循环冷却水用绿色阻垢剂的研究进展
何振波 张厉 高铭心 栾玲玉
摘要:近年来,为缓解水资源匮乏,循环冷却水系统得到了广泛应用。冷却水中通常含有钙、镁等多种矿物离子,容易形成不溶性盐,在设备表面结垢。使用阻垢剂是解决结垢问题最有效的方法之一。综述了近年来国内外绿色阻垢剂的研究进展,介绍了绿色阻垢剂的开发与应用现状,分析了不同类型阻垢剂的特点和阻垢性能,从螯合增溶、晶格畸变和凝聚分散作用等方面阐述了阻垢机理,可为未来绿色阻垢剂研究发展提供借鉴。
关键词:循环冷却水;绿色阻垢剂;阻垢性能;阻垢机理
中图分类号:X52 文献标志码:A 文章编号:1002-4026(2023)05-0102-19
Research progress of green scale inhibitors for circulating cooling water
HE Zhenbo, ZHANG Li, GAO Mingxin, LUAN Lingyu*
(Shandong Analysis and Test Center,Qilu University of Technology(Shandong Academy of Sciences), Jinan 250014, China)
Abstract∶Recently, circulating cooling water systems have been widely used to alleviate water shortage.However, cooling water usually contains various mineral ions,such as calcium and magnesium, which can easily form insoluble salts and scale on the surface of the equipment. The use of scale inhibitors in cooling water systems is one of the most effective methods to solve the scaling problem. In this paper, the recent research progress on green scale inhibitors at home and abroad was reviewed. The development and applications of green scale inhibitors were introduced here. The characteristics and scale inhibition performance of different types of scale inhibitors are also analyzed.Moreover,the scale inhibition mechanism was explained from different aspects,such as chelation and solubilization, coagulation and dispersion, and lattice distortion.Therefore,this review would provide an excellent reference for future research and development of green scale inhibitors.
Key words∶circulating cooling water; green scale inhibitor; scale inhibition performance; scale inhibition mechanism
随着水资源日益短缺,循环冷却水系统被广泛应用于各行业。循环冷却水中通常含有许多的钙、镁等矿物离子,随着冷却水的反复使用,水中的离子浓度不断升高[1],盐类达到过饱和状态,形成不溶性盐,在设备管道表面结垢,引起金属设备腐蚀。另外,钙垢吸附在设备表面,会降低系统的换热效率,甚至缩短设备的使用寿命[2-3]。因此,寻找有效、快速的方法来防止或消除结垢已迫在眉睫。
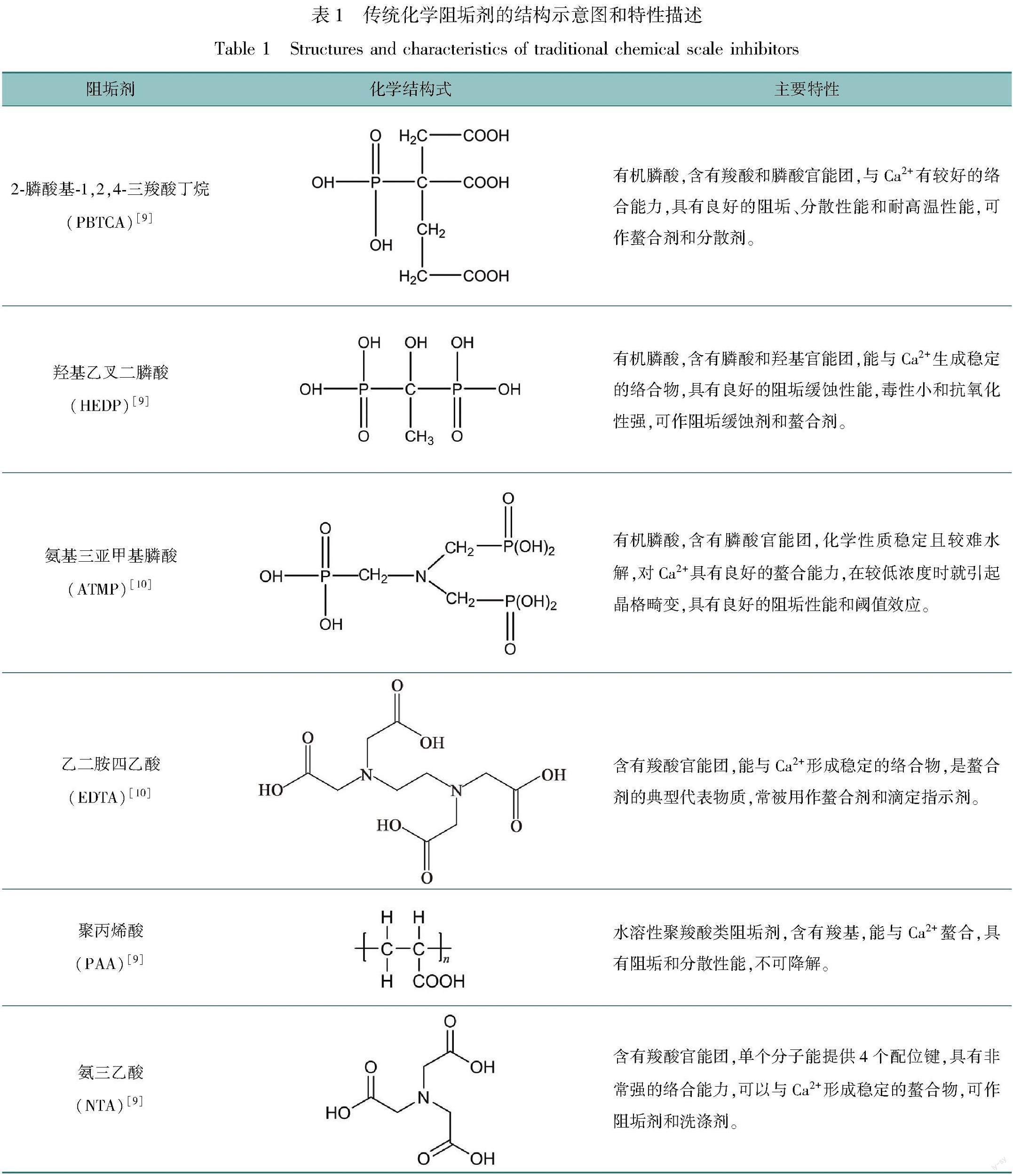
目前,在循环冷却水系统中添加化学阻垢剂是经济、有效的方法之一[4-5]。化学阻垢剂种类较多,根据聚合单体成分可分为天然有机聚合物阻垢剂和合成聚合物阻垢剂[6-8]。阻垢剂通常由含有膦酸基、羧基、磺酸基、酰胺基、醚键和羟基等多种官能团的单体聚合而成,其阻垢机理主要以螯合增溶、晶格畸变和凝聚分散为主。表1列举了常见的几种传统化学阻垢剂的结构和特性。
近年来,膦酸、羧酸、磺酸和醚键等官能团被证实能够抑制钙垢晶体成核[11-13],特别是膦酸官能团,其对阻止CaCO3的沉积具有显著作用[14]。氨基三亚甲基膦酸(ATMP)和2-膦酸基-1,2,4-三羧基丁烷(PBTCA)等是典型的含膦阻垢剂,对CaCO3的形成有着良好的抑制效果[9]。然而,含磷阻垢剂会引起水体富营养化,在应用中日益受到限制[15]。此外,经研究证明,聚丙烯酸[16]、聚马来酸及其盐类[17],因含有羧基等官能团具有良好的阻垢性能,但由于不易生物降解受到应用限制[18]。随着人们环保意识的增强,水体排放标准逐渐严格,开发新型的无磷、低毒、易生物降解的高效绿色阻垢剂[19]将会成为研究热点。
绿色阻垢剂可分为天然有机阻垢剂和人工合成的绿色聚合物阻垢剂。目前,天然有机阻垢剂主要包括基于生物提取物及其衍生物阻垢劑,因来源丰富、环保以及生物可降解等特点受到广泛关注。人工合成的绿色聚合物阻垢剂主要包括聚天冬氨酸(PASP)和聚环氧琥珀酸(PESA)类阻垢剂,因具有良好的阻垢性能、无磷、低毒以及可生物降解等优点被广泛应用。近年来,随着技术的发展和可持续战略的提出,对高效绿色阻垢剂的研究越来越多[20-24]。本文对近年来国内外绿色阻垢剂的研究进展、开发与应用现状进行了探讨分析,并阐述了它们对钙垢的阻垢性能和作用机理。
1 天然有机阻垢剂
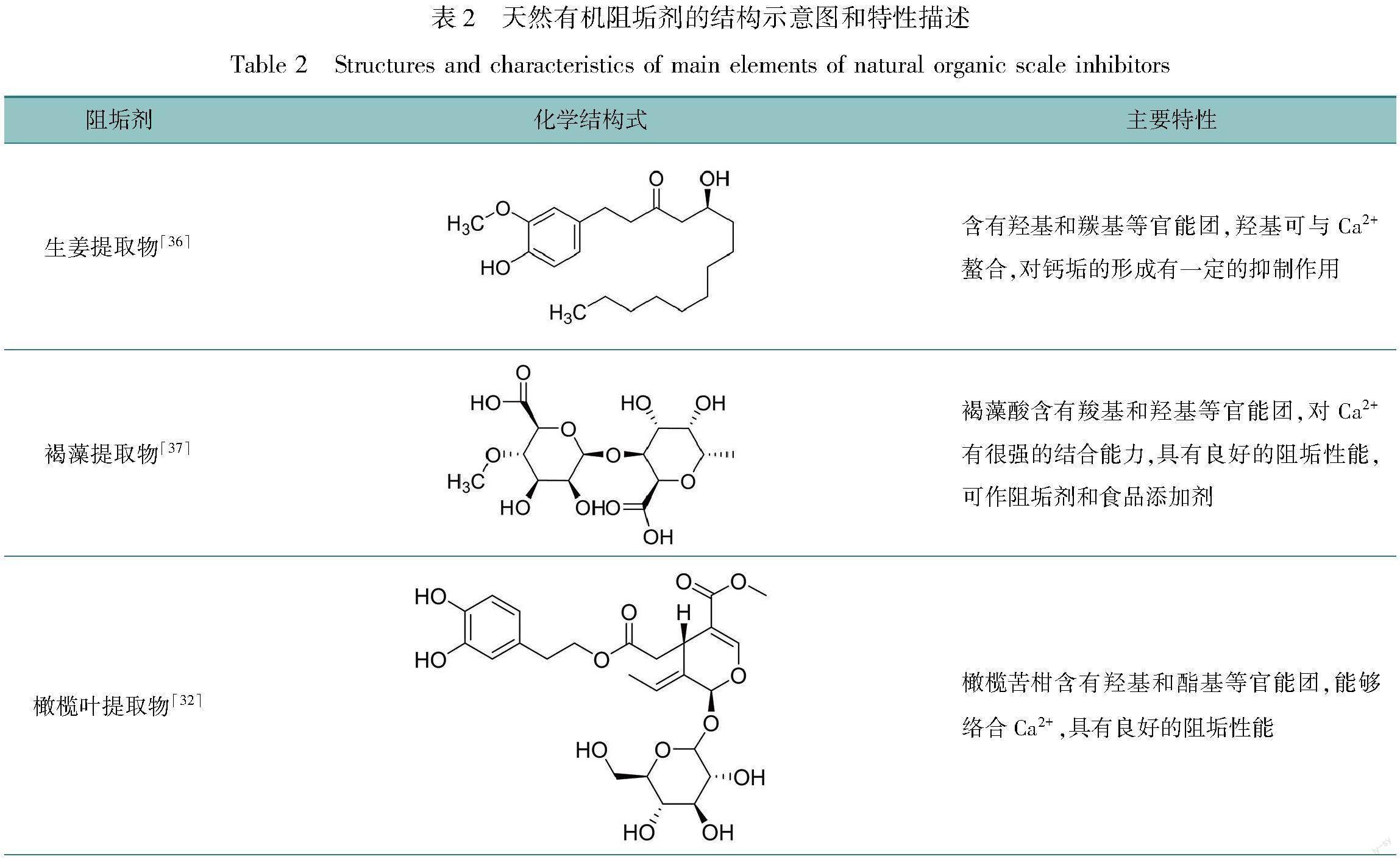
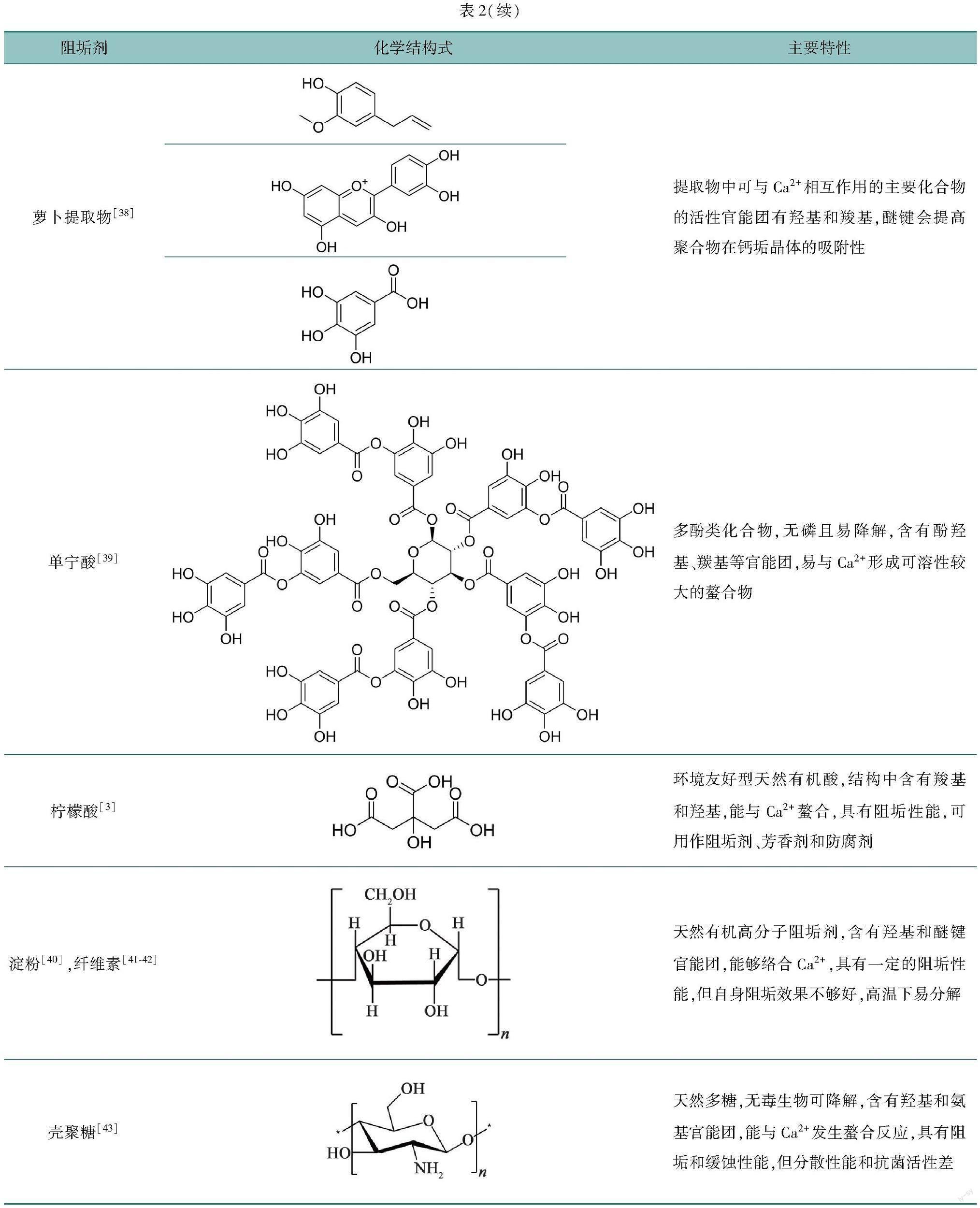
近年来,学者们已对部分生物提取物进行了阻垢性能的研究[25-27],植物提取物是天然有机阻垢剂的主要来源,物料丰富,提取工艺简单,且无毒,无生物积累,作为绿色阻垢剂具有非常可观的前景[28-30]。这些提取物的来源有杏仁叶[31]、橄榄叶[32]、槲皮素[33]、拳参[34]和甘文叶[35]等,其中含有类黄酮、有机酸等化合物,可以通过其结构中的某些特定基团与钙离子反应,从而起到阻垢的作用。表2列举了几种天然有机阻垢剂的结构和特性。
1.1 基于生物提取物的阻垢剂
羧甲基菊粉(CMI)是一种可从堆心菊根部分离出的多糖,具有无毒、可再生和生物可降解等特性[44-45]。Zhang等[46]利用分子动力学(MD)模拟CMI与CaCO3晶体相互作用,结果表明CMI能够有效地吸附在CaCO3表面,抑制CaCO3生长。分析发现CMI的氢原子和方解石表面的氧原子形成大量氢键,有助于CMI吸附在CaCO3晶体表面。此外,CMI的羧基氧原子与方解石表面钙原子的距离为2.4~2.6 ,与Ca—O键长2.39 十分相近,表明羧基和Ca2+之间形成了较强的离子键,抑制CaCO3晶体的形成。另外,Kirboga等[47-48]发现CMI在方解石晶体生长过程中可以诱导其形成不同的晶型。Boels等[49]将CMI与亚甲基膦酸(NTMP)和羟基乙叉二膦酸(HEDP)进行比较,CMI也能够表现出较好的阻垢能力。
Hamdona等[36]利用生姜提取物制作阻垢剂,并进行膜矿物结垢实验,发现在60 ℃和pH约为6.5时,100 mg/L质量浓度的生姜提取物对CaSO4的阻垢率为98.80%。生姜富含多种化学成分,包括酚类化合物、多糖和有机酸等。由此认为羟基能与Ca2+结合,对CaSO4的形成起到抑制作用。此外,生姜提取物中还含有羧基,与Ca2+有良好的螯合能力,这也可促进其阻垢能力。需要注意的是,该提取物在弱酸和低温时阻垢效果较好,但可能不适用于实际循环水系统中高温和弱碱的水体环境。
Khamis等[37]制备了一种褐藻提取物绿色阻垢剂,通过计时电流法、电化学阻抗谱技术和美国腐蚀工程师协会(national association of corrosion engineers,NACE)测试评估其阻垢性能。结果表明,褐藻提取液在质量浓度为15 mg/L和200 mg/L时,对CaSO4和CaCO3阻垢效率分别为100%和80%。褐藻提取物富含褐藻酸和水溶性1,3;1,6-3-D-葡聚糖[50-51],结构中的羟基和羧基对钙离子具有很强的结合能力[52],可以破坏CaSO4和CaCO3的晶体结构,使它们变得蓬松,从而不易吸附在设备表面,使提取物具有良好的阻垢效果。但是,与HEDP相比,达到相同阻垢效果时,所需投加量远大于HEDP。
