基于放射组学评估胸肌指数与乳腺癌骨转移患者预后关系的研究
吴海啸 马文娟 李之珺 张超
摘要:目的 利用放射组学精准测量胸肌肌肉含量,分析胸肌指数(PMI)与乳腺癌骨转移(BCBM)患者预后的相关性。方法 收集197例BCBM患者年龄、病理分型、远处转移、放疗和随访等数据。基于胸部CT结果计算PMI,利用受试者工作特征(ROC)曲线确定PMI的最佳临界值,并分析不同PMI水平患者的临床病理特征和预后差异。结果 197例BCBM患者根据PMI最佳临界值分为低PMI组(≤6.295,104例)和高PMI组(>6.295,93例)。与高PMI组相比,低PMI组患者的Ki-67≥14%比例、脑转移率、血清Ca2+浓度和死亡比例增加,病理分型和骨转移部位分布差异有统计学意义(P<0.05)。低PMI组和高PMI组患者的1、3、5年总生存率分别为67.3%、17.3%、5.8%和96.8%、84.9%、36.6%,累积总生存率差异有统计学意义(Log-rank χ2=82.329,P<0.01)。多因素Cox回归分析显示,在校正了病理分型、骨转移部位、脑转移和Ca2+等因素后,低PMI组患者的死亡风险是高PMI组患者的3.954倍。结论 PMI下降可增加BCBM患者的死亡風险,BCBM治疗过程中应鼓励对患者进行个性化康复训练和营养支持干预。
关键词:乳腺肿瘤;预后;骨转移;胸肌指数
中图分类号:R737.9文献标志码:ADOI:10.11958/20230241
Relationship between pectoralis muscle index and prognosis of breast cancer patients with bone metastases based on radiomics
WU Haixiao MA Wenjuan LI ZhijunZHANG Chao
1 Department of Bone and Soft Tissue Tumor, 2 Department of Breast Imaging, 3 Department of Radiology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer
Prevention and Therapy, Tianjin's Clinical Research Center for Cancer, Tianjin 300060, China
Abstract: Objective To accurately calculate the pectoralis muscle mass and clarify the influence of pectoralis muscle index (PMI) on the survival and prognosis of breast cancer patients with bone metastasis (BCBM) using radiomics. Methods Clinical data of 197 BCBM patients were collected, including age, pathological classification, distant metastasis, radiotherapy and 5-year follow-up data. The PMI was calculated based on chest CT results. The optimal cutoff value for PMI was determined by receiver operating characteristic (ROC) curve, and patients were divided into the low-PMI and the high-PMI groups. Then the clinical and pathological characteristics and prognostic differences of patients with different levels of PMI were analyzed. Results According to the optimal cutoff value for PMI, 197 BCBM patients were divided into the low PMI group (≤6.295, 104 cases) and the high PMI group (>6.295, 93 cases). Compared with the high PMI group, there were significant differences in the proportion of Ki-67≥14%, distribution of bone metastasis sites, and serum Ca2+ concentration in the low PMI group (P<0.05). The 1-, 3- and 5-year overall survival rates of patients were 67.3%, 17.3%, 5.8% and 96.8%, 84.9% and 36.6% in the low PMI group and the high PMI group, respectively, and the cumulative overall survival rate difference was statistically significant (Log-rank χ2=82.329, P<0.001). The multivariate Cox regression analysis showed that after adjusting pathological classification, site of bone metastasis, brain metastasis and Ca2+, the risk of death in the low PMI group was 3.954 times higher than that in the high PMI group. Conclusion The decrease in PMI can increase the risk of death in BCBM patients. Personalized rehabilitation training and nutritional support intervention should be encouraged for patients during the BCBM treatment process.
