in Fig.3(a).As is seen, all the samples show only one Raman scattering peak located 1333.5 cm-1with a small full width at half maximum (FWHM), indicating that the prepared doped diamond films have high quality, and the scattering peaks associated with the graphite phase structure are not observed.In addition, relative to the standard Raman peak position(1332.8 cm-1)of the diamond,the Raman peak of the samples is all blue-shifted,which should be due to the residual stress caused by doping in the film.Figure 3(b)shows the extracted FWHM of the Raman scattering spectra for boron-laughing gas co-doped diamond films.By adding a small amount of laughing gas(N2O/C=2.5%),the FWHM increases significantly.This may be due to the N and O substituting C in the diamond lattice,and the lattice mismatch leads to the decrease of crystal quality.With further increase of the laughing gas concentration, the FWHM is first reduced and then increased, and the crystal quality of the diamond film is optimized at N2O/C ratio with 7.5%.We believe that with the addition of nitrogen, the film quality gradually degrades.[35]With the increase of oxygen, the film quality improves first and then degrades.[41,42]Therefore, the phenomenon that the quality of co-doped diamond film improves first and then decreases should be a result of the synergistic effect of N and O on the growth regulation for the boron-doped diamond film.
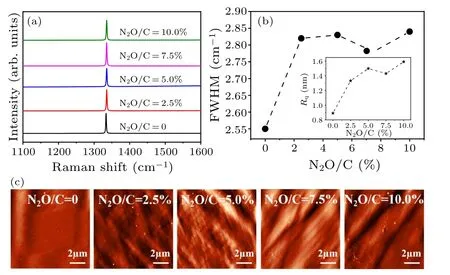
The surface morphology of diamond films is also an important means to evaluate the crystal quality,so we conducted an AFM measurement on the boron-doped diamond films at different laughing gas concentrations,and the obtained surface morphologies are shown in Fig.3(c).The surface of the single boron-doped sample is relatively flatter.With the increase of laughing gas concentration,the“step”flow slowly appears on the surface of the sample.The rms surface roughness(Rq)of the boron-doped diamond films at different laughing gas concentrations is shown in the inset of Fig.3(b).TheRqof single boron-doped films is smaller.When laughing gas is added,Rqgenerally rises to more than 1.2 nm,indicating that the incorporation of laughing gas reduces the surface quality of diamond.In the boron-laughing gas co-doped diamond film,with the increase of laughing gas concentration,Rqdecreases first and then increases,reaching the minimum at the N2O/C ratio of 7.5%, indicating a better surface quality.This optimized point is consistent with the change of Raman FWHM shown in Fig.3(b),which indicates that the film quality improves first and then decreases.
After laughing gas incorporation, nitrogen will replace the carbon in the diamond lattice to form the substitutional nitrogen, and part of the substitutional nitrogen will further combine with the surrounding vacancy to form the NV color center.In order to evaluate the laughing gas incorporation in the samples, the PL measurement has been employed first.Figure 4(a) shows the PL spectra of all the samples excited by a 514-nm laser.A sharp Raman peak at 552 nm labeled as R is from the diamond lattice,which is used as a reference to normalize all the spectra.Besides the R peak,two peaks at 575 nm and 637 nm,corresponding to the NV0and NV-emissions,have been clearly observed for all the co-doped samples.In fact,we can get the signal of NV-related emissions in borondoped sample with no nitrous oxide.The signals originate from unintentional source(s).Typical sources can be residual nitrogen inside the chamber or the nitrogen in the substrate that diffuses into the grown film.In order to quantify the effect of the laughing gas incorporation on the NV center luminescence for the boron-doped diamond films, the intensities of NV0, NV-and NV0+ NV-in the PL spectra are extracted as shown in Fig.4(b).The intensity of NV center increases as the N2O/C ratio increases from 0 to 5.0%.However, the intensity of the NV center decreases monotonically with further increase of the laughing gas concentration.The analysis shows that,when the concentration of laughing gas is low,the enhancement effect with NV center formation due to nitrogen increase will be greater than the inhibitory effect of oxygen in the boron-doped diamond film.However,the incorporation of excessive N2O will significantly enhance the inhibitory effect of oxygen,which greatly reduces the concentration of both intentional and unintentional nitrogen incorporation.Therefore,with the increase of the N2O concentration,the luminescence intensity related to the NV centers shows a trend of increasing first and decreasing then.The intensity is even lower for the N2O/C∼10%sample than the one with unintentional nitrogen doping.