Vasyliev等[38]利用乙醇浸渍法制备了萝卜提取物溶液(RCE),分别用计时电流法和热结垢技术测试了RCE对CaCO3的阻垢性能。RCE的体积分数为10 mL/L时,阻垢率接近100%,即使在
100 ℃的高温下,也能有78.7%的阻垢效率,表现出良好的耐温性。RCE中含有酚类衍生物,结构中有大量的羟基和羧基,與Ca2+形成水溶性络合物,阻止CaCO3的沉积。此外,端羧基花青素具有更大的表面积,可以促进RCE与更多的钙离子螯合,提高了其阻垢效果。同时,RCE还具有良好的缓蚀性能。
近年来,除了采用植物提取物作为阻垢剂外,一些学者也对动植物蛋白和微生物提取物进行了研究。Mady等[27]利用动态法研究了动植物蛋白(如肉蛋白、大豆、小麦和乳清等)的阻垢性能。结果发现,牛奶蛋白对CaCO3和CaSO4的阻垢效果最好,还发现通过蛋白胨与马来酸酐的开环反应引入羧基后,阻垢性能得到了显著地提高。这可能由于增加羧基的影响,或者是未反应水解的马来酸酐和蛋白质之间的协同作用。此外,需要注意的是,动植物蛋白的使用是否会容易滋养细菌和藻类,导致水体污染,这将需要进一步研究。
随着微生物在化学领域的日益普及,微生物提取物在阻垢方面的研究也越来越受到重视,蜡样芽孢杆菌就是典型代表之一。Li等[26]利用蜡样芽孢杆菌分泌的可溶性胞外聚合物(s-EPS)制作阻垢剂,并探究了其阻垢性能和机理。在70 ℃,pH为8时,质量浓度为80 mg/L的s-EPS对CaCO3阻垢效率为87.60%。这可能是因为s-EPS由多糖、蛋白质和腐殖酸类物质组成,富含羧基、羟基、氨基和酰胺官能团,对Ca2+有优异的螯合能力[53]。此外,s-EPS可以通过范德瓦耳斯或静电相互作用紧密吸附在CaCO3晶体表面生长位点上[54],导致CaCO3的生物矿化,从而抑制CaCO3晶体的生长。最近,Gao等[55]利用s-EPS和沸石咪唑骨架-8(ZIF-8)[JP]合成了一种新型亲水性阻垢剂,即ZIF-8@s-EPS。实验结果表明,在温度为20 ℃条件下,质量浓度仅为20 mg/L的ZIF-8@s-EPS对CaCO3的阻垢率就能达到98.63%。ZIF具有高比表面积、耐高温性和化学稳定性,这将有助于保护DNA、蛋白质、酶等生物大分子免受失活或变性[56],也有助于ZIF-8@s-EPS对CaCO3生物矿化[57]。此外,研究表明,ZIF-8@s-EPS还表现出防污和抗菌的多功能性能,这将为解决实际循环水系统中含有污垢和微生物问题提供新的思路。
1.2 基于改性的天然有机阻垢剂
天然提取物阻垢剂所需投加量大、杂质含量高、在高温环境下易分解,且阻垢效果并不十分理想[58-59]。此外,大部分天然高分子物质,如淀粉、纤维素和壳聚糖等属于多糖物质,它们本身通常表现出较差的阻垢效果[60]。因此,需要对天然有机物质进行改性研究,进而提高阻垢性能[61-63]。
1.2.1 柠檬酸
柠檬酸(CA)是一种天然有机酸,价格低廉,存在于各种水果和蔬菜中,常在食品工业中被用作芳香剂或饮料防腐剂[64]。此外,它也是一种环境友好型化合物,由于其结构中存在羧基,能与Ca2+螯合[65],被用作阻垢剂[66-67]。
Yuan等[68]利用CA分别与天冬氨酸、谷氨酸和甘氨酸合成了
姜黃-柠檬酸-天冬氨酸聚合物(PCCA)、姜黄-柠檬酸-谷氨酸聚合物(PCCG)和姜黄-柠檬酸-甘氨酸聚合物(PCCD),并通过静态阻垢实验研究了它们阻垢性能, PCCA的阻垢性能最好,这可能是由于氨基酸种类对聚合物的阻垢性能起到了重要作用,因为酸性越强越容易解离出—COO-,越有利于阻垢。此外,质量浓度仅为4 mg/L的PCCA对CaSO4的最大抑制率为99.7%,在20 mg/L的用量下,对CaCO3的抑制率可达98.8%。PCCA良好的阻垢效果跟羧基与Ca2+的强亲和力[69]和酰胺基在晶体表面强吸附力有关,能有效抑制钙垢的生长。另外,PCCA不仅投加量少,还适用于高温、高硬度、高SO2-4浓度的水环境中,并能长时间抑制CaSO4垢的形成。
Zhang等[70]研制了10-甲基吖啶碘化铵(MAI)和柠檬酸钠(SC)组成的环境友好型缓蚀阻垢剂(MAI-SC),[JP]并通过电化学和静态阻垢实验研究了其阻垢性能。结果表明,在MAI与SC的最佳配比下,缓蚀率高达92.7%(mMAI:mSC=1[JX-*4]:[JX*4]2),CaCO3的阻垢率为98.3% (mMAI:mSC=1[JX-*4]:[JX*4]3)。这可能是由于MAI-SC中羧基的增加,加强了与Ca2+的螯合能力,附着并干扰钙垢晶体的生长,破坏晶体结构,起到良好的阻垢作用。此外,量子化学计算表明,MAI-SC主要对方解石的(104)和(110)面以及球霰石的(002)和(020)面起抑制作用。
Zhao等[3]通过缩聚反应成功地制备了聚柠檬酸(PCA),实验结果表明,PCA用量为25 mg/L时,阻垢率可达98.8%。这可能是由于PCA分子吸附在生长的CaSO4晶面的活性位点上,使CaSO4晶格发生扭曲,抑制其生长,从而达到良好的阻垢效果。相同条件下,与PASP、PESA和HEDP相比,PCA的阻垢效果最好。此外,PCA中可水解为柠檬酸,且易生物降解[71]。然而,在85 ℃时,阻垢效果明显下降,表明PCA耐温性不好,不适用于高温环境中。
1.2.2 淀粉
众所周知,淀粉是一种天然高分子阻垢剂[63],来源十分广泛,但其水溶性低,阻垢性能不大理想,所以众多学者对淀粉进行了氧化或接枝等改性[49,72]。氧化淀粉(OS)是一种氧化改性淀粉[60],将淀粉上的部分羟甲基氧化成羧基[73],从而增强了其阻垢分散性能。
Chen等[74]采用分子动力学(molecular dynamics,MD)模拟和量子化学计算对OS的阻垢性能和机理进行分析,结果发现,OS主要通过羧基与Ca2+结合形成络合物,并可以吸附在晶体表面的活性位点上,使钙垢发生晶格畸变,从而抑制钙垢的生长。径向分布函数(RDF)分析结果表明,OS和晶体表面之间能够形成离子键,使晶体表面变形,对钙垢产生抑制效应。但是OS的用量太大,成本也会增加,因此,也需要对OS改性或与其他材料结合。
Yu等[54]以聚琥珀酰亚胺(PSI)和OS为原料合成了一种新型高效二元阻垢剂,即PASP/OS,并研究了其阻垢性能。在50 ℃,PASP/OS质量浓度为8 mg/L时,对CaCO3的抑制率能够达到100%,即使在100 ℃时,阻垢率也能够达到83.57%,表现出良好的耐高温性。PASP/OS的阻垢机理包括三个方面:PASP/OS的羧基和羟基可以与Ca2+螯合形成可溶性螯合物,防止钙垢形成;通过静电作用吸附悬浮在溶液中的新形成的垢颗粒表面,使垢颗粒表面具有相同的负电荷相互排斥,达到分散的作用;PASP/OS的氮原子和氧原子上的孤对电子可以吸附在钙垢晶体的活性位点上,从而破坏原有的晶体结构[63,75-76]。此外,他们还研究了加热时间、pH和Ca2+浓度对PASP/OS阻垢效果的影响,结果表明,与PASP相比,PASP/OS在高碱、高硬度等条件下,仍具有较好的抑制效果,且表现出良好的可持续性阻垢效果。
1.2.3 纤维素
纤维素是世界上最丰富的天然有机物,占植物含碳量的50%以上。纤维素及其衍生物广泛应用于食品、医药、缓蚀阻垢剂等领域[77-79]。羧甲基纤维素(CMC)是其最简单的线性链结构衍生物之一,由于引入了羧基,也表现出良好的阻垢性能[61-62,80]。
随着研究的深入,很多学者通过对CMC进行接枝等化学改性来提高其阻垢性能[55,81-85]。Yu等[85]通过羧甲基纤维素(CMC)与丙烯酸(AA)的接枝共聚,合成了一种高效的纤维素阻垢剂——羧甲基纤维素接枝聚丙烯酸(CMC-g-PAA),并通过反渗透和静态试验系统地研究了其阻垢性能。在接枝率相近的情况下,由于含有更多的活性抑制位点,平均接枝链数越高,CMC-g-PAA的阻垢性能越好。然而,在接枝率相同的情况下,接枝链较长的CMC-g-PAA会增强架桥絮凝效果[85-87],反而削弱了分散效果,导致其阻垢性能变差。当CMC与AA的质量比为1:0.3,引发剂质量为0.5 g时,得到的CMC-g-PAA接枝率为59%,阻垢性能最好。在pH为8.0、温度为70 ℃和Ca2+浓度为75 mmol/L条件下,6 mg/L的CMC-g-PAA的阻垢率能够达到95.5%。与CMC相比,一方面, CMC-g-PAA的两个相邻的接枝链对Ca2+的螯合作用具有协同作用,这增强了CMC-g-PAA的稳定性[49,62,63]。另一方面,含氮量较高的CMC-g-PAA中存在较多的端基,使CMC-g-PAA的活性更高,增强了与钙垢的相互作用,从而表现出更好的阻垢性能。但需要注意的是,聚丙烯酸难以降解,可能使CMC-g-PAA的生物降解性变差,这将有待进一步研究[88-89]。