Key words: breast neoplasms; prognosis; bone metastasis; pectoralis muscle index
骨是乳腺癌最常见的转移部位,60%~80%的乳腺癌患者可发生骨转移(breast cancer bone metastasis,BCBM),5年生存率仅为22.8%[1-3]。骨相关事件是骨转移发生后最常见的临床表现,可显著降低患者的生活质量[4-5]。晚期肿瘤患者由于能量摄入减少、负氮平衡和静止能量消耗的增加,肌肉系统逐渐发生结构和功能性的改变,从而对患者的治疗耐受性和生活质量产生负面影响[6]。前期有研究表明,胸肌指数(pectoralis muscle index,PMI)与淋巴瘤、非小细胞肺癌和宫颈癌患者的预后密切相关,同时与患者手术并发症、治疗费用和住院时间有关[7-9]。目前,CT是分析骨骼肌肉系统疾病的首选方法,较传统的检测方式更为简便、准确,而放射组学技术可通过图像识别技术提取CT影像中肌肉特征,准确计算肌肉面积。因此,本研究利用放射组学技术提取BCBM患者胸部CT图像中胸肌形态特征,旨在探讨PMI与患者死亡的关系。
1 对象与方法
1.1 研究对象 纳入2010年1月—2017年12月就诊于天津医科大学肿瘤医院的197例BCBM患者,年龄47~61岁,中位年龄54岁。纳入标准:原发性乳腺癌,影像学检查确诊为骨转移,确诊骨转移时有清晰的胸部CT图像,病例资料详尽。排除标准:病理诊断难以明确和(或)多重恶性肿瘤患者,双乳癌,男性乳腺癌,随访信息不完整,拒绝参加本研究者。本研究经天津医科大学肿瘤研究所和天津医科大学肿瘤医院医院伦理委员会批准(审批号:bc2021245),所有患者均签署知情同意书。
1.2 资料收集 根据患者临床和病理特征,收集年龄、原发性乳腺癌病理、雌激素受体(estrogen receptor,ER)状态、孕激素受体(progesterone receptor,PR)状态、人类表皮生长因子受体2(human epidermal growth factor receptor2,HER2)状态、Ki-67状态、骨转移部位、骨轉移数目、骨转移灶放疗史、应用骨保护剂、内脏转移和Ca2+浓度等指标。总生存期为乳腺癌患者确诊骨转移至死亡或随访结束,随访结束日期为死亡或2022年1月。
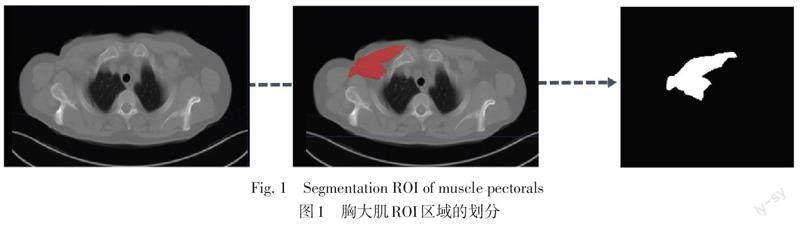
1.3 PMI测量 所有患者均进行胸部常规CT平扫。患者取仰卧位,扫描范围从胸廓入口至肋膈角。数据均以DCM形式导出,用于后续的放射组学特征提取。胸部CT图像均通过开源软件ITK-SNAP 3.6.0版本手动分割第2胸椎胸肋水平健侧胸肌的感兴趣区域(regions of interest,ROI),提取过程见图1。ROI分割由2名经验丰富的影像医师完成,影像医师均不了解分割对象的具体情况。利用PyRadiomics软件自动计算放射组学特征,精确提取CT图像中胸大肌的图形特征,计算胸大肌面积(cm2)。PMI=胸大肌面积/身高2(cm2/m2)。
1.4 统计学方法 采用SPSS 26.0和R 4.2.0进行数据分析。不符合正态分布的计量资料以中位数和四分位数[M(P25,P75)]表示,组间比较采用Mann-Whitney U检验,通过绘制受试者工作特征(ROC)曲线确定PMI预测患者死亡的最佳临界值,并计算对应的曲线下面积(AUC)、敏感度和特异度。计数资料采用例(%)表示,组间比较采用χ2检验。采用Kaplan-Meier法绘制生存曲线,单因素和多因素Cox回归分析PMI对患者预后的影响。P<0.05为差异有统计学意义。
2 结果
2.1 随访结果及患者分组 截至末次随访结束时,197例BCBM患者中157例死亡,40例患者存活。根据ROC曲线,确定PMI预测患者死亡的最佳临界值为6.295,测量曲线下面积(AUC)为0.767,敏感度为62.4%,特异度为85.0%,见图2。依据PMI最佳临界值,将患者分为低PMI组(≤6.295,104例)和高PMI组(>6.295,93例)。
2.2 低PMI组和高PMI组患者的临床病理特征比较 与高PMI组相比,低PMI组患者的Ki-67≥14%比例、脑转移率、血清Ca2+浓度和死亡比例增加(P<0.05),2组病理分型和骨转移部位分布差异亦有统计学意义(P<0.05),见表1。