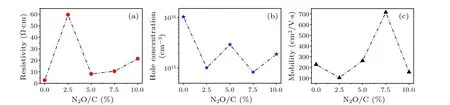
Moreover,electrical properties are also important for testing the doping results of the diamond films.Figure 5(a)shows the resistivity change of boron-doped diamond films at room temperature with different laughing gas concentrations.The resistivity of the single boron-doped film is very low at about 2.5 Ω·cm.When a small amount of laughing gas is incorporated (N2O/C=2.5%), the resistivity is rapidly increased to 60 Ω·cm.The sharp increase of resistivity may come from two reasons: (1) The incorporation with laughing gas leads to worse film crystal quality.(2) The compensation due to doping substitutional nitrogen results in a lower hole concentration.However, with the laughing gas further adding, there is a strong inhibitory effect of oxygen on nitrogen.The resistivity will decrease due to the improvement with film quality and the decrease of the donor compensation behavior related to nitrogen.
Figure 5(b) shows the hole concentration change of boron-doped diamond film at room temperature with different laughing gas concentrations.With the increasing N2O/C ratio, the hole concentration decreases quickly from about 1×1016cm-3to 1×1015cm-3.At the same time, nitrogen/oxygen co-incorporation may also lead to an increase in acceptor activation energy, which could result in decrease in hole concentration and significant increase in resistivity.The measurement results of mobility for all the samples at room temperature are shown in Fig.5(c).In single boron-doped films, the hole mobility is about 250 cm2/V·s.When a small amount of laughing gas is incorporated (N2O/C=2.5%),the mobility shows a significant decrease from 250 cm2/V·s to 100 cm2/V·s.This change with mobility is generally considered to be caused by the change of crystal quality.When the concentration of laughing gas incorporation is further increased to 7.5%, the mobility rapidly improves from 100 cm2/V·s to 700 cm2/V·s.However, when the laughing gas concentration continues to increase to 10.0%,the mobility again decreases to 150 cm2/V·s.Therefore,the growth behavior of nitrogen/oxygen co-doping regulation is complex.It is noted that the mobility results are in agreement with the crystal quality and surface morphology[Figs.3(a)–3(c)],so we believe that the large mobility (700 cm2/V·s) is associated with diamond film crystal quality improvement and oxygen inhibition behavior on nitrogen at the specific doping concentration(N2O/C=7.5%).The improved crystal quality thus limits the scattering from lattice.Therefore, high mobility is achieved then.
To further explore the mechanism with this optimized doping concentration on the growth behavior of boron-doped diamond films, the Hall effect measurement has been carried out at variable temperatures from 290 K to 900 K,and the electrical properties of single boron and boron-laughing gas codoping diamond films are further studied.Figure 6(a) shows the resistivity changes of boron-doped diamond films under different laughing gas concentrations.As the measured temperature increases,the resistivity decreases monotonically for boron-doped and boron/laughing gas co-doped diamond films.This is due to the fact that more acceptors are activated and the hole concentrations gradually increase as the temperature rising.Moreover, with the increase of temperature, the resistivity decrease with the single boron-doped sample is relatively small,while the boron-laughing gas co-doping sample decreases greatly.The resistivity of the single boron-doped sample is 3 Ω·cm at room temperature,and when the temperature approaches 900 K,the resistivity decreases to 0.15 Ω·cm with a relative decrease of 95%.However,the sample with the N2O/C ratio of 2.5%shows the resistivity of 60 Ω·cm at room temperature and 0.3 Ω·cm at temperature rising to 900 K with a decrease of 99.5%.