Zhao等[90]利用动态阻垢试验和MD模拟研究了温度对羧甲基纤维素钠(SCMC)阻垢性能的影响,结果表明,温度在293~343 K内,当SCMC加入量为20 mg/L时,CaCO3的污垢热阻降低,阻垢效率会随着温度的升高而增加,343 K时的最高阻垢率为99.8%。这可能是由于随着温度的升高,SCMC与方解石面的结合能以及SCMC与Ca2+结合的几率均增大,从而能有效地阻止CaCO3垢在传热表面的生长。RDF结果表明,SCMC与方解石面绝大部分是通过氢键和化学键结合,且主要以羧基中的氧原子和晶面钙原子之间形成的离子键为主,这种强相互作用会使方解石晶格畸变,阻碍方解石晶体的正常生长。此外,SCMC在方解石(104)[JP]面上的MD吸附构型以及结合能与实验结果相一致,这些发现有利于指导高效水处理阻垢剂的开发。
1.2.4 其他改性天然有机阻垢剂
除了对上述天然有机聚合物阻垢剂研究外,还有学者对单宁酸、壳聚糖等进行了改性研究,并考察了它们的阻垢性能。单宁和壳聚糖具有许多活性基团,如羟基,可以很容易地通过在其主干上引入各种官能团进行化学修饰,如酯化、醚化和接枝共聚,以克服溶解性差的问题[91-95]。
Zhang等[96]采用电导法和静态试验对羧甲基季铵盐低聚壳聚糖(CM-QAOC)的阻垢性能进行评价,在Ca2+质量浓度为240 mg/L 和pH为8.0条件下,投加量为50 mg/L的CM-QAOC的阻垢效率可以超过98%,但与含磷阻垢剂相比,阻垢效果偏差。Zeng等[97]以聚琥珀酰亚胺和壳聚糖为原料,合成了聚天冬氨酸/壳聚糖接枝共聚物(PASP/CS),静态阻垢实验结果表明,当阻垢剂质量浓度为8 mg/L时,PASP/CS对碳酸钙垢的抑制效率为92%, 然而PASP 仅有68%。此外,相比PASP,PASP/CS的耐温性、耐盐性和耐碱性明显得到提高,且对Ca2(PO4)3也表现出优异的阻垢效果。但是,PASP/CS的合成过程中耗能巨大,会增加生产成本且不利于生产应用。Maher等[43]利用胍基对壳聚糖改性并成功合成了壳聚糖双胍盐酸盐(CG),实验结果表明,CG质量浓度为10~15 mg/L时,对CaCO3和CaSO4的阻垢性能最好。胍基中含有胺基结构,羟基和氨基协同作用不但有助于改善壳聚糖的溶解性和抗菌活性,还能提高CG在钙垢晶体表面的吸附能力,增强壳聚糖的阻垢能力。CG的阻垢机理为两种:一是羟基与Ca2+螯合,增加了Ca2+的溶解度,延缓钙垢结晶;二是CG吸附在钙垢晶体表面,占据生长活性位点,引起晶格畸变,抑制钙垢的形成。
Cui等[39]基于自由基聚合原理,以衣康酸、2-丙烯酰胺-2-甲基丙磺酸和单宁酸为单体,成功制备了一种共聚物阻垢剂。研究发现,当反应时间为3.5 h,反应温度为75 ℃,单宁酸加入量为4 g,引发剂用量为单体总质量的5%时,共聚物的阻垢性能最好。由于含有单宁酸,分子结构中相邻的羟基可与Ca2+形成溶解性较大的络合物,增大了Ca2+的溶解度,延缓CaCO3晶体的形成。共聚物中还含有羧基、磺酸基和酰胺基,这些官能团能够促进阻垢剂吸附在钙垢晶体表面生长活性位点上,阻止钙垢晶体正常生长,从而起到抑制钙垢形成的作用。此外,合成的共聚物阻垢剂即使在高温、高硬度和高碱性的水环境中也能表现出较好的阻垢效果,具有更大的应用前景。
2 人工合成的绿色聚合物阻垢剂
随着公众对环境保护的关注,环保型无磷绿色阻垢剂成为了众多学者关注的热点。聚天冬氨酸(PASP)和聚环氧琥珀酸(PESA)是典型的人工合成绿色聚合物阻垢剂[98-99],由于它们显示出良好的阻垢性能和生物降解性得到国内外广泛研究和应用[18,100]。表3列举了几种研究较多的绿色阻垢剂的结构和特性。
2.1 聚天冬氨酸类
PASP是一种环境友好型多功能聚合材料,因其无毒、无磷和良好的生物降解性而呈现出良好的发展前景[104-105]。同时,PASP分子中含有羧基,具有良好的螯合能力和分散性[18,106-107]。然而,PASP在高温环境下阻垢性能并不好[108],且当溶液中Ca2+浓度较高时,PASP的阻垢效果也不是很好[109],这些因素限制了PASP的应用范围。研究发现,通过在其侧链引入羟基、磺酸基和氨基等基团对PASP进行化学改性后,能够提高PASP的阻垢性能,以及在高温、高硬度环境下的稳定性[110-113]。
Zhang等[114]通過酪氨酸或色氨酸同时引入羧基和磺酸基团,与PASP接枝共聚制备了改性聚天冬氨酸阻垢剂(Tyr-SA-PASP和Trp-SA-PASP)。静态阻垢实验结果表明,当Tyr-SA-PASP和Trp-SA-PASP的投加质量浓度分别仅为4 mg/L和5 mg/L时,最大阻垢效率都能够达到98%,并且还能延长CaSO4结晶诱导时间。通过差分吸收光谱和密度泛函理论(DFT)分析,Ca2+通过与Tyr-SA-PASP和Trp-SA-PASP分子的配位作用,导致Tyr-SA-PASP和Trp-SA-PASP分子去质子化,这将破坏CaSO4的晶体结构,从而提高Tyr-SA-PASP和Trp-SA-PASP的阻垢性能。此外,磺酸基也具有配位和分散效果,在一定程度上也能够提高阻垢效果。与常用阻垢剂PASP、PAPEMP, JH-907相比,达到最大阻垢效率时,Trp-SA-PASP的投加量却少很多。
Zhang等[109]以PSI和尿素为原料,合成了聚天冬氨酸/尿素接枝共聚物(PASP/Urea)。结果表明,在溶液pH为9、温度为80 ℃条件下,当PASP/Urea质量浓度为10 mg/L时,对CaCO3的阻垢率为93%;质量浓度为4 mg/L时,对CaSO4的阻垢率提高到了97%,质量浓度为12 mg/L时,对Ca3(PO4)2的阻垢率高达100%,表明PASP/Urea能适用于含有多种钙盐离子的复杂水体环境。这可能是因为PASP/Urea结构中的大量羧基,是抑制钙垢形成的主要官能团,具有优异的螯合能力和分散性能[108,115]。此外,与PASP相比,PASP/Urea中同时含有羟基离子和酰胺基。不仅羟基能够与Ca2+结合,而且具有孤对电子的酰胺基中的氮原子也可以与Ca2+螯合,延缓水垢的形成,提高PASP/Urea的阻垢性能[23,116]。
Chen等[117]以聚天冬氨酸(PASP)和氧化石墨烯为原料合成了一种聚天冬氨酸/氧化石墨烯接枝共聚物(PASP/GO)阻垢剂。结果表明,同时引入了羧基和羟基后,在80 ℃,pH为8时,向250 mg/L Ca2+溶液中加入8 mg/L PASP/GO,对CaCO3的阻垢效率为100%;在70 ℃,pH为7时,10 mg/L PASP/GO加入到6 800 mg/L Ca2+溶液中,对CaSO4的阻垢效率也高达100%,且阻垢性能都要强于PASP,这可能是由于同浓度的PASP/GO含有的羧基和羟基的数量要多于PASP,而且在高温、高钙或高碱的环境中,PASP/GO的阻垢性能都非常好,但PASP/GO的制备工艺条件复杂,在实际生产过程中较困难。此外,MD模拟分析结果表明,PASP/GO的阻垢机理为螯合、晶格畸变和分散作用。值得注意的是,计算机模拟和实验相结合不仅能够保证实验的准确性,还能够节省大量的人力和财力,并且计算机模拟还可以对实验有一定的预测性和指导性。
2.2 聚环氧琥珀酸类
PESA是20世纪90年代初分别由美国宝洁公司和贝茨公司合成的一种阻垢缓蚀剂。由于其结构中含有羧基和醚基,PESA不但可以和Ca2+发生螯合反应,还能够表现出良好的分散和阻垢性能[118-119]。此外,PESA因其无氮、无磷和可生物降解的特点已被广泛应用[120-122]。Li等[123]通过MD模拟和DFT对PESA和PASP进行了阻垢性能和机理研究,结果发现,PESA的阻垢性能和分散性都要优于PASP。然而,PESA存在投加量大、Ca2+耐受性差和耐温性差等缺点[78,124],所以需要对PESA进行改性研究,弥补这些不足。
Zhang等[125]以马来酸酐和精氨酸为原料成功合成了一种新型绿色阻垢剂——精氨酸改性聚环氧琥珀酸(Arg-PESA),并利用静态阻垢测试和MD模拟对Arg-PESA阻垢性能和机理进行研究。结果表明,在80 ℃和60 ℃时,6 mg/L的Arg-PESA对250 mg/L Ca2+溶液中CaCO3的阻垢率都可达到100%,而此时PESA阻垢率分别只有60%和80%。因为经过左旋精氨酸接枝后,酰胺、氨基和羧基的存在增加了分子的电负性,更容易与Ca2+螯合。且Arg-PESA更易与吸附在活性位点上,引起晶格畸变,阻碍晶体的正常生长,达到更好的阻垢效果。此外,与PESA相比,即使在弱碱、Ca2+浓度较高的条件下,Arg-PESA也能够表现出良好的阻垢性能,且对Fe2O3有更好的分散性能。
