The diameter of beech snags is an important factor for saproxylic beetle richness: Implications for forest management and conservation
Václav Zumr, Oto Nakládal, Lukáˇs Bílek, Jiˇrí Remeˇs
Czech University of Life Sciences Prague, Faculty of Forestry and Wood Sciences, Kamýcká 129, Praha 6 - Suchdol, 16500, Czech Republic
Keywords:Deadwood Coleoptera Biodiversity Active management Endangered beetles
ABSTRACT Snags are an important component of beech forests that promote biodiversity.However, their occurrence is completely marginal in managed stands.Creating snags in these stands would greatly enhance biodiversity.We investigated whether snag dimensions were important for saproxylic beetle richness since they were easily transferable parameters to forest management and assessed the presence of other snag microhabitats affecting beetle communities.Data collection was performed using passive flight traps placed on thirty snags in a recent beech reserve.A total of 6706 adults belonging to 231 saproxylic species (53 Red List species, 23%) were captured.The results showed that the most important snag parameters were the diameter(thickness)and canopy openness of the surrounding stands.The occurrence of Fomes fomentarius, the volume of snag and decay class 3 were marginally significant in terms of the preference of all saproxylic species.Alpha diversity was reduced by an advanced degree of decay and a surprisingly deep stem cavity.After dividing snag thickness into categories(<35 cm; 35-70 cm and >70 cm DBH),we found that categories with snag diameter greater than 35 cm showed little differences in all saproxylic and Red List species richness and diversity indices and exhibited the highest similarity in beetle communities.Regarding recommendations to forest managers in terms of optimization and simplification of practical procedures, we suggest actively creating high stumps to act as snags greater than 35 cm in DBH diameter to promote biodiversity in beech management stands.
1.Introduction
Deadwood (DW) is a crucial element of natural forests for carbon sequestration(Martin et al.,2021),water storage,anti-erosion function,nutrient cycling, soil prosperity (Paletto and Tosi, 2010; Dhiedt et al.,2019; Klamerus-Iwan et al., 2020) and biodiversity (Parajuli and Markwith,2023).Deadwood is a key habitat for various saproxylic groups of invertebrates (Gao et al., 2015; Zumr et al., 2021), fungi (Friess et al.,2019; Yang et al., 2021), bryophytes, lichens, and vascular plants (Dittrich et al.,2014;Hofmeister et al.,2016)and for cavity-nesting bird and bats (Ettwein et al., 2020; Basile et al., 2023a), in recent studied deadwood may be important also for nonsaproxylic invertebrates taxa (Seibold et al., 2016; Graf et al., 2022).The often-studied group of invertebrates are saproxylic beetles(Seibold et al.,2015b).These beetles are among the most threatened in forests(Cálix et al.,2018).Saproxylic beetles, during at least part of their development, are associated with dead wood of different types and qualities.This is true even when they are linked to another obligate saproxylic species,such as mycetophagous beetles bound to wood-destroying fungi (Speight, 1989).Deadwood is found in a very low volume in today's commercial forests(Puletti et al.,2019).A stand managed for timber production contains only approximately 10 m3·ha-1of DW(Fridman and Walheim,2000;Kapusta et al.,2020).These volumes are well below the observed optimal DW volumes in temperate deciduous and mixed forests which is 30-50 m3·ha-1(Müller and Bütler,2010).Moreover,forests with low DW volumes pose a challenge for saproxylic organisms(Bujoczek and Bujoczek,2022).This is the reason why more and more saproxylic species are becoming endangered (Cálix et al., 2018).The consistent application of salvage logging also impacts many other animals (Thorn et al., 2018; Basile et al.,2023a).Large volumes of DW are typically reported in old-growth forest reserves (Christensen et al., 2005).Meanwhile, saproxylic beetles respond strongly to DW volumes (Gao et al., 2015; Müller et al., 2015),and,therefore,it is essential to enrich DW in managed stands to increase saproxylic beetle species abundance(Doerfler et al.,2018,2020),partly also in nonsaproxylic species (Seibold et al., 2016).DW is thought to increase forest biodiversity by 30%-70% (Dudley and Vallauri, 2005;Graf et al.,2022).European beech(Fagus sylvatica L.)is one of the most important native tree species in Europe and is an essential component of most forests in Central Europe (Brunet et al., 2010).Beech and mixed forests have been converted to intensively managed coniferous forests over the last few centuries (Hahn and Fanta, 2001).However, this has disrupted the important continuity of the forest environment (Mollier et al.,2022),which is crucial for the most endangered species,referred to as primary forest relicts (Eckelt et al., 2018).Management stands differ greatly from the natural state in terms of their structure, including DW volumes(Christensen et al.,2005)and microhabitats(Winter and Möller,2008;Vuidot et al.,2011;Asbeck et al.,2022),which also affects species composition in forests(Zumr et al.,2022a).Natural forests and protected forests are very biodiverse (Schneider et al., 2021; Zumr et al., 2022b).Müller et al.(2013)stated that saproxylic beetle species richness in beech forests represented 70% of the total Central European saproxylic beetle species.The distribution of lying and standing DW in natural forests corresponds to a ratio of approximately 70%::30%(lying::standing),and standing DW volumes are higher in protected forests(Christensen et al.,2005; Tavankar et al., 2021).Standing DW exhibits lower moisture content,which promotes biodiversity because saproxylic beetle diversity decreases with higher wood moisture content (Macagno et al., 2015).Most microhabitats important for saproxylic beetles,e.g.,fruiting bodies of fungi (Friess et al., 2019) and cavities (Henneberg et al., 2021), host standing log snags in forests (Vuidot et al., 2011; Paillet et al., 2017).Therefore, standing DW is richer regarding saproxylic beetle species compared to logs lying on the forest floor (Kappes and Topp, 2004;Bouget et al., 2012).Standing DW in beech forests highly influences saproxylic beetle richness (Redolfi De Zan et al., 2014) and the abundance of threatened species (Gibb et al., 2006; Bouget et al., 2014), as well as that of birds and bats (Roberge et al., 2008; Tillon et al., 2016;Urkijo-Letona et al., 2020; Basile et al., 2021).Actively created high stumps are almost identical to natural stumps in terms of the species composition of beetles(Jonsell et al.,2004).Stump diameter is one of the essential variables used in forest management.Zumr et al.(2021)recommend the creation of the equivalent of snags in the form of high stumps to support saproxylic beetles.At the same time, snags do not present major barriers to silvicultural management when compared to lying logs.Snags decompose much more slowly than lying DW (Vacek et al., 2015; Bradford et al., 2021) and, thus, could potentially perform environmental functions over a much longer period.