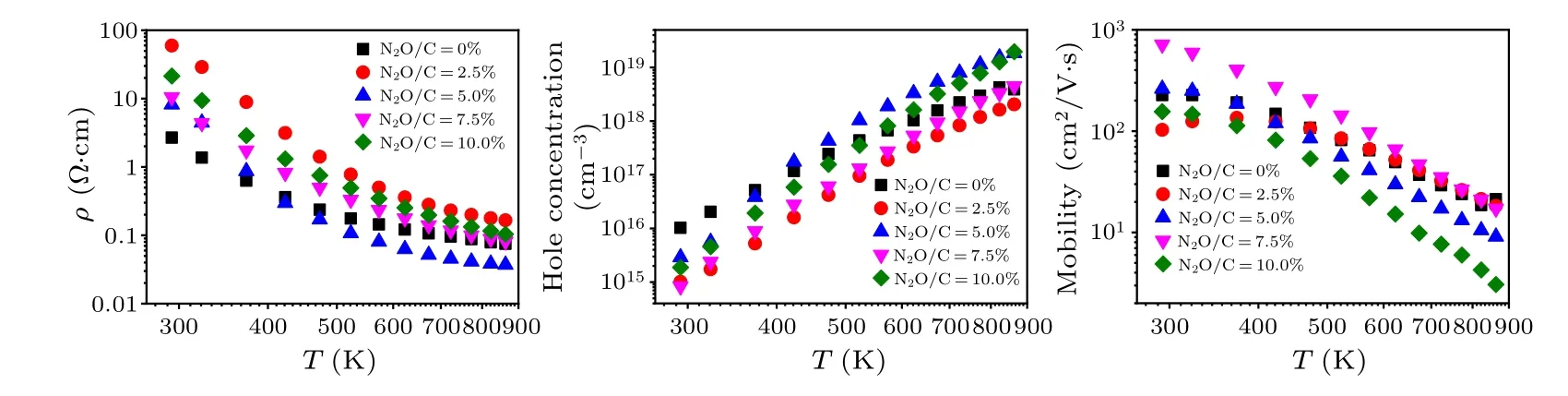
Figure 6(b)shows the hole concentration change of boron doped film under different laughing gas concentrations, with the temperature range from 290 K to 900 K.As the temperature increases, the hole concentration of all films increases,which is also responsible for the decreased resistivity with the film[Fig.6(a)].The hole concentration of single boron-doped samples increases from 1×1016cm-3at room temperature to 2.5×1018cm-3at 900 K, with a great change in two orders of magnitude.However, the hole concentration of the boronlaughing gas co-doped film increases from 1015cm-3at room temperature to 1018–1019cm-3at 900 K, and the increase is 1–2 orders of magnitude higher than the single boron-doped samples.As the temperature rises, the hole concentration increases gradually for all the samples, and no jump conductivity is observed, indicating that the defect concentration in the film is relatively low.The change of variable temperature hole concentration is consistent with the change of resistivity,which may be caused by the change of acceptor concentration,donor concentration and activation energy.
Besides the measurement of resistivity and hole concentration for different temperatures, we also performed the mobility measurement with the temperature from 290 K to 900 K as shown in Fig.6(c).For the film with N2O/C ratio of 2.5%,the mobility increases from 100 cm2/V·s to 135 cm2/V·s with temperatures from 290 K to 370 K.However, with the temperature increasing from 370 K to 900 K, the mobility decreases from 135 cm2/V·s to 18 cm2/V·s with the maximum value(135 cm2/V·s)at 370 K.This is due to the range of 290–370 K, the carrier scattering is dominated by ionized impurity scattering,with the temperature further rising from 370 K to 900 K, the carrier ionization impurity scattering weakens,and the optical and acoustic phonon scattering plays a major role.However, the mobility of the remaining samples decreases monotonically with increasing temperature, because during the overall temperature change, the carrier scattering is mainly dominated by optical and acoustic phonon scattering.Among these samples,the film with N2O/C ratio of 7.5%has the highest mobility, reaching 700 cm2/V·s at room temperature.Notably, as the temperature increases to 900 K,the mobility decreases to about 20 cm2/V·s.