Huang等[124]通过缩水甘油基和环氧琥珀酸酯的共聚反应合成了具有线性和超支化的聚环氧琥珀酸(HBP),并评价HBPs对碳酸钙的阻垢性能。结果表明,当Ca2+为125 mg/L时,HBPs质量浓度为15 mg/L对CaCO3阻垢率高达95.9%,远高于长链PESA(L-PESA)的阻垢率。此外,在高碱度、高硬度的条件下,HBPs的阻垢效果也都要优于L-PESA。与L-PESA相比,HBPs具有三维立体结构,可以提高与Ca2+的结合能力[109],而且在相同分子量下,HBPs醚键的数量要多于L-PESA,带有孤对电子的醚基有利于HBPs吸附在CaCO3晶體表面,因此,即使HBPs在较低的浓度也可以表现出优异的阻垢性能[126]。机理研究表明,HBPs不仅可以通过提高CaCO3的溶解度和抑制晶核的形成来延长诱导期,而且在晶体生长过程中,可以吸附在晶体表面活性位点上,从而对晶体生长造成干扰和抑制。此外,HBPs可生物降解,虽然生物降解性比L-PESA差,但也可以在天然水中降解。
Shi等[121]利用衣康酸(IA)和环氧琥珀酸(ESA)合成了一种聚衣康酸-环氧琥珀酸(PIA-co-ESA)新型阻垢剂。通过单因素试验确定,当单体配比(nIA: nESA)为4:1,引发剂用量为11%,反应温度为85 ℃,反应时间为4 h时,PIA-co-ESA表现出最优异的阻垢性能。静态试验结果表明,Ca2+为125 mg/L时,PIA-co-ESA为18 mg/L时对CaCO3阻垢效率高达100%。阻垢机理可能为PIA-co-ESA占据晶体表面活性位点,使晶体表面发生扭曲,导致晶格畸变,抑制晶体的生长。
Yan等[127]以ESA、IA和甲基丙烯磺酸钠(SMAS)为原料,合成了一种新型无磷无氮阻垢剂(ESA/IA/SMAS),并探究了其阻垢性能。在80 ℃和pH为9时,向600 mg/L的Ca2+溶液中加入20 mg/L的ESA/IA/SMAS,10 h对CaCO3阻垢率为100%。在70 ℃时,向5 000 mg/L的Ca2+溶液中加入10 mg/L的ESA/IA/SMAS,对CaSO4阻垢率为100%。这可能是由于羧基和磺酸基团的存在,ESA/IA/SMAS能够吸附在钙垢颗粒表面,使得钙垢颗粒表面电荷密度和颗粒间的排斥力增加,从而阻止了碳酸钙晶体的形成和生长。此外,羧基和磺酸基团能够与Ca2+相互作用,促使ESA/IA/SMAS占据晶体生长的活性中心,扰乱晶体的正常生长。值得注意的是,ESA/IA/SMAS具有良好的生物可降解性,在第21天时,生物可降解率达到近63.3%。
2.3 其他新型绿色阻垢剂
除了上述绿色阻垢剂外,部分学者在碳纳米技术方向的研究也为绿色阻垢剂研究提供了新的思路。
碳纳米材料具有高比表面积和良好的吸附能力。Wan等[128]利用碳纳米颗粒合成了一种新型纳米流体阻垢剂。实验结果表明,碳纳米颗粒可以抑制热交换装置表面CaCO3垢的形成,具有良好的阻垢效果,当其质量浓度为75 mg/L时,阻垢效率可达97.31%。同时,碳纳米颗粒的阻垢作用主要是改变CaCO3晶体形态,能将CaCO3晶体从方解石转变为文石,使其难以黏附在设备表面。
碳纳米管的强吸附能力可以使阻垢剂具有保留时间长和高吸附浓度等优点。Teng等[129]利用乙二胺四乙酸(EDTA)处理多壁碳纳米管(MWCNT)纳米流体得到一种MWCNT-EDTA阻垢剂,并评估其对碳酸钙结垢的抑制效果。研究发现,MWCNT-EDTA能够延长钙垢结晶时间,从而起到抑制钙垢的作用。这是由于EDTA中含有大量羧基,可以螯合Ca2+,减少其与CO2-3的有效碰撞,延长结晶诱导期。此外,MWCNT中还含有羟基,这也有助于提高MWCNT-EDTA的阻垢性能。
碳量子点(CQDs)具有高比表面积,含有羧基和羟基等亲水性基团。Hao等[130]采用柠檬酸热分解法合成了羧基碳量子点(CCQDs),通过0~80 ℃静态阻垢试验,在CCQDs加入量较低的情况下,对CaSO4阻垢率可达100%,然而,柠檬酸在同等条件的阻垢率仅有20%。此外,CCQDs对BaSO4也具有优异的阻垢性能,而且相比柠檬酸其耐温和耐碱性有了巨大的提高。同时,CCQDs具有物料丰富、低毒性、生物相容和环境友好等优点。
3 阻垢机理
3.1 钙垢的形成机理
在循环水系统中,大部分循环水来自于经过处理后的污水,其中含有Ca2+、Mg2+、CO2-3、SO2-4等离子,在重复使用过程中水分不断挥发,水中无机盐浓度持续升高,容易形成不溶性盐,从水溶液中析出,在设备表面沉积形成钙垢。钙垢的形成主要分三個过程:
(1)形成过饱和溶液。冷却水在循环过程中蒸发,Ca2+在水中不断浓缩积累,当钙盐的浓度达到其溶解度时,溶液将达到过饱和状态。此外,温度的升高会降低钙盐的溶解度,这将进一步促进溶液达到过饱和状态。
(2)晶核形成。当钙盐浓度超过其溶解度时,离子开始碰撞形成离子对并聚集成微核。少部分微核会成为成核中心,然后离子团开始以有序的方式排列,形成稳定的晶核。
(3)晶体生长。当晶核形成后,成垢离子不断向晶核聚集,晶核逐渐成长成微晶颗粒,这一过程是不可逆的[131],微晶颗粒由于布朗运动不断地碰撞聚集在一起,使得晶体不断生长,从而形成钙垢。
因此,扰乱其中一个或多个过程都能够有效地延缓或抑制钙垢的形成。目前使用的阻垢剂所具备的功能就是影响这些过程,抑制晶核或晶体的形成,扰乱晶体的生长,从而达到阻垢的效果。
3.2 常见的阻垢机理
阻垢剂的作用机理取决于其化学性质,无论是螯合剂还是阈值阻垢剂,它可以通过一个或多个机制发挥阻垢作用。一般而言,阻垢剂中通常含有丰富的官能团,通过干扰一个或多个结晶阶段来影响钙垢形成,例如膦酸基、羧基、羟基和磺酸基等,通过一种或多种抑制机理,如螯合、分散、晶格畸变和阈值效应[131-134],与钙垢相互作用达到抑制结垢的效果。
3.2.1 螯合增溶作用
在结晶诱导期,含有羧酸、膦酸或磺酸基团等官能团的阻垢剂,如EDTA、ATMP[24]和2-丙烯酰胺-2-甲基磺酸(AMPS)[24]等,在水溶液中能够发生电离形成含有负电荷的官能团或分子长链,它们会和溶液体系中的Ca2+、Mg2+等金属阳离子形成配位键,形成可溶性的螯合物,提高了Ca2+在水溶液中的溶解度以及降低了Ca2+与CO2-3、SO2-4等阴离子碰撞的几率,在一定程度上能够延缓和抑制钙垢的形成[63,121]。根据化学计量比,阻垢剂分子结合的Ca2+越多,Ca2+与阻垢剂负离子间的配位键越强,阻垢效果越好。但是,螯合剂只能在一定程度的过饱和溶液中防止结垢,如果平衡系统被破坏,则会开始沉淀。
3.2.2 凝聚分散作用
凝聚分散作用是指阻垢剂通过减少CaCO3微晶颗粒相互之间的碰撞凝聚,降低微晶成垢的形成速度,从而达到阻垢的效果。阴离子型聚合物阻垢剂能够在溶液中电离出带有电负性的基团,如羧酸类阻垢剂,含有电负性的基团能与微晶颗粒相互碰撞并吸附在微晶表面,使微晶表面带有大量的负电荷,由于同种电荷相互排斥,静电斥力作用能够阻止微晶颗粒之间的有效碰撞、生长和沉积,有效地将微晶颗粒分散在水体中,达到阻垢效果[3,135]。
3.2.3 晶格畸变作用
在晶体生长阶段,阻垢剂分子可以吸附在钙垢晶体表面,占据生长活性位点,使晶体表面发生扭曲,导致晶格畸变,从而抑制晶体生长[118]。当阻垢剂分子中含有羟基、羧基、氨基或磺酸基等[54,111,122]具有螯合作用的官能团时,这些官能团中的氧原子会与晶体表面的钙原子以化学键的方式结合,占据晶体表面活性位点,引起晶格畸变,阻碍晶体的正常生长[90]。此外,阻垢剂能够吸附并包裹在晶体表面,减少钙垢微晶之间的有效碰撞,导致晶体的有序性发生改变,使原有的晶体结构变得不稳定,钙垢变得疏松多孔,容易被水溶液冲走,从而起到抑制钙垢生成的作用。
4 总结与展望
随着国家环保法规的完善,水体排放标准日趋严格,传统化学阻垢剂因其会给环境带来二次污染,已经不能满足当前发展要求,研发新型、高效绿色阻垢剂将会成为未来发展趋势。近年来,植物提取物类阻垢剂因来源广泛、无毒不含磷、生物可降解性以及良好的阻垢性能具有巨大的发展前景。但也要注意到植物提取物目前存在不确定成分较多、提取工艺复杂、添加量大、适应性较差等主要问题,而且对其阻垢机理的研究也不够深入。PASP和PESA类人工合成的阻垢剂在进行改性后,即使在高温、高硬度和高碱环境下,也能够表现出不俗的阻垢性能,这将有助于提高它们在实际循环水系统的实用性。为了落实可持续发展理念,防止生态环境受到污染和破坏,预计今后新型、高效绿色阻垢剂的研究会朝以下几个方向发展:
(1)坚持“绿色”发展理念。在阻垢剂的研究过程中,不仅要求最终阻垢剂产品无毒无害,而且在原材料和生产过程中都应坚持绿色、环保发展理念。