The aim of our study was to identify and quantify the effect of the variables that most influence the species richness of saproxylic beetles on snags.These variables included snag characteristics and canopy openness.Concerning the possible application of the results to forestry practice, a next question was whether there were differences between snag classes with small, medium and large snag thickness (Gossner et al.,2013).The results of the study could provide guidance that would effectively promote biodiversity of saproxylic beetles in managed beech stands.These findings should also contribute to establishing thresholds needed to maintain biodiversity in forest ecosystems (Tsikas and Karanikola,2022;Oettel et al.,2022).
2.Materials and methods
2.1.Study area
The study area was located in Central Bohemia,30-40 km southeast of Prague,in the National Nature Reserve(NNR)Beechwood of Vodĕrady(49°58′15.117′′N, 14°46′56.541′′E) at an altitude from 345 to 501 m a.s.l.The parent rock was granite, and the most represented soil categories are oligotrophic and mesotrophic Cambisols.The mean annual temperature was 7.9°C,and the mean annual precipitation was 671 mm(Climate station Ondˇrejov; Czech Hydrometeorological Institute).The duration of the vegetation period with the mean temperature above 10°C was more than 158 days.The predominant forest community was an acidophilous beech forest.The National Nature Reserve Vodˇeradské buˇciny was established in 1955 on a total area of 658 ha with the objective to protect extended old beechwoods with semi-natural stand structures (mainly associations Luzulo-Fagetum and Asperulo-Fagetum)and scattered geomorphological peri-glacial phenomena.The study area has been excluded from normal forest management since 1955.The territory was valuable due to its large areas of senescent beech stands supporting regional biodiversity and natural forest stand dynamics.These old beech stands were established by natural regeneration.Between 1820 and 1850,almost 500 ha of the area was regenerated using the three-phase shelterwood felling(Bílek et al.,2009,2014).
2.2.Environmental variables
The study was conducted in an unmanaged beech forest.Traps were placed on snags of a similar overall development stage(Fig.1).The snags were classified into three classes according to the modified methodology of Brunet and Isacsson(2009a):
1.Living snags and recently dead snags without fruitbodies of Fomes fomentarius and with hard dead wood(n=8)
2.Dead snags with fruitbodies of F.fomentarius and slightly decayed wood(n= 15)
3.Dead snags without fruitbodies of F.fomentarius and moderately or highly decayed wood(n=7)
The height of the snags (h-meters) was measured using a digital altimeter with a VERTEX ultrasonic rangefinder with an accuracy of 0.1 m.The thickness of the trunk, expressed as diameter at breast height(DBH-centimeters), was measured using a forestry belt initially in the field as circumference (O) and then converted to a diameterThe volume of snags was estimated according to the formula for an ellipsoid cone according to Brunet and Isacsson(2009a).The volume of the snag was calculated by the formula(V=volume,d=DBH,h=snag height in meters).The canopy openness was determined using hemispherical photographs taken with a fisheye lens over each trap.The analysis was performed using Gap Light Analyzer software.The software converted the transmittance of sunlight through the crown canopy into a percentage for each trap location.The presence/absence of a fallen log was recorded as a part of the broken stem lying around a studied snag,according to Brunet and Isacsson (2009a).Furthermore, we used the classification of four types of microhabitats, according to Winter and Möller(2008)and Paillet et al.(2017).1)Cavities with an entrance >5 cm formed mainly by woodpecker species (Dryocopus martius, Dendrocopos sp., Picus viridis).2) Deep stem cavities with mould and rotten wood in the base of the tree.3)Fruitbodies of F.fomentarius were white rot fungi decomposing polysaccharide (cellulose) as well as lignin.F.fomentarius was an important habitat for a high number of arthropods and represents one of the elements of the old-growth beech forest(Müller et al.,2007;Friess et al.,2019).4)Bark loss:patches with bark loss of at least 5 cm were caused mainly by bark stripping after the tree has died or by the natural falling of surrounding trees.The recorded characteristics of the snags were shown in Table 1.