Moreover, for a p-type semiconductor, the general description of the hole concentrationPat temperatureTis given by the neutrality equation[41]
whereEais the ionization energy of the acceptor,kBis the Boltzmann constant,gdis the acceptor degeneracy,NAis the acceptor concentration,NDis the compensating donor concentration,Mvis the effective density of states of the valence band.Using this equation to fit the hole concentration data shown in Fig.6(b),we can obtain the values ofNA,ND,andEa.

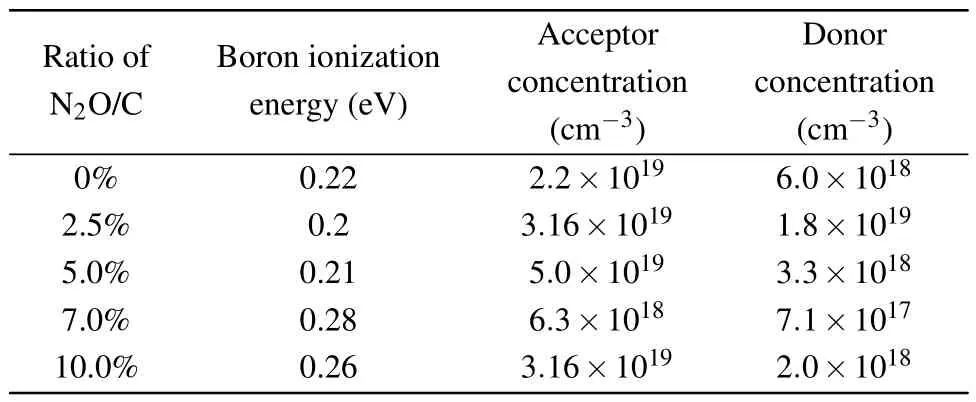
Ratio of Boron ionization Acceptor Donor N2O/C energy(eV) concentration concentration(cm-3) (cm-3)0% 0.22 2.2×1019 6.0×1018 2.5% 0.2 3.16×1019 1.8×1019 5.0% 0.21 5.0×1019 3.3×1018 7.0% 0.28 6.3×1018 7.1×1017 10.0% 0.26 3.16×1019 2.0×1018
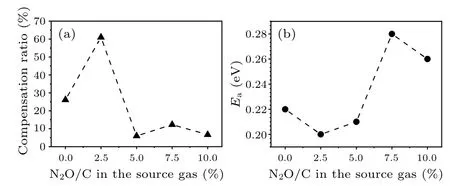
Figure 7(a) shows the hole concentration and the fitted curve for all the samples.It can be seen that the data could be well fitted by the theoretical model.The fitted results are drawn in Figs.7(b)–7(c) and Fig.8(b), as also listed in Table 1.For boron-doped diamond (N2O/C=0%), the fitted boron ionization energy (Ea), acceptor concentration (NA),and donor concentration (ND) are 0.22 eV, 2.2×1019cm-3,and 6.0×1018cm-3, respectively.The activation energy is consistent with the data reported in the literature for this doping concentration.[43,44]Grotjohnet al.suggested that the activation energy decreases from 0.36 eV to 0.05 eV as the acceptor concentration increases from 1016cm-3to 1020cm-3according to Fourier infrared spectroscopy.[43]Also, Faggioet al.reported that the activation energy decreases from 0.23 eV to 0.04 eV with acceptor concentration increases from 1018cm-3to 1020cm-3.[44]Based on Refs.[43,44],it can be concluded that the acceptor concentration and the corresponding activation energy are basically inversely related.This is exactly what we have seen in comparison of Fig.7(b) with Fig.8(b).The acceptor concentration shows an up-down-up trend versus the N2O/C ratio while the activation energy shows an opposite result.