(2)追求阻垢剂多功能化。单一阻垢剂已不能满足实际复杂水体的除垢要求,可以结合实际情况对单体进行接枝聚合、氧化或者酯化等化学改性,不仅能够提高其阻垢性能,还能使得聚合物具有分散、缓蚀或抗菌等功能。但需要注意的是,在引入其他官能团后,如磺酸基,可能会降低阻垢剂的生物可降解性。
(3)深入了解阻垢机理。目前对阻垢机理的研究主要基于传统的研究手段,包括紫外-可见光谱、扫描电子显微镜和X光衍射等,侧重于宏观尺度的表征,以推测微观机理,对阻垢机理研究不够深入,特别是植物提取物的阻垢机理还不够明确。可以结合分子动力学模拟、密度泛函理论计算和阻垢机理之间的关系,从原子间相互作用的微观角度去分析和设计分子结构,阐述结构与性质的关系。
(4)融合納米新技术。碳纳米材料,如碳量子点(CQDs),与阻垢剂一起使用能够提高阻垢剂的阻垢性能。碳纳米材料在阻垢剂中的应用具有广泛的前景,建议以碳纳米材料为载体,将更多种类的阻垢剂融入到碳纳米材料中,研究其阻垢性能和探究其阻垢机理,以提高其阻垢和分散性能,适应实际复杂的水体环境。
(5)贯彻产品经济化。在满足所需功能的反应单体之间,尽可能寻求低毒、易生物降解和经济的绿色单体。在阻垢剂选择方面,应满足原料易得,制备简单,价格低廉,易于运输和贮备,制备工艺简单,所需耗能小的要求。与缓蚀剂、杀菌剂并用时,阻垢效果应不明显下降,且不影响缓蚀和杀菌的效果。
参考文献:
[1]RAHMANI K, JADIDIAN R, HAGHTALAB S. Evaluation of inhibitors and biocides on thecorrosion, scaling and biofouling control of carbon steel and copper-nickel alloys in a power plant cooling water system[J]. Desalination, 2016, 393: 174-185. DOI: 10.1016/j.desal.2015.07.026.
[2]ZHU T Z, WANG L D, SUN W, et al. The role of corrosion inhibition in the mitigation of CaCO3 scaling on steel surface[J]. Corrosion Science, 2018, 140: 182-195. DOI: 10.1016/j.corsci.2018.06.003.
[3]ZHAO Y Z, JIA L L, LIU K Y, et al. Inhibition of calcium sulfate scale by poly (citric acid)[J]. Desalination, 2016, 392: 1-7.[JP] DOI: 10.1016/j.desal.2016.04.010.
[4]CAN H K, NER G. Water-soluble anhydride containing alternating copolymers as scale inhibitors[J]. Desalination, 2015, 355: 225-232. DOI: 10.1016/j.desal.2014.11.001.
[5]WANG C, SHEN T, LI S, et al. Investigation of influence of low phosphorous co-polymer antiscalant on calcium sulfate dihydrate crystal morphologies[J]. Desalination, 2014, 348: 89-93. DOI: 10.1016/j.desal.2014.06.017.
[6]MIGAHED M A, RASHWAN S M,KAMEL M M, et al. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants[J]. Journal of Molecular Liquids, 2016, 224: 849-858. DOI: 10.1016/j.molliq.2016.10.091.
[7]LIU G Q, ZHOU Y M, HUANG J Y, et al. Acrylic acid-allylpolyethoxy carboxylate copolymer as an effective inhibitor for calcium phosphate and iron(III) scales in cooling water systems[J]. CLEAN - Soil, Air, Water, 2015, 43(7): 989-994. DOI: 10.1002/clen.201100569.
[8]BUTT F H, RAHMAN F, BADURUTHAMAL U. Evaluation of SHMP and advanced scale inhibitors for control of CaSO4, SrSO4, and CaCO3 scales in RO desalination[J]. Desalination, 1997, 109(3): 323-332. DOI: 10.1016/s0011-9164(97)00078-7.
[9]ZUO Z, YANG W, ZHANG K,et al. Effect of scale inhibitors on the structure and morphology of CaCO3 crystal electrochemically deposited on TA1 alloy[J]. Journal of Colloid and Interface Science, 2020, 562: 558-566. DOI: 10.1016/j.jcis.2019.11.078.
[10]JI Y, CHEN Y, LE J X, et al. Highly effective scale inhibition performance of amino trimethylenephosphonic acid on calcium carbonate[J]. Desalination, 2017, 422: 165-173. DOI: 10.1016/j.desal.2017.08.027.
[11]LUPU C, ARVIDSON R S, LUTTGE A, et al. Phosphonate mediated surface reaction and reorganization: Implications for the mechanism controlling cement hydration inhibition[J]. Chemical Communications (Cambridge, England), 2005(18): 2354-2356. DOI: 10.1039/b500192g.
[12]LI X, GAO B, YUE Q, et al. Effect of six kinds of scale inhibitors on calcium carbonate precipitation in high salinity wastewater at high temperatures[J]. Journal of Environmental Sciences, 2015, 29: 124-130. DOI: 10.1016/j.jes.2014.09.027.
[13]SHEIKHI A, LI N, VAN DE VEN T G M, et al. Macromolecule-based platforms for developing tailor-made formulations for scale inhibition[J]. Environmental Science: Water Research & Technology, 2016, 2(1): 71-84. DOI: 10.1039/C5EW00158G.
[14]ZUO Y W, SUN Y, YANG W Z, et al. Performance and mechanism of 1-hydroxy ethylidene-1, 1-diphosphonic acid and 2-phosphonobutane-1, 2, 4-tricarboxylic acid in the inhibition of calcium carbonate scale[J]. Journal of Molecular Liquids, 2021, 334: 116093. DOI: 10.1016/j.molliq.2021.116093.