2.3 PMI对患者死亡的Cox回归分析结果 在未校正其他影响因素时,低PMI组BCBM患者发生死亡的风险是高PMI患者的4.318倍;在校正了病理分型、骨转移部位、脑转移和Ca2+等因素后,低PMI组BCBM患者发生死亡的风险是高PMI组患者的3.954倍,见表2。
2.4 生存分析结果 197例患者1、3、5年总生存率分别为81.2%、48.7%、20.3%,中位生存期为34.8个月(95%CI:29.5~40.2)。低PMI组和高PMI组患者1、3、5年总生存率分别为67.3%、17.3%、5.8%和96.8%、84.9%、36.6%,累积总生存率差异有统计学意义(Log-rank χ2=82.329,P<0.01),见图3。
3 讨论
随着靶向治疗、免疫治疗以及内分泌治疗的飞速发展,晚期乳腺癌患者的生存期显著延长,骨相关事件的发生风险也相应提高。然而,骨转移患者的总生存率仍较低。本研究结果显示,至末次随访结束时,所纳入BCBM患者的1年生存率为81.2%,5年生存率为20.3%,这与既往研究[10]报道一致。
本研究中,低PMI是BCBM患者死亡的独立影响因素。前期研究报道,肌肉指数与淋巴瘤、肺癌、卵巢癌等疾病的预后显著相关[7-8,11],同时还是患者发生摔倒、骨折、术后并发症和早死的独立影响因素[12-13]。此外,骨骼肌含量下降在非小细胞肺癌患者中较常见,即使在正常BMI患者中,随着骨骼肌含量的下降,患者的死亡率也随之上升[14]。另有研究指出,肌肉含量下降不仅与年龄相关,且与慢性消耗性疾病密切相关,此类疾病可导致肌肉含量的变化,而肌肉含量的变化同样可促进疾病的进展[15]。因此,基于本研究和前期研究成果,笔者认为骨骼肌含量对于恶性肿瘤患者的预后预测具有重要意义。骨骼肌含量的测定与评价通常取患者常用的较大肌群肌肉,例如胸大肌、臀大肌、下肢肌群等,以便更好地反映全身骨骼肌含量。由于胸部CT是恶性肿瘤患者复查时常进行的检查,相较肉眼评价,通过影像组学技术提取CT图像中胸大肌的肌肉特征更加精确,并且可实现高通量检测。
目前,晚期惡性肿瘤患者肌肉含量改变发生的具体机制尚未被揭示。但多种证据表明,肌肉含量的改变与患者机体免疫系统失衡(激素分泌失调、慢性炎症和氧化应激),新陈代谢紊乱(蛋白质分解增加、脂肪堆积、骨质流失等),以及日常体力活动减少和营养状况不良等息息相关[16-17]。因此,早期识别肌肉含量下降的高危患者,并制定早期干预措施,通过合理的康复锻炼和运动训练进行及时干预,有助于降低晚期恶性肿瘤患者的死亡风险。
本研究多因素分析结果提示,病理分型、脑转移、四肢和中轴骨转移均与BCBM的预后有关。而前期有研究表明,年龄、单发骨转移、脑和内脏转移、HR、HER2和碱性磷酸酶等多种因素可影响BCBM患者的预后[18]。与既往研究相比,本研究结果支持四肢和中轴骨转移是BCBM患者的重要预后因素。分析其原因可能是四肢骨和中轴骨转移均可引起患者病理性骨折、脊髓压迫、截瘫等严重骨相关事件,以及继发的下肢深静脉血栓、褥疮和坠积性肺炎等并发症,严重影响患者的生存时间和生活质量。
综上,本研究阐述了不同PMI水平BCBM患者的预后特征,发现低PMI水平会显著增加BCBM患者的死亡风险。对于BCBM患者应进行必要的康复锻炼和运动指导,以维持患者的肌肉水平,进而降低患者的死亡风险并提高生活质量。
参考文献
[1] ALLEMANI C,MATSUDA T,DI CARLO V,et al. Global surveillance of trends in cancer survival 2000-14(CONCORD-3):analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries[J]. Lancet,2018,391(10125):1023-1075. doi:10.1016/S0140-6736(17)33326-3.
[2] CHEN H M,CHEN F P,YANG K C,et al. Association of bone metastasis with early-stage breast cancer in women with and without precancer osteoporosis according to osteoporosis therapy status[J]. JAMA Netw Open,2019,2(3):e190429. doi:10.1001/jamanetworkopen.2019.0429.