The reason why the acceptor concentration has such a trend could be easily understood as stated in the following.With adding a small amount of laughing gas(N2O/C=2.5%),the acceptor concentration increases slightly as shown in Fig.7(b).It is possibly due to the incorporation of large size oxygen ions and nitrogen ions, which could effectively balance and compensate the tension stress caused by B ions.Therefore,the doping efficiency of boron in the diamond film is improved and the acceptor concentration increases,leading to the activation energy reducing from 0.22 eV to 0.20 eV.The compensation (ND/NA) is calculated based on the acceptor and compensation donor concentrations as shown in Fig.8(a).After the incorporation of a small amount of laughing gas(N2O/C=2.5%), even the acceptor concentration increases,the increase of compensation donor concentration results in the compensation from 26%to 61%,thus the hole concentration in Fig.5(b) reduces an order of magnitude at room temperature.When the N2O/C ratio increases further (>2.5%),the incorporation of excessive laughing gas will significantly enhance the inhibition of oxygen and reduce the incorporation efficiency of nitrogen,leading to the decrease of nitrogenrelated compensation donor and the decrease of compensation degree, which helps to improve the acceptor concentration in the film.At the same time, the acceptor activation energy is still small (N2O/C=5.0%), thus increasing the hole concentration at room temperature.When the N2O/C ratio increases to 7.5%, the inhibitory effect of oxygen on nitrogen gradually dominates, which leads to a well improved crystal and surface quality and reduced acceptor and compensation (nitrogen) concentrations.Both the acceptor and compensated donor concentrations reach relative minima 6.5×1018cm-3and 8.0×1017cm-3in the film, respectively.The large reduction in the acceptor concentration results in the activation energy increasing from 0.21 eV to 0.28 eV.Therefore,the decrease of the acceptor concentration,the increase of activation energy and compensation ratio reduce the hole concentration at room temperature.The results of NV center luminescence measurement show that the relative luminescence intensity at this laughing gas concentration(N2O/C=7.5%)is strong,indicating that the oxygen doping in the laughing gas can effectively inhibit the formation of the defects related to the non-radiative recombination process, which is conducive to improving crystal quality and leads to the significant increase with hole mobility∼700 cm2/V·s[Fig.5(c)].With further increase in the ratio to 10%,the etching effect of oxygen makes the crystal and surface quality worse, which is beneficial for the incorporation of boron acceptors.As a result,the acceptor concentration raises again.
4.Conclusion
In summary, the regulation of laughing gas on the properties of lightly boron-doped diamond is fully investigated.With adding appropriate laughing gas, boron-doped diamond films with relatively high crystal quality and structural properties,as well as relatively smooth growth surface,are obtained.More importantly, the regulation of laughing gas on boron doped diamond film growth realizes relatively low compensation donor concentration and compensation doping control under the optimization conditions.This improves the electrical performance of boron doped diamond films,especially the hole mobility, which provides a possible way to further improve the performance and application level for photoelectric devices.
Acknowledgments
Project supported by the National Key R&D Program of China(Grant Nos.2018YFB0406502,2017YFF0210800,and 2017YFB0403003),the National Natural Science Foundation of China (Grant Nos.61974059, 61674077, and 61774081),the Natural Science Foundation of Jiangsu Province (Grant No.BK20160065), and the Fundamental Research Funds for the Central Universities.
——江苏邳州老年大学校歌
- Chinese Physics B的其它文章
- The application of quantum coherence as a resource
- Special breathing structures induced by bright solitons collision in a binary dipolar Bose–Einstein condensates
- Effect of short-term plasticity on working memory
- Directional-to-random transition of cell cluster migration
- Effect of mono-/divalent metal ions on the conductivity characteristics of DNA solutions transferring through a microfluidic channel
- Off-diagonal approach to the exact solution of quantum integrable systems