[15]GUO X R,QIU F X,DONG K,et al. Preparation and application of copolymer modified with the palygorskite as inhibitor for calcium carbonate scale[J]. Applied Clay Science, 2014, 99: 187-193. DOI: 10.1016/j.clay.2014.06.031.
[16]SHAKKTHIVEL P, VASUDEVAN T. Acrylic acid-diphenylamine sulphonic acid copolymer threshold inhibitor for sulphate and carbonate scales in cooling water systems[J]. Desalination, 2006, 197(1/2/3): 179-189. DOI: 10.1016/j.desal.2005.12.023.
[17]AMJAD Z, KOUTSOUKOS P G. Evaluation of maleic acid based polymers as scale inhibitors and dispersants for industrial water applications[J]. Desalination, 2014, 335(1): 55-63. DOI: 10.1016/j.desal.2013.12.012.
[18] YANG L, YANG W, XU B, et al. Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor[J]. Desalination, 2017, 416: 166-174. DOI: 10.1016/j.desal.2017.05.010.
[19]CHAUSSEMIER M, POURMOHTASHAM E, GELUS D, et al. State of art of natural inhibitors of calcium carbonate scaling: a review article[J]. Desalination, 2015, 356: 47-55. DOI: 10.1016/j.desal.2014.10.014.
[20]ZHANG H X, WANG F, JIN X H, et al. A botanical polysaccharide extracted from abandoned corn stalks: modification and evaluation of its scale inhibition and dispersion performance[J]. Desalination, 2013, 326: 55-61. DOI: 10.1016/j.desal.2013.07.015.
[21]LIU D,DONG W B,LI F T,et al. Comparative performance of polyepoxysuccinic acid and polyaspartic acid on scaling inhibition by static and rapid controlled precipitation methods[J]. Desalination, 2012, 304: 1-10. DOI: 10.1016/j.desal.2012.07.032.
[22]SHI W Y, DING C, YAN J L, et al. Molecular dynamics simulation for interaction of PESA and acrylic copolymers with calcite crystal surfaces[J]. Desalination, 2012, 291: 8-14. DOI: 10.1016/j.desal.2012.01.019.
[23]GUO X, QIU F, DONG K, et al. Preparation, characterization and scale performance of scale inhibitor copolymer modification with chitosan[J]. Journal of Industrial and Engineering Chemistry, 2012, 18(6): 2177-2183. DOI: 10.1016/j.jiec.2012.06.015.
[24]ZHANG B, ZHOU D, LV X, et al. Synthesis of polyaspartic acid/3-amino-1H-1, 2, 4-triazole-5-carboxylic acid hydrate graft copolymer and evaluation of its corrosion inhibition and scale inhibition performance[J]. Desalination, 2013, 327: 32-38. DOI: 10.1016/j.desal.2013.08.005.
[25]LOURTEAU T, BERRICHE H, KCILI K, et al. Scale inhibition effect of Hylocereus undatus solution on calcium carbonate formation[J]. Journal of Crystal Growth, 2019, 524: 125161. DOI: 10.1016/j.jcrysgro.2019.125161.
[26]LI S L, QU Q, LI L, et al. Bacillus cereus s-EPS as a dual bio-functional corrosion and scale inhibitor in artificial seawater[J]. Water Research, 2019, 166: 115094. DOI: 10.1016/j.watres.2019.115094.
[27]MADY M F, KELLAND M A. Study on various readily available proteins as new green scale inhibitors for oil field scale control[J]. Energy & Fuels, 2017, 31(6): 5940-5947. DOI: 10.1021/acs.energyfuels.7b00508.
[28]CASTILLO L A, TORIN E V, GARCIA J A, et al. New product for inhibition of calcium carbonate scale in natural gas and oil facilities based on Aloe vera: Application in venezuelan oilfields[C]//All Days. Cartagena de Indias, Colombia: SPE, 2009. DOI: 10.2118/123007-ms.
[29]ABDEL-GABER A M, ABD-EL-NABEY B A, KHAMIS E, et al. A natural extract as scale and corrosion inhibitor for steel surface in brine solution[J]. Desalination, 2011, 278(1/2/3): 337-342. DOI: 10.1016/j.desal.2011.05.048.
[30]ABD-EL-KHALEK D E, ABD-EL-NABEY B A, ABDEL-KAWI M A, et al. Investigation of a novel environmentally friendly inhibitor for calcium carbonate scaling in cooling water[J]. Desalination and Water Treatment, 2016, 57(7): 2870-2876. DOI: 10.1080/19443994.2014.987174.
[31]KHALED R H, ABDEL-GABER A M,RAHAL H T, et al. A potential green anti-scaling and corrosion inhibitor for mild steel in brine solution[J]. International Journal of Electrochemical Science, 2020, 15(7): 6790-6801. DOI: 10.20964/2020.07.54.
[32]AIDOUD R, KAHOUL A, NAAMOUNE F. Inhibition of calcium carbonate deposition on stainless steel using olive leaf extract as a green inhibitor[J]. Environmental Technology, 2017, 38(1): 14-22. DOI: 10.1080/09593330.2016.1183716.
[33]GHIZELLAOUI S, BOUMAGOURA M, RHOUATI S, et al. Inhibition of CaCO3 growth in hard water by quercetin as green inhibitor[J]. Water and Environment Journal, 2020, 34(S1): 263-272. DOI: 10.1111/wej.12524.
[34]MOHAMMADI Z, RAHSEPAR M. The use of green Bistorta Officinalis extract for effective inhibition of corrosion and scale formation problems in cooling water system[J]. Journal of Alloys and Compounds, 2019, 770: 669-678. DOI: 10.1016/j.jallcom.2018.08.198.
[35]A S, M. Inhibition of calcium oxalate crystallization by graft copolymers[J]. Crystal Growth & Design, 2009, 9(5): 2159-2167. DOI: 10.1021/cg800802z.
[36]HAMDONA S K, EL-AASSAR A H M, AHMED A E M M, et al. Enhancing anti-scaling resistances of aromatic polyamide reverse osmosis membranes using a new natural materials inhibitor[J]. Chemical Engineering and Processing-Process Intensification, 2021, 164: 108404. DOI:10.1016/j.cep.2021.108404.
[37]KHAMIS E, ABD-EL-KHALEK D E, ABDEL-KAWI M A, et al. New application of brown sea algae as an alternative to phosphorous-containing antiscalant[J]. Environmental Technology, 2022, 43(4): 595-604. DOI: 10.1080/09593330.2020.1797898.
[38]VASYLIEV G, VOROBYOVA V, ZHUK T. Raphanus sativus L. extract as a scale and corrosion inhibitor for mild steel in tap water[J]. Journal of Chemistry, 2020, 2020: 1-9. DOI: 10.1155/2020/5089758.
[39]CUI C C, ZHANG S G. Preparation, characterization and performance evaluation of a novel scale inhibiting and dispersing copolymer containing natural tannin[J]. Journal of Polymers and the Environment, 2020, 28(7): 1869-1879. DOI: 10.1007/s10924-020-01730-x.
[40]CHEN Y, CHEN X S, LIANG Y N, et al. Synthesis of polyaspartic acid-oxidized starch copolymer and evaluation of its inhibition performance and dispersion capacity[J]. Journal of Dispersion Science and Technology, 2021, 42(13): 1926-1935. DOI: 10.1080/01932691.2020.1791172.
[41]XU Z, ZHAO Y, WANG J, et al. Inhibition of calcium carbonate fouling on heat transfer surface using sodium carboxymethyl cellulose[J]. Applied Thermal Engineering, 2019, 148: 1074-1080.
[42]SHAHINI M H, RAMEZANZADEH B, MOHAMMADLOO H E. Recent advances in biopolymers/carbohydrate polymers as effective corrosion inhibitive macro-molecules: a review study from experimental and theoretical views[J]. Journal of Molecular Liquids, 2021, 325: 115110. DOI: 10.1016/j.molliq.2020.115110.
[43]MAHER Y A, ALI M E A, SALAMA H E, et al. Preparation, characterization and evaluation of chitosan biguanidine hydrochloride as a novel antiscalant during membrane desalination process[J]. Arabian Journal of Chemistry, 2020, 13(1): 2964-2981. DOI: 10.1016/j.arabjc.2018.08.006.
[44]LIU J, WILLFR S, XU C L. A review of bioactive plant polysaccharides: biological activities, functionalization, and biomedical applications[J]. Bioactive Carbohydrates and Dietary Fibre, 2015, 5(1): 31-61. DOI: 10.1016/j.bcdf.2014.12.001.
[45]PRO D, HUGUET S, ARKOUN M, et al. From algal polysaccharides to cyclodextrins to stabilize a urease inhibitor[J]. Carbohydrate Polymers, 2014, 112: 145-151. DOI: 10.1016/j.carbpol.2014.05.075.
[46]ZHANG H P, LUO X G, LIN X Y, et al. Biodegradable carboxymethyl inulin as a scale inhibitor for calcite crystal growth: Molecular level understanding[J]. Desalination, 2016, 381: 1-7. DOI: 10.1016/j.desal.2015.11.029.
[47]KIRBOGA S, NER M. Investigation of calcium carbonate precipitation in the presence of carboxymethyl inulin[J]. CrystEngComm, 2013, 15(18): 3678-3686. DOI: 10.1039/C3CE27022J.
[48]KIRBOGA S, NER M. The inhibitory effects of carboxymethyl inulin on the seeded growth of calcium carbonate[J]. Colloids and Surfaces B: Biointerfaces, 2012, 91: 18-25. DOI: 10.1016/j.colsurfb.2011.10.031.
[49]BOELS L, WITKAMP G. Carboxymethyl inulin biopolymers: a green alternative for phosphonate calcium carbonate growth inhibitors[J]. Crystal Growth & Design, 2011, 11(9): 4155-4165. DOI: 10.1021/CG2007183.
[50]SHEVCHENKO N M, ANASTYUK S D, GERASIMENKO N I, et al. Polysaccharide and lipid composition of the brown seaweed Laminaria gurjanovae[J]. Russian Journal of Bioorganic Chemistry, 2007, 33(1): 88-98. DOI: 10.1134/S1068162007010116.
[51]OBLUCHINSKAYA E D. Comparative chemical composition of the Barents Sea brown algae[J]. Applied Biochemistry and Microbiology, 2008, 44(3): 305-309. DOI: 10.1134/S0003683808030149.
[52]ITUEN E, AKARANTA O, JAMES A. Evaluation of performance of corrosion inhibitors using adsorption isotherm models: an overview[J]. Chemical Science International Journal, 2017, 18(1): 1-34. DOI: 10.9734/csji/2017/28976.
[53]HE C S, DING R R, CHEN J Q, et al. Interactions between nanoscale zero valent iron and extracellular polymeric substances of anaerobic sludge[J]. Water Research, 2020, 178: 115817. DOI: 10.1016/j.watres.2020.115817.