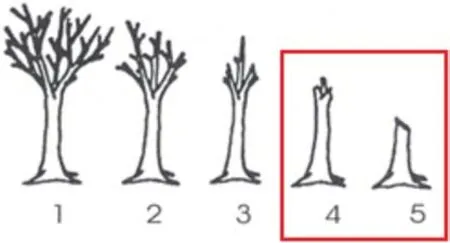
Fig.1.The developmental stages of the snags used for beetle trapping were those indicated by the red square.Snag decay development classes were according to Hayden et al.(1995).
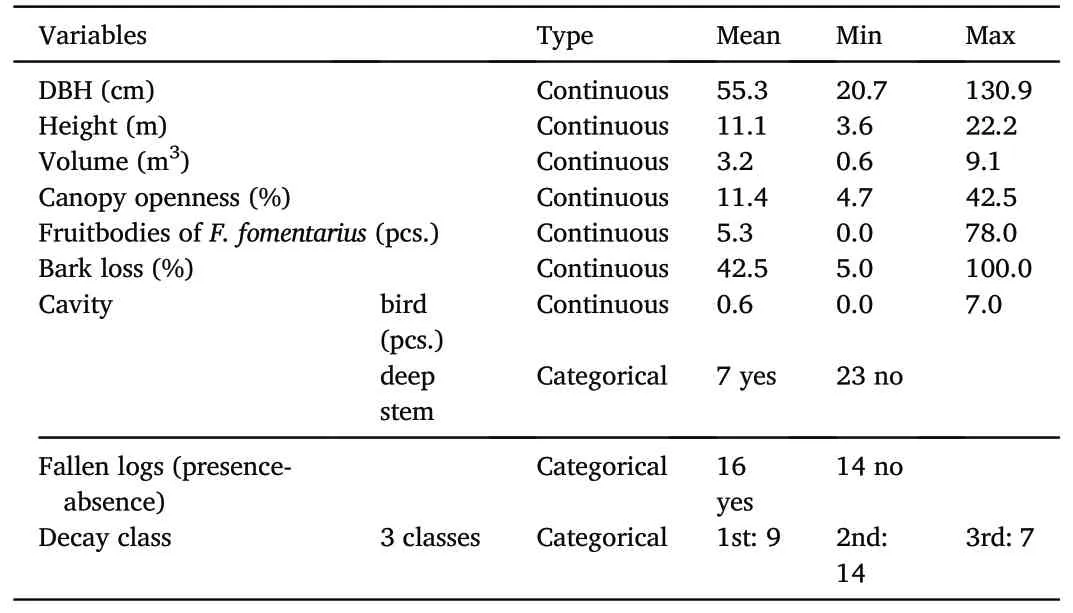
Table 1Snag characteristics.
2.3.Beetle sampling
Data collection was carried out in 2022.An unbaited flight intercept trap was used to collect entomological material (Fig.2).The trap consisted of a roof, a plexiglass barrier, a funnel, and a catch basin.The assembled trap measured 90-100 cm in length.A total of 30 traps were used,which were active during the period from April to September,and materials were collected regularly every 2-3 weeks.Traps were placed directly on beech tree trunks(trap-south direction,one trap per trunk)at a height of 1.5 m with a minimum spacing of 25 m,and the vast majority were separated by a much greater distance.The average distance was 100 m,and the distribution of traps was not uniform due to the irregular distribution of snags.The preservative solution was propylene glycol(1:1.5),with a drop of detergent to break surface tension.The collected material was identified to the species level.The families Staphylinidae and Scydmanidae were not identified due to the high degree of difficulty of determination and lack of experts.However, excluding the family Staphylinidae would not bias the results of the study, because it was highly correlated with other saproxylic beetle species (Parmain et al.,2015).Species were categorized as saproxylic species or nonsaproxylic species according to Schmidl and Bußler (2004) and Seibold et al.(2015a).Captured species that were not included in these lists were classified as nonsaproxylic species and were not included in further analyses.Following Seibold et al.(2015a), we classified species by microhabitat guilds of larvae: 1) dead wood and bark substrates, 2) cavities,and 3) fungi.The taxonomy and nomenclature of the species corresponded to the concept of Zicha (2022) (http://www.biolib.cz).The species were further classified as critically endangered(CR),endangered(EN), vulnerable (VU), or near threatened (NT) according to the IUCN Red List of Endangered Species of the Czech Republic, Invertebrates(Hejda et al.,2017).

Fig.2.Traps hung on the snag.