[3] MA W,WANG X,XU G,et al. Distant metastasis prediction via a multi-feature fusion model in breast cancer[J]. Aging(Albany NY),2020,12(18):18151-18162. doi:10.18632/aging.103630.
[4] LIANG Y,ZHANG H,SONG X,et al. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets[J]. Semin Cancer Biol,2020,60:14-27. doi:10.1016/j.semcancer.2019.08.012.
[5] AHMAD A. Breast cancer metastasis and drug resistance[M]. 2nd ed. Switzerland:Springer Nature Switzerland AG,2019:105.
[6] MATHIEU M,GUILLOT P,RIAUDEL T,et al. Association between bone mineral density and fat mass independent of lean mass and physical activity in women aged 75 or older[J]. Nutrients,2021,13(6):1994. doi:10.3390/nu13061994.
[7] GO S I,PARK M J,PARK S,et al. Cachexia index as a potential biomarker for cancer cachexia and a prognostic indicator in diffuse large B-cell lymphoma[J]. J Cachexia Sarcopenia Muscle,2021,12(6):2211-2219. doi:10.1002/jcsm.12837.
[8] SUN C,ANRAKU M,KAWAHARA T,et al. Respiratory strength and pectoralis muscle mass as measures of sarcopenia: Relation to outcomes in resected non-small cell lung cancer[J]. J Thorac Cardiovasc Surg,2022,163(3):779-787.e2. doi:10.1016/j.jtcvs.2020.10.133.
[9] DE SANTANA F M,PREMAOR M O,TANIGAVA N Y,et al. Low muscle mass in older adults and mortality:A systematic review and meta-analysis[J]. Exp Gerontol,2021,152:111461. doi:10.1016/j.exger.2021.111461.
[10] LI C,LIU M,LI J,et al. Machine learning predicts the prognosis of breast cancer patients with initial bone metastases[J]. Front Public Health,2022,10:1003976. doi:10.3389/fpubh.2022.1003976.
[11] JIN Y,MA X,YANG Z,et al. Low L3 skeletal muscle index associated with the clinicopathological characteristics and prognosis of ovarian cancer: a meta-analysis[J]. J Cachexia Sarcopenia Muscle,2023,14(2):697-705. doi:10.1002/jcsm.13175.
[12] GO S I,PARK M J,SONG H N,et al. A comparison of pectoralis versus lumbar skeletal muscle indices for defining sarcopenia in diffuse large B-cell lymphoma - two are better than one[J]. Oncotarget,2017,8(29):47007-47019. doi:10.18632/oncotarget.16552.
[13] WU H,XU G,LI Z,et al. Nomogram predicting leukopenia in osteosarcoma after high-dose methotrexate chemotherapy[J]. Aging (Albany NY),2022,14(12):5023-5033. doi:10.18632/aging.203978.
[14] BYE A,SJ?BLOM B,WENTZEL-LARSEN T,et al. Muscle mass and association to quality of life in non-small cell lung cancer patients[J]. J Cachexia Sarcopenia Muscle,2017,8(5):759-767. doi:10.1002/jcsm.12206.
[15] CRUZ-JENTOFT A J,SAYER A A. Sarcopenia[J]. Lancet,2019,393(10191):2636-2646. doi:10.1016/S0140-6736(19)31138-9.
[16] KIRK B,MILLER S,ZANKER J,et al. A clinical guide to the pathophysiology, diagnosis and treatment of osteosarcopenia[J]. Maturitas,2020,140:27-33. doi:10.1016/j.maturitas.2020.05.012.
[17] PETERMANN-ROCHA F,FERGUSON L D,GRAY S R,et al. Association of sarcopenia with incident osteoporosis:A prospective study of 168,682 UK biobank participants[J]. J Cachexia Sarcopenia Muscle,2021,12(5):1179-1188. doi:10.1002/jcsm.12757.
[18] 王艳辉,李建华,柳雅慧,等. 不同分子亚型乳腺癌首发骨转移患者的临床特征和预后分析[J]. 天津医药,2021,49(5):499-504. WANG Y H,LI J H,LIU Y H,et al. Analysis of clinical features and prognosis of patients with first-episode bone metastasis of different molecular subtypes of breast cancer[J]. Tianjin Med J,2021,49(5):499-504. doi:10.11958/20203031.
(2023-02-24收稿 2023-05-15修回)
(本文編辑 胡小宁)