[54]YU W, WANG Y W, LI A M, et al. Evaluation of the structural morphology of starch-graft-poly(acrylic acid) on its scale-inhibition efficiency[J]. Water Research, 2018, 141: 86-95. DOI: 10.1016/j.watres.2018.04.021.
[55]GAO R X, LI Y, ZHU T T, et al. ZIF-8@s-EPS as a novel hydrophilic multifunctional biomaterial for efficient scale inhibition, antibacterial and antifouling in water treatment[J]. The Science of the Total Environment, 2021, 773: 145706. DOI: 10.1016/j.scitotenv.2021.145706.
[56]CHEN G S, HUANG S M, KOU X X, et al. A convenient and versatile amino-acid-boosted biomimetic strategy for the nondestructive encapsulation of biomacromolecules within metal-organic frameworks[J]. Angewandte Chemie International Edition, 2019, 58(5): 1463-1467. DOI: 10.1002/anie.201813060.
[57]DOONAN C, RICC R, LIANG K, et al. Metal-organic frameworks at the biointerface: synthetic strategies and applications[J]. Accounts of Chemical Research, 2017, 50(6): 1423-1432. DOI: 10.1021/acs.accounts.7b00090.
[58]OUYANG X P, QIU X Q, LOU H M, et al. Corrosion and scale inhibition properties of sodium lignosulfonate and its potential application in recirculating cooling water system[J]. Industrial & Engineering Chemistry Research, 2006, 45(16): 5716-5721. DOI: 10.1021/ie0513189.
[59]張惠欣, 葛丽环, 周宏勇, 等. 羧烷基-季铵两性壳聚糖的制备及其阻垢杀菌性能[J]. 化工进展, 2011, 30(9): 2055-2059. DOI: 10.16085/j.issn.1000-6613.2011.09.016.
[60]GUO X R, QIU F X, DONG K, et al. Scale inhibitor copolymer modified with oxidized starch: synthesis and performance on scale inhibition[J]. Polymer-Plastics Technology and Engineering, 2013, 52(3): 261-267. DOI: 10.1080/03602559.2012.747206.
[61]GONCHARUK V V, KAVITSKAYA A A, SKILSKAYA M D. Sodium carboxymethyl cellulose as an inhibitor of scale formation in nanofiltration of hard artesian waters[J]. Desalination and Water Treatment, 2012, 47(1/2/3): 235-242. DOI: 10.1080/19443994.2012.696408.
[62]YU W, SONG D, LI A, et al. Control of gypsum-dominated scaling in reverse osmosis system using carboxymethyl cellulose[J]. Journal of Membrane Science, 2019, 577: 20-30. DOI: 10.1016/j.memsci.2019.01.053.
[63]WANG Y, LI A, YANG H. Effects of substitution degree and molecular weight of carboxymethyl starch on its scale inhibition[J]. Desalination, 2017, 408: 60-69. DOI: 10.1016/j.desal.2017.01.006.
[64]PRISCIANDARO M, MAZZIOTTI DI CELSO G, LANCIA A, et al. Citric acid as a green additive to retard calcium carbonate scales on process equipment[J]. The Canadian Journal of Chemical Engineering, 2020, 98(9): 1973-1979. DOI: 10.1002/cjce.23783.
[65]SINN C G, DIMOVA R, ANTONIETTI M. Isothermal titration calorimetry of the polyelectrolyte/water interaction and binding of Ca2+: effects determining the quality of polymeric scale inhibitors[J]. Macromolecules, 2004, 37(9): 3444-3450. DOI: 10.1021/ma030550s.
[66]WADA N, YAMASHITA K, UMEGAKI T. Effects of carboxylic acids on calcite formation in the presence of Mg2+ ions[J]. Journal of Colloid and Interface Science, 1999, 212(2): 357-364. DOI: 10.1006/jcis.1998.6067.
[67]GHIZELLAOUI S, SEMINERAS H. Inhibition of scale formation by electrochemical means in the presence of a green inhibitor: citric acid[J]. Journal of Materials and Environmental Science, 2017, 8(6): 2105-2111.
[68]YUAN X J, DONG S Y, ZHENG Q, et al. Novel and efficient curcumin based fluorescent polymer for scale and corrosion inhibition[J]. Chemical Engineering Journal, 2020, 389: 124296. DOI: 10.1016/j.cej.2020.124296.
[69]AL-SABAGH A M, EL BASIONY N M, SADEEK S A,
et al. Scale and corrosion inhibition performance of the newly synthesized anionic surfactant in desalination plants: experimental, and theoretical investigations[J]. Desalination, 2018, 437: 45-58. DOI: 10.1016/j.desal.2018.01.036.
[70]ZHANG W W, LI H J, CHEN L W, et al. Performance and mechanism of a composite scaling-corrosion inhibitor used in seawater: 10-Methylacridinium iodide and sodium citrate[J]. Desalination, 2020, 486: 114482. DOI: 10.1016/j.desal.2020.114482.
[71]TSUTSUMI N, OYA M, SAKAI W. Biodegradable network polyesters from gluconolactone and citric acid[J]. Macromolecules, 2004, 37(16): 5971-5976. DOI: 10.1021/ma049607g.
[72]DU Q, WANG Y, LI A, et al Scale-inhibition and flocculation dual-functionality of poly(acrylic acid) grafted starch[J]. Journal of Environmental Management, 2018, 210: 273-279. DOI: 10.1016/j.jenvman.2018.01.016.
[73]YU Y, WANG Y N, DING W, et al. Preparation of highly-oxidized starch using hydrogen peroxide and its application as a novel ligand for zirconium tanning of leather[J]. Carbohydrate Polymers, 2017, 174: 823-829. DOI: 10.1016/j.carbpol.2017.06.114.
[74]CHEN X S, CHEN Y, CUI J J, et al. Molecular dynamics simulation and DFT calculation of “green” scale and corrosion inhibitor[J]. Computational Materials Science, 2021, 188: 110229. DOI: 10.1016/j.commatsci.2020.110229.
[75]BUTLER M F, GLASER N, WEAVER A C, et al. Calcium carbonate crystallization in the presence of biopolymers[J]. Crystal Growth & Design, 2006, 6(3): 781-794. DOI: 10.1021/cg050436w.
[76]DIETZSCH M, BARZ M, SCHLER T, et al. PAA-PAMPS copolymers as an efficient tool to control CaCO3 scale formation[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2013, 29(9): 3080-3088. DOI: 10.1021/la4000044.
[77]KAZI S N, DUFFY G G, CHEN X D. Fouling mitigation of heat exchangers with natural fibres[J]. Applied Thermal Engineering, 2013, 50(1): 1142-1148. DOI: 10.1016/j.applthermaleng.2012.08.042.
[78]WANG C, ZHU D Y, WANG X K. Low-phosphorus maleic acid and sodium ρ-styrenesulfonate copolymer as calcium carbonate scale inhibitor[J]. Journal of Applied Polymer Science, 2010, 115(4): 2149-2155. DOI: 10.1002/app.31300.
[79]LOPEZ C G, ROGERS S E, COLBY R H, et al. Structure of sodium carboxymethyl cellulose aqueous solutions: A SANS and rheology study[J]. Journal of Polymer Science Part B, Polymer Physics, 2015, 53(7): 492-501. DOI: 10.1002/polb.23657.
[80]JEDVERT K, HEINZE T. Cellulose modification and shaping: a review[J]. Journal of Polymer Engineering, 2017, 37(9): 845-860. DOI: 10.1515/polyeng-2016-0272.
[81]LI W Z, HUANG S Y, XU D J, et al. Molecular dynamics simulations of the characteristics of sodium carboxymethyl cellulose with different degrees of substitution in a salt solution[J]. Cellulose, 2017, 24(9): 3619-3633. DOI: 10.1007/s10570-017-1364-0.
[82]SHUI T, FENG S H, CHEN G, et al. Synthesis of sodium carboxymethyl cellulose using bleached crude cellulose fractionated from cornstalk[J]. Biomass and Bioenergy, 2017, 105: 51-58. DOI: 10.1016/j.biombioe.2017.06.016.
[83]TENG K H, KAZI S N,
AMIRI A, et al. Calcium carbonate fouling on double-pipe heat exchanger with different heat exchanging surfaces[J]. Powder Technology, 2017, 315: 216-226. DOI: 10.1016/j.powtec.2017.03.057.
[84]XU Z M, ZHAO Y, HE J J, et al. Fouling characterization of calcium carbonate on heat transfer surfaces with sodium carboxymethyl cellulose as an inhibitor[J]. International Journal of Thermal Sciences, 2021, 162: 106790. DOI: 10.1016/j.ijthermalsci.2020.106790.
[85]YU W, CHEN W, YANG H. Evaluation of structural effects on the antiscaling performance of various graft cellulose-based antiscalants in RO membrane scaling control[J]. Journal of Membrane Science, 2021, 620: 118893. DOI: 10.1016/j.memsci.2020.118893.
[86]QIANG X, SHENG Z, ZHANG H. Study on scale inhibition performances and interaction mechanism of modified collagen[J]. Desalination, 2013, 309: 237-242. DOI: 10.1016/j.desal.2012.10.025.
[87]HU P, XI Z, LI Y, et al. Evaluation of the structural factors for the flocculation performance of a co-graft cationic starch-based flocculant[J]. Chemosphere, 2020, 240: 124866. DOI: 10.1016/j.chemosphere.2019.124866.
[88]MISHRA S, SAXENA P, DEORE D A. Studies on antiscaling effect of polyacrylic acid on boiler[J]. Polymer-Plastics Technology and Engineering, 2005, 44(8/9): 1389-1398. DOI: 10.1080/03602550500209754.
[89]YANG Q F, GU A Z, DING J, et al. Effects of PAA and PBTCA on CaCO3 scaling in pool boiling system[J]. Chinese Journal of Chemical Engineering, 2002, 10(2): 190-197.
[90]ZHAO Y, XU Z M, WANG B B, et al. Scale inhibition performance of sodium carboxymethyl cellulose on heat transfer surface at various temperatures: Experiments and molecular dynamics simulation[J]. International Journal of Heat and Mass Transfer, 2019, 141: 457-463. DOI: 10.1016/j.ijheatmasstransfer.2019.06.091.
[91]BOLTO B, GREGORY J. Organic polyelectrolytes in water treatment[J]. Water Research, 2007, 41(11): 2301-2324. DOI: 10.1016/j.watres.2007.03.012.
[92]RINAUDO M. Chitin and chitosan: Properties and applications[J]. ChemInform, 2007, 38(27): 603-632. DOI: 10.1002/chin.200727270.
[93]ZHU F. Composition, structure, physicochemical properties, and modifications of cassava starch[J]. Carbohydrate Polymers, 2015, 122: 456-480. DOI: 10.1016/j.carbpol.2014.10.063.
[94]YANG R, LI H J, HUANG M, et al. A review on chitosan-based flocculants and their applications in water treatment[J]. Water Research, 2016, 95: 59-89. DOI: 10.1016/j.watres.2016.02.068.