2.4.Data analysis
2.4.1.Microhabitats evaluation
We used ordination analyses to evaluate microhabitats for species preferences of saproxylic beetles.This approach evaluated individual beetle species and plotted them in ordination space.A forward selection method based on linear(gradient 1.9 SD units long) constrained redundancy analysis(RDA)was used to express the preference of all saproxylic beetle species to environmental conditions.Thus, the best subset of environmental variables was selected to summarize the variability in beetle species composition (all saproxylic species; Red List species).Abundance data of all saproxylic species were centered and logtransformed.Group Red List species were used in forward-constrained unimodal (gradient 4.7 SD units long) canonical correspondence analysis (CCA) without log-transformed abundance, using centered data.Calculations with 4999 unrestricted Monte Carlo permutations were used for all analyses.Contour plots of the number of beetle species and contour plots of the Shannon-Wiener index were generated for visualization of both model groups.Canoco 5 software(ˇSmilauer and Lepˇs,2014)was used for ordination analyses.The number of species captured in traps(alpha diversity)was also analyzed.For this evaluation,we used stepwise parsimony of a generalized linear model (Poisson distribution, log link function) based on the lowest value of the Akaike information criterion(Akaike,1978).The model was used for two dependent variables:species richness of all saproxylic beetles and Red List species.The significance of the variables was tested at the 0.05 confidence level.Analyses were performed in Statistica 13 software(StatSof,Inc.).
2.4.2.Thickness classes
Each snag was sorted into the thickness class following Gossner et al.(2013), into small, medium, and large (<35 cm; 35-70 cm; >70 cm)diameter at DBH of snag classes.In this frame, we analyzed alpha (α),beta(β),and gamma(γ)diversity of saproxylic beetle species.
The number of species per trap(alpha diversity)captured in thickness classes of snags was evaluated by the generalized linear model with Poisson error distribution (log link function) using package glmmTMB(Brooks et al.,2017).These data were analyzed using the post-hoc Tukey multiple comparison test within the package emmeans(Lenth,2023).To study the similarity of saproxylic beetle communities (beta diversity)living on the surveyed snags,we expressed the similarity/dissimilarity of the investigated thickness classes using nonmetric multidimensional scaling (NMDS), which plots similarities in saproxylic beetle species communities.This method was appropriate for ecology to study communities(Minchin,1987;ˇSmilauer and Lepˇs,2014).For NMDS analysis,we used“metaMDS”function implemented in the“vegan”package with an abundance approach Bray-Curtis distance with two dimensions(Oksanen et al., 2022).The significance of the difference among thickness classes was calculated by permutational multivariate analysis of variance using the function“adonis2”in the package“vegan”with 9999 permutations (Anderson, 2001).Analyses were performed in R version 4.3.1(R Core Team,2023).
We generated species cumulative curves(gamma diversity)based on the approach Chao et al.(2014).Abundance data was used for the assessment of Red list species, and for all saproxylic species incidence data were used according to the thickness class of snag.Data were replicated with 200 bootstraps, similar to Chao and Jost (2012).This rarefaction-extrapolation approach,which estimated the rate of increase in species per number of samples,was often used in studies(e.g.Seibold et al.,2018;Weiss et al.,2021).The diversity indices were based on Chao et al.(2014)with Hill numbers:q =0(species richness)and q=2 (the inverse of Simpson's concentration index).Hill’s numbers have distinct advantages over other diversity indices (Chao et al., 2014).Subsequently,we also used a coverage-based approach according to Chao and Jost (2012) to determine the sample coverage and estimate of species richness(q=0)of saproxylic beetles on the beech snags.Analyses were performed in Inext software(Chao et al.,2016).
Indicator species analysis (IndVal) was used to identify species that indicate specific classes of snag diameter(Dufr^ene and Legendre,1997).Based on this approach, it is possible to detect species that reliably indicate habitat affiliation(ˇSmilauer and Lepˇs, 2014).Saproxylic beetle species with more than three samples’ incidences were included in the analyses.We used presence-absence values(binary).The IndVal analyses were calculated using the CANOCO 5 software (ˇSmilauer and Lepˇs,2014).
3.Results
A total of 6706 individuals of 231 saproxylic beetle species(Supplementary Materials)were recorded on the snags.Among them,53 Red List(23%)species were found.Two were CR:Ennearthron pruinosulum,Hylis cariniceps; ten were EN: Cerylon deplanatum, Corticeus fraxini, Eucnemis capucina, Hylis olexai, Isorhipis melasoides, Melandrya caraboides, Pycnomerus terebrans, Synchita separanda, Synchita undata, Synchita variegata
and other 15 were VU, 26 were NT.The saproxylic species Ptilinus pectinicornis (Ptinidae) was the most abundant species, with 1367 adults.Curculinidae (including Scolytidae), Cerambycidae, Ciidae and Tenebriniodae were the most species-rich families (Fig.3).High sample coverage of 96%was recorded with thirty traps.Extrapolation to 100%sample coverage would require 200 traps.The estimated species richness of the snags was 306 saproxylic beetle species (lower confidence level 267 - upper confidence level 346).The last 4% of sample coverage corresponded to a 31%increase in species richness.
3.1.Microhabitats response
The alpha diversity response of the model groups to the environmental snag variables was significant for all saproxylic species (Df = 6;AIC=233.58;χ2=96.31;p <0.0001)and Red List species(Df=5;AIC=160.08;χ2=51.39;p <0.0001)based on the regression model results(Table 2).Multivariate analysis of species preferences explained a significant proportion(40.1%)of the variability using a forward selection of the significant variables(Fig.4,Table 3)from a global permutation test with all variables, which was 55.9% (pF = 2.1; p = 0.0002).The preference for Red List species CCA explained 15.7%(Fig.5,Table 3)of the variability in the dataset,which was conclusively explained by a minority of the global permutation test of 44.8% (pF = 1.3; p = 0.006).The Shannon-Wiener index showed species richness preferences in both groups (Figs.4 and 5).The diversity index in all saproxylic species increased with canopy openness,while in Red List species,this trend was observed with increasing volume of snags.
3.2.Thickness classes
Captured numbers of species per trap (alpha diversity) were significantly different between thickness classes for tested groups - all the saproxylic species (χ2= 18.40, Df = 2, p <0.001) and for the Red List species(χ2=24.70,Df=2,p <0.0001).Large and medium snags were similar, but differences increased between large and small snags for all species(Fig.6).Species curves according to Hill numbers(q=0,2)were very similar (gamma diversity) in the medium and large thickness classes,showing conclusively higher values compared to the small snag class.Species cumulative richness did not differ between the medium and large classes(Fig.7).According to NMDS,thickness classes were most similar in species composition(beta diversity)for medium and large snags.Small snags had the lowest degree of intersection within these classes.However,communities were marginal insignificant p=0.06(Fig.8).Red List species were the most abundant in medium and large snag;however,the large snag had significantly the highest Simpon’s diversity index(Fig.7).A total of 113(78%)of the 144 species tested were found to be indicative of the snag size class.The majority of species were associated with large snags 56(50%),medium snags 37(33%)and small snags 20(27%).For Red List species,the preference of indicator species was similar patterns(Table 4).
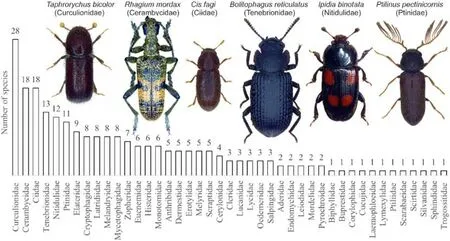
Fig.3.The richness of saproxylic families recorded during the study.The six richest families were shown with their most abundant species(photos by Leica and edited with CorelDRAW, May 2021).
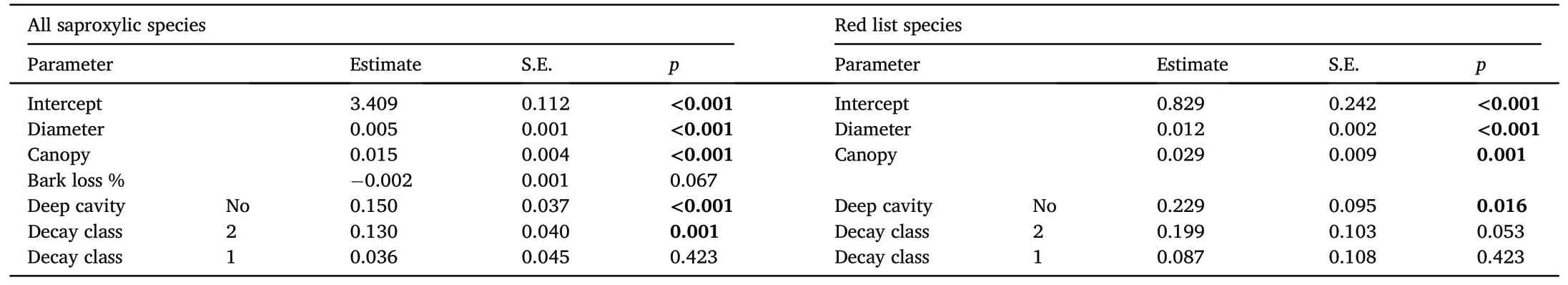
Table 2Results of the importance of environmental variables on the alpha diversity of the studied beetle groups.A sigma-restricted generalized linear model with a Poisson distribution stepwise parsimony model according to AIC values was used.Significant differences are marked (p <0.05).S.E., Standard error.
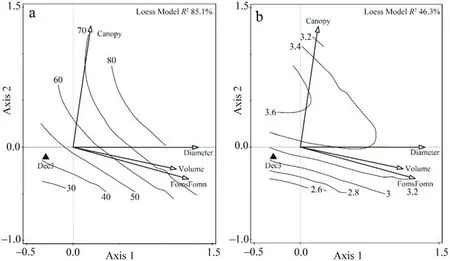
Fig.4.The isolane trend of a) preference of species richness of all saproxylic species and b) Shannon-Wiener diversity indices of samples by stepwise selected variables.RDA ordination diagram of the first two axes was shown (Axis 1 eigenvalues 0.1802; Axis 2 eigenvalues 0.1105).