[95]SHAK K P Y, WU T Y. Synthesis and characterization of a plant-based seed gum via etherification for effective treatment of high-strength agro-industrial wastewater[J]. Chemical Engineering Journal, 2017, 307: 928-938. DOI: 10.1016/j.cej.2016.08.045.
[96]ZHANG H X, CAI Z Y, JIN X H, et al. Preparation of modified oligochitosan and evaluation of its scale inhibition and fluorescence properties[J]. Journal of Applied Polymer Science, 2015, 132(37): 42518. DOI: 10.1002/app.42518.
[97]ZENG D F, CHEN T S, ZHOU S J. Synthesis of polyaspartic acid/chitosan graft copolymer and evaluation of its scale inhibition and corrosion inhibition performance[J]. International Journal of Electrochemical Science, 2015, 10(11): 9513-9527. DOI: 10.1016/S1452-3981(23)11197-7.
[98]ZOU Z Y, BERTINETTI L, POLITI Y, et al. Control of polymorph selection in amorphous calcium carbonate crystallization by poly(aspartic acid): two different mechanisms[J]. Small (Weinheim an Der Bergstrasse, Germany), 2017, 13(21): 10.1002/smll.201603100. DOI: 10.1002/smll.201603100.
[99]PRAMANIK B K, GAO Y H, FAN L H, et al. Antiscaling effect of polyaspartic acid and its derivative for RO membranes used for saline wastewater and brackish water desalination[J]. Desalination, 2017, 404: 224-229. DOI: 10.1016/j.desal.2016.11.019.[JP][ZK)]
[100][ZK(#]HASSON D, SHEMER H, SHER A. State of the art of friendly “green” scale control inhibitors: a review article[J]. Industrial & Engineering Chemistry Research, 2011, 50(12): 7601-7607. DOI: 10.1021/ie200370v.
[101]CHHIM N, HADDAD E, NEVEUX T, et al. Performance of green antiscalants and their mixtures in controlled calcium carbonate precipitation conditions reproducing industrial cooling circuits[J]. Water Research, 2020, 186: 116334. DOI: 10.1016/j.watres.2020.116334.
[102]MITHIL KUMAR N, GUPTA S K, JAGADEESH D, et al. Development of poly(aspartic acid-co-malic acid) composites for calcium carbonate and sulphate scale inhibition[J]. Environmental Technology, 2015, 36(10): 1281-1290. DOI: 10.1080/09593330.2014.984773.
[103]MARTINOD A, NEVILLE A, EUVRAD M, et al, et al. Electrodeposition of a calcareous layer: effects of green inhibitors[J].Chemical Engineering Science, 2009, 64(10):2413-2421.DOI:10.1016/j.ces.2009.01.024.
[104]QUAN Z H, CHEN Y C, WANG X R, et al. Experimental study on scale inhibition performance of a green scale inhibitor polyaspartic acid[J]. Science in China Series B: Chemistry, 2008, 51(7): 695-699. DOI: 10.1007/s11426-008-0063-y.
[105]吴新世, 孙波, 王菁, 等. 一种新型高聚物生物可降解性评价[J]. 南开大学学报(自然科学版), 2009, 42(4): 13-17.
[106]TOUIR R, CENOUI M, BAKRI M E, et al. Sodium gluconate as corrosion and scale inhibitor of ordinary steel in simulated cooling water[J]. Corrosion Science, 2008, 50(6): 1530-1537. DOI: 10.1016/j.corsci.2008.02.011.
[107]LING L, ZHOU Y M, HUANG J Y, et al. Carboxylate-terminated double-hydrophilic block copolymer as an effective and environmental inhibitor in cooling water systems[J]. Desalination, 2012, 304: 33-40. DOI: 10.1016/j.desal.2012.07.014.
[108]CHEN J X, XU L H, HAN J, et al. Synthesis of modified polyaspartic acid and evaluation of its scale inhibition and dispersion capacity[J]. Desalination, 2015, 358: 42-48. DOI: 10.1016/j.desal.2014.11.010.
[109]ZHANG Y, YIN H Q, ZHANG Q S, et al. Synthesis and characterization of novel polyaspartic acid/urea graft copolymer with acylamino group and its scale inhibition performance[J]. Desalination, 2016, 395: 92-98. DOI: 10.1016/j.desal.2016.05.020.
[110]余吉良, 王志坤, 霍然, 等. 弱堿环境中碳酸钙垢阻垢剂的阻垢性能与阻垢机理[J]. 油田化学, 2017, 34(4): 699-704. DOI: 10.19346/j.cnki.1000-4092.2017.04.026.
[111]ZHAO L N, ZHOU Y M, YAO Q Z, et al. Calcium scale inhibition of stimulated oilfield produced water using polyaspartic acid/aminomethanesulfonic acid[J]. ChemistrySelect, 2021, 6(15): 3692-3701. DOI: 10.1002/slct.202100853.
[112]CHEN J X, CHEN F J, HAN J, et al. Evaluation of scale and corrosion inhibition of modified polyaspartic acid[J]. Chemical Engineering & Technology, 2020, 43(6): 1048-1058. DOI: 10.1002/ceat.201900518.
[113]GUO X Y, ZHAO X W, XU Y H, et al. The synthesis of polyaspartic acid derivative PASP-Im and investigation of its scale inhibition performance and mechanism in industrial circulating water[J]. RSC Advances, 2020, 10(55): 33595-33601. DOI: 10.1039/d0ra06592g.
[114]ZHANG S P, QU H J, YANG Z, et al. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate[J]. Desalination, 2017, 419: 152-159. DOI: 10.1016/j.desal.2017.06.016.
[115]ZHANG B R, HE C J, WANG C, et al. Synergistic corrosion inhibition of environment-friendly inhibitors on the corrosion of carbon steel in soft water[J]. Corrosion Science, 2015, 94: 6-20. DOI: 10.1016/j.corsci.2014.11.035.
[116]XU Y, ZHAO L L, WANG L N, et al. Synthesis of polyaspartic acid-melamine grafted copolymer and evaluation of its scale inhibition performance and dispersion capacity for ferric oxide[J]. Desalination, 2012, 286: 285-289. DOI: 10.1016/j.desal.2011.11.036.
[117]CHEN Y, CHEN X S, LIANG Y N. Synthesis of polyaspartic acid/graphene oxide grafted copolymer and evaluation of scale inhibition and dispersion performance[J]. Diamond and Related Materials, 2020, 108: 107949. DOI: 10.1016/j.diamond.2020.107949.
[118]LI C, ZHANG C, ZHANG W. The inhibition effect mechanisms of four scale inhibitors on the formation and crystal growth of CaCO3 in solution[J]. Scientific Reports, 2019, 9(1): 13366.DOI:10.1038/s41598-019-50012-7.
[119]ZHOU X H, SUN Y H, WANG Y Z. Inhibition and dispersion of polyepoxysuccinate as a scale inhibitor[J]. Journal of Environmental Sciences, 2011, 23: S159-S161. DOI: 10.1016/S1001-0742(11)61102-9.
[120]LIU C, ZHENG Y F, YANG S Y, et al. Exploration of a novel depressant polyepoxysuccinic acid for the flotation separation of pentlandite from lizardite slimes[J]. Applied Clay Science, 2021, 202: 105939. DOI: 10.1016/j.clay.2020.105939.
[121]SHI W Y, XU W, CANG H, et al. Design and synthesis of biodegradable antiscalant based on MD simulation of antiscale mechanism: a case of itaconic acid-epoxysuccinate copolymer[J]. Computational Materials Science, 2017, 136: 118-125. DOI: 10.1016/j.commatsci.2017.04.035.
[122]ZUO Y W, YANG W Z, ZHANG K G, et al. Experimental and theoretical studies of carboxylic polymers with low molecular weight as inhibitors for calcium carbonate scale[J]. Crystals, 2020, 10(5): 406. DOI: 10.3390/cryst10050406.
[123]LI C J, ZHANG C Y, ZHANG W P. The inhibitory effects of four inhibitors on the solution adsorption of CaCO3 on Fe3O4 and Fe2O3 surfaces[J]. Scientific Reports, 2019, 9: 13724. DOI: 10.1038/s41598-019-50127-x.
[124]HUANG H H, YAO Q, JIAO Q, et al. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism[J]. Journal of Saudi Chemical Society, 2019, 23(1): 61-74. DOI: 10.1016/j.jscs.2018.04.003.
[125]ZHANG K F, CHEN F J, HAN J, et al. Evaluation of arginine-modified polyepoxysuccinic acid as anti-scaling and anti-corrosion agent[J]. Chemical Engineering & Technology, 2021, 44(6): 1131-1140. DOI: 10.1002/ceat.202000576.
[126]HUANG H H, YAO Q, LIU B L, et al. Synthesis and characterization of scale and corrosion inhibitors with hyper-branched structure and the mechanism[J]. New Journal of Chemistry, 2017, 41(20): 12205-12217. DOI: 10.1039/C7NJ02201H.
[127]YAN M F, TAN Q Q, LIU Z, et al. Synthesis and application of a phosphorous-free and non-nitrogen polymer as an environmentally friendly scale inhibition and dispersion agent in simulated cooling water systems[J]. ACS Omega, 2020, 5(25): 15487-15494. DOI: 10.1021/acsomega.0c01620.
[128]WAN C, WANG L T, SHA J Y, et al. Effect of carbon nanoparticles on the crystallization of calcium carbonate in aqueous solution[J]. Nanomaterials (Basel, Switzerland), 2019, 9(2): 179. DOI: 10.3390/nano9020179.
[129]TENG K H, AMIRI A, KAZI S N, et al. Fouling mitigation on heat exchanger surfaces by EDTA-treated MWCNT-based water nanofluids[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 60: 445-452. DOI: 10.1016/j.jtice.2015.11.006.
[130]HAO J, LI L Y, ZHAO W W, et al. Synthesis and application of CCQDs as a novel type of environmentally friendly scale inhibitor[J]. ACS Applied Materials & Interfaces, 2019, 11(9): 9277-9282. DOI: 10.1021/acsami.8b19015.
[131]ALROOMI Y M, HUSSAIN K F. Potential kinetic model for scaling and scale inhibition mechanism[J]. Desalination, 2016, 393: 186-195. DOI: 10.1016/j.desal.2015.07.025.
[132]AMJAD Z. Mineral scales in biological and industrial systems[J]. Crc Press, 2013, 10.1201/b1: 77-102.
[133]RAHMAN F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination[J]. Desalination, 2013, 319: 79-84. DOI: 10.1016/j.desal.2013.03.027.
[134]DOBBERSCHTZ S, NIELSEN M R, SAND K K, et al. The mechanisms of crystal growth inhibition by organic and inorganic inhibitors[J]. Nature Communications, 2018, 9: 1578. DOI: 10.1038/s41467-018-04022-0.
[135]ZHENG Z, YU Z P, YANG M D, et al. Substituent group variations directing the molecular packing, electronic structure, and aggregation-induced emission property of isophorone derivatives[J]. The Journal of Organic Chemistry, 2013, 78(7): 3222-3234. DOI: 10.1021/jo400116j.