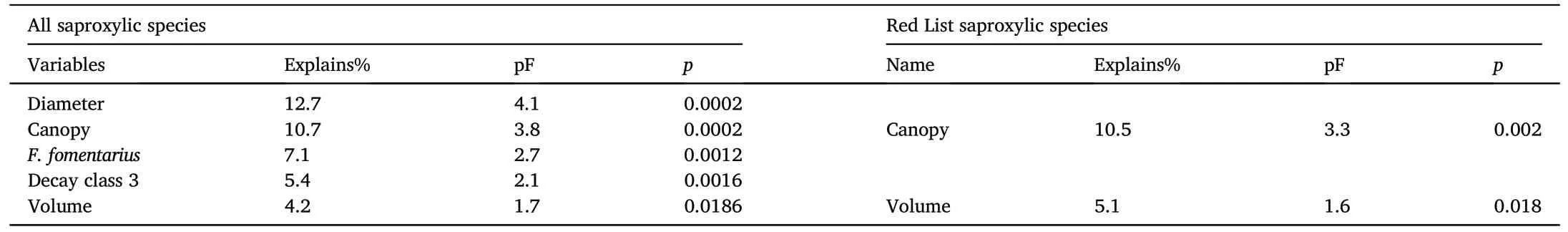
Table 3Result of forward selection multivariate analysis of significant variables for beetle species of model groups related to variables of the snag habitats by multivariate analysis.
3.3.Guild composition
The most common species captured were wood-bark-dwelling (153 species), the next group was fungicolous (66 species), and the last was cavity-dwelling (13 species), as shown in Table 5.For the Wood-bark group, confirmatory variables explained 35.9% of the global permutation test explained variation 57.1% (pF = 2.2; p = 0.0002).For the Fungicolous group, confirmatory variables explained 36.0% of the total explained variation 53.0% (pF = 2.1; p = 0.0004), Cavity explained 24.7%of the total explained variation 45.3%(pF=1.6;p =0.019).
4.Discussion
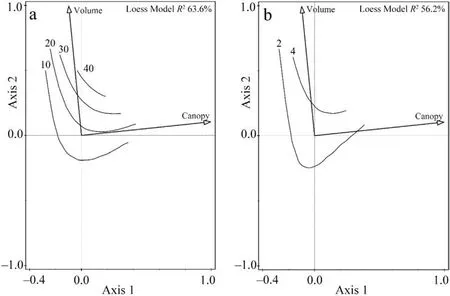
Fig.5.The isolane trend of a)species richness of Red List species and b)Shannon-Wiener diversity indices of samples by stepwise selected variables.RDA ordination diagram of the first two axes was shown (Axis 1 eigenvalues 0.640; Axis 2 eigenvalues 0.306).

Fig.6.Number of species recorded in the study among thickness classes.Solid lines in the box were median values;the box indicated the interquartile range(Q1-Q3)and whiskers min-max values.The letters above bars indicated significant differences by multiple comparison post hoc Tukey test.
Our study found that snag characteristics strongly influenced the saproxylic beetles attached to snags.The most important parameters were the thickness of the snag trunk and the openness of the stand patch.Lindhe et al.(2005)also found a similar pattern on high stumps.Thus,we assume that natural snags are comparable to actively formed high stumps in this respect(Jonsell et al.,2004).The openness of the patch was a key factor for high beetle abundance, as confirmed by, e.g.Nakládal et al.(2022)and Lettenmaier et al.(2022).In contrast to some authors(Jonsell et al.,2004;Brunet and Isacsson,2009a)who found snag thickness to be a variable of minimal importance,this variable's importance was crucial in our study.Our finding corresponded with Rappa et al.(2022),as well as beetle biomass increasing with tree thickness.The snag decomposition phase had a marginal but negative effect on alpha diversity,while Brunet and Isacsson (2009a,b) found the snag decomposition phase to be the most important negative variable.However, even the advanced decomposition phase represented a life niche for a number of species, which was also why we detected it as a conclusively marginally important variable in species preferences in our study,especially from the group of saproxylic species inhabiting DW substrates.This trend was mainly observed in shaded advanced decomposed snags.This result was consistent with the finding of Jonsell et al.(1998), saying that higher decomposition stages can be inhabited by shade-dwelling saproxylic beetle species.We did not expect deep stem cavities to negatively affect saproxylic beetle alpha diversity,as they are widely known to be one of the most important microhabitats for this group of beetles(Müller et al.,2014; Henneberg et al., 2021).This could be explained by the fact that cavity-dwelling species very rarely leave their cavity, e.g.,cavity-dwelling species of Elateridae (Mertlik, 2019a, 2019b).Nevertheless,Hennenberg et al.(2021)noted that cavity communities are also influenced by stand-level structure.The low mobility of cavity species and rare species(Brunet and Isacsson,2009b)and the study area being a recent reserve with a history of managed stands may have lost the continuity of suitable cavity microhabitats, and recolonization of these microhabitats may only occur over a longer period.Continuity is an important parameter for saproxylic species(Brin et al.,2016),especially for rare species (Eckelt et al., 2018).Increasing environmental temperature allows faster recolonization of suitable habitats,but cavity-nesting species hardly respond to this change(Della Rocca and Milanesi,2020).Other explanations for the negative effect of deep stem cavities may be that they occurred mainly on advanced decomposed snags, generally leading to lower species richness (Brunet and Isacsson, 2009a), and cavity characteristics may also be less suitable for saproxylic species(Cuff et al.,2021).The abundance of F.fomentarius fruiting bodies was found to be a less important but statistically conclusive parameter for the group of all saproxylic species.The most central attribute was for the fungicolous guild of species mainly of the family Ciidae and, e.g., the species Bolitophagus reticulatus.Our findings support the conclusions of Friess et al.(2019),who suggests that snags represent an essential element that creates a refuge for many species, especially the fungicolous guild, but also for other saproxylic species in terms of wood decomposition.However, these fungal species are rarely present in beech-managed stands(Müller et al.,2007).The absence of F.fomentarius may partially explain why intensively managed beech stands are biologically poor (Müller et al.,2008;Roth et al.,2019).Endangered species preferences have been linked to canopy openness,snag thickness and volume.These parameters are the main reasons saproxylic species are red-listed and are related to the life history strategies of these species.The importance of large volume and thick snag dimensions for endangered species are also found by Müller et al.(2010) and Procházka and Schlaghamerský (2019).The most threatened species depend on the sunlit massive dimension of DW(Seibold et al.,2015a).
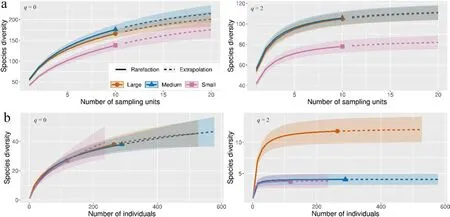
Fig.7.Sample-size-based rarefaction and extrapolation sampling gamma diversity curve showing Hill's numbers.(a) All saproxylic species, incidence data; (b) Red List species,abundance data.q=0(species richness)and q=2(the inverse of Simpson’s concentration index)of model groups of saproxylic species by the diameter classes of snags.Colored shaded areas are the 95%confidence intervals.Solid symbols represent the total number of study samples(a)and total number of species(b).
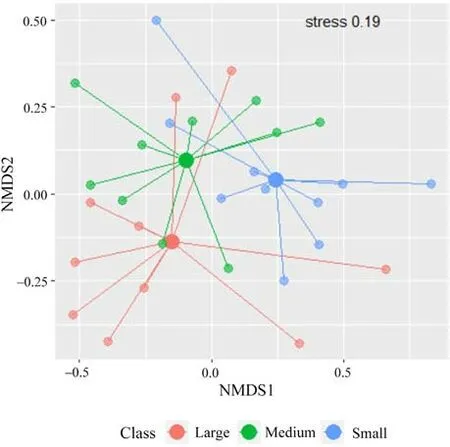
Fig.8.The nonmetric multidimensional metric(NMDS)shows the similarity of samples based on thickness classes.Bray-Curtis distance was used.Solid points indicate centroids.
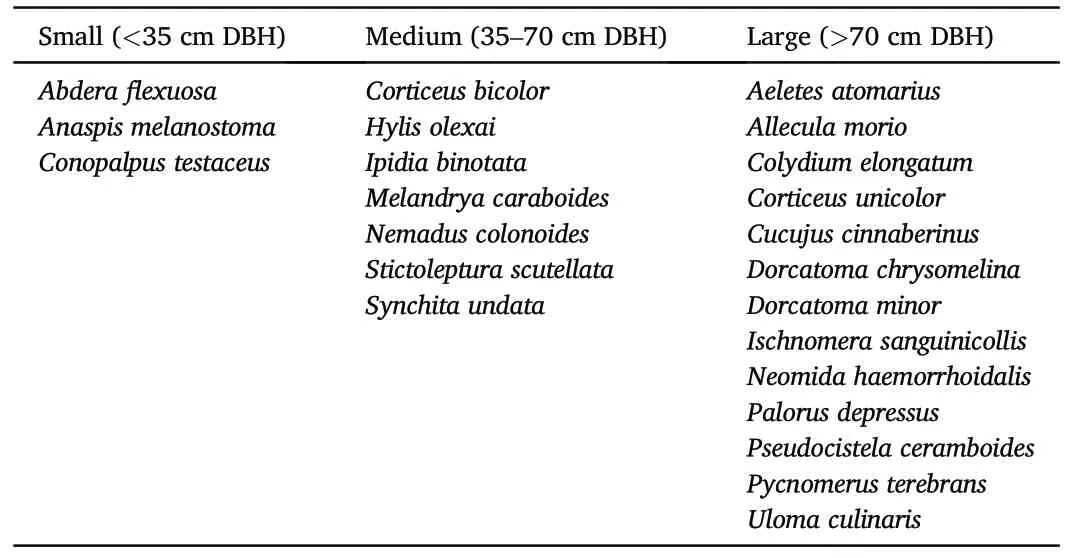
Table 4Characteristic Red List species affiliated with the thickness classes of the beech snags.Binomial distribution was used, and a minimum of three incidences of species were included for IndVal analyses (Dufr^ene and Legendre, 1997).
4.1.Implications for management
Conservation-oriented forestry aims to maintain populations of forest organisms by improving the conservation value of managed forests(Gibb et al.,2006).Stem diameter is the main dendrometric variable in forestry management.It is easily transferable to the active promotion of biodiversity in managed stands.According to our results,we recommend the use of high stumps as an artificial equivalent of a snag greater than 35 cm DBH in to promote saproxylic beetle species richness.From an economic point of view,the same level of snag dimensions is indicated(Zumr et al.,2021).This dimension will provide many other animals with a broader range of habitat options.Snags greater than 30 cm DBH have the most significant potential to occur in cavity-dwelling bird communities(Smith et al., 2008).At measurements greater than 30 cm, the white-backed woodpecker occupancy probability on snags increases significantly(Ettwein et al.,2020),as does the occurrence of tree fungi(Urkijo-Letona et al.,2020).Therefore,snags greater than 25 cm DBH are recommended to support cavity-nesting birds such as woodpeckers(Bush et al.,2009).Our findings may lead to a redirection of targeted attention to support biodiversity in beech management forests,as simple methodology is verywelcome in practical forestry management.This could lead to a very rapid increase in snag numbers, as they are almost absent in managed forests (Zumr and Remeˇs, 2020), and the occurrence of snags greater than 30 cm in forest management stands at almost zero(Sweeney et al.,2010;Kapusta et al.,2020).Larger snag dimensions are critical because they are key attributes for the long-term persistence of the snag in the forest stand (Parish et al., 2010; Oettel et al., 2023), thus promoting biodiversity in forests in the longer term.In managed stands,this would create valuable longer-term DW microhabitats for many saproxylic species, even in terms of creating new habitats in a no-connectivity environment that saproxylic beetles will gradually colonize (Janssen et al.,2016; Basile et al., 2023).However, the creation and maintenance of good quality DW habitat is,in many cases,more important for saproxylic organisms than the explicit maintenance of the forest continuity(Komonen and Müller,2018).At the same time, at the established minimum level (>35 cm DBH) and above, snag may provide a refuge for scarce saproxylic species in beech forests.One can agree with the high preference of endangered species for large deadwood diameters.Also,in several species,the highest preference for species occurrence was found to be in the range of 30-60 cm DBH, e.g., Cucujus cinnaberinus (Scopoli 1763), Rosalia alpina(Linnaeus 1758),and Rhysodes sulcatus(Farbricius 1787)(Cizek et al.,2009;Kostanjsek et al.,2018;Jaworski et al.,2019).Many authors have recommended a DW volume for saproxylic beetles in beech forests with an intersection of DW values of 40 m3·ha-1(Müller and Bütler, 2010; Gossner et al., 2013; Procházka and Schlaghamerský,2019).The average snag volume in our study greater than 35 cm was 4 m3, or 10 snag pieces·ha-1.A similar recommendation for maintaining ecological processes was suggested for an average of 9 large snags·ha-1(Marage and Lemperiere,2005).This may be an economically viable rate for forest management companies, as a snag greater than 35 cm DBH occurs in a beech reserve at 30 snags·ha-1(Keren et al.,2018).

Table 5Result of forward selection multivariate analysis of significant variables for beetle species of model groups related to variables of the snag habitats by multivariate analysis.
5.Conclusion
Our study identified a subattribute of forest stands that largely influences saproxylic beetle biodiversity.The search for applicable approaches transferable to forestry practice is essential for sustainable forest management and conservation.An unprecedented insect decline has been observed in recent decades(Kunin,2019;Wagner et al.,2021),especially in forest ecosystems (Seibold et al., 2019).Therefore, efforts should be made to increase the amount of DW and focus on elements missing in managed stands, which are very often snags (Bujoczek and Bujoczek, 2022).Targeted creation of high stumps as the equivalent of snags greater than 35 cm DBH in beech-managed forests can be highly beneficial in promoting biodiversity.Combined with cyclical management practices that create diverse niches in managed stands, this approach can synergistically affect saproxylic beetle richness.
Funding
This research was supported by grant No.QK23020008, funded by the Ministry of Agriculture of the Czech Republic.
Authors’contributions
Conceptualization, V.Z.; Methodology, V.Z.and O.N.; Field work,V.Z., O.N.and J.R.; Investigation, V.Z.and O.N.; Analyses: V.Z.; Writing-original draft preparation, V.Z.and J.R.; Writing-review and editing,V.Z.,O.N.,J.R.,and L.B..All authors have read and agreed to the published version of the manuscript.
Data availability
Data are available on request from the authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the following experts for help in the identification of some beetle families: Jan Horák (Praha): Scraptiidae, Mordelidae;Pavel Průdek (Brno): Cerylonidae, Ciidae, Corylophagidae, Cryptophagidae,Latridiidae,Monotomidae;Josef Jelínek(Praha):Nitidulidae.The English language of the manuscript has been proofread by certified American Journal Experts.We also thank anonymous reviewers for valuable comments to improve the manuscript.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2023.100143.
- Forest Ecosystems的其它文章
- Growth phenology adjusts to seasonal changes in water availability in coexisting evergreen and deciduous mediterranean oaks
- Assessing spatiotemporal variations of forest carbon density using bi-temporal discrete aerial laser scanning data in Chinese boreal forests
- Stabilizing forest productivity and resilience at multiple scales
- Legacy effects of historical woodland changes on contemporary plant functional composition
- Habitat heterogeneity and biotic interactions mediate climate influences on seedling survival in a temperate forest
- Trees species’ dispersal mode and habitat heterogeneity shape negative density dependence in a temperate forest

