含1,3,4-噁二唑片段的胡椒碱类衍生物合成及杀螨活性
李绍晨, 王燕燕, 方珊珊, 王 振, 徐 晖, 吕 敏
(西北农林科技大学 植物保护学院,陕西 杨凌 712100)
朱砂叶螨Tetranychus cinnabarinusBoisduval广泛分布于我国农作物种植区,是一种危害严重的植食性害螨,其具有生长周期短、繁殖能力强、易产生抗药性等特点,是最难防治的有害生物之一[1-2]。目前,化学杀螨剂仍然是防治害螨发生的主要手段,但随着人们食品安全及环境保护意识的提高,部分高毒杀螨剂 (如三氯杀螨醇) 已被禁止使用,并且长时间使用一种杀螨剂,极易使害螨产生严重的抗药性。螺螨酯和螺甲螨酯是拜耳公司研发的两种杀螨剂。封云涛等用螺螨酯筛选山楂叶螨T.viennensis21 代,发现螺螨酯防治山楂叶螨存在抗性风险,抗螺螨酯的山楂叶螨品系会产生交互抗性[3];螺甲螨酯对二斑叶螨T.urticae和加州新小绥螨Neoseiulus californicus生长速率影响的试验表明,螺甲螨酯对捕食螨无害,但对叶螨有剧毒,可在10 d 内实现种群抑制[4]。因此,研发高效、低毒、环境友好的新型杀螨剂已成为农业发展亟待解决的问题。植物源天然产物因具有环境友好、作用方式独特且不易产生抗药性等特点,已经成为新农药研发的重要来源。
胡椒碱 (1,piperine,图式1) 是从胡椒科植物中分离提取的一种桂皮酰胺类生物碱,具有广泛的生物活性[5]。如在医学领域,胡椒碱具有抗癌、消炎、抗癫痫等生物活性[6-7];在农业领域,胡椒碱对多种农业害虫具有较好的生物活性[8-12]。因此,胡椒碱常作为先导化合物用于药物研发。此外,噁二唑是一种含有氮原子和氧原子的五元杂环,可通过多种非共价键与生物系统中的多种酶和受体结合,因此含有噁二唑杂环的衍生物一般具有多种生物活性[13]。例如,2-酰肼-1,3,4-噁二唑类化合物 (2,图式1) 对多种病原菌具有抑制作用[14];Yang 等将1,3,4-噁二唑引入诺卡酮,得到一系列含噁二唑杂环的诺卡酮类化合物 (3,图式1),其中部分化合物对小菜蛾3 龄幼虫显示出较好的杀虫活性[15]。目前,商品化的含有噁二唑结构的农药有杀虫剂噁虫酮(metoxadiazone)、除草剂噁草酮(oxadiazon)和杀线虫剂tioxazafen 等。基于此,本研究以胡椒碱为先导化合物,通过1,4-二溴丁烷桥接2-巯基-5-苯基-1,3,4-噁二唑杂环活性片段,合成一系列含1,3,4-噁二唑杂环的胡椒碱类衍生物,并评价其对朱砂叶螨T.cinnabarinus的触杀活性。
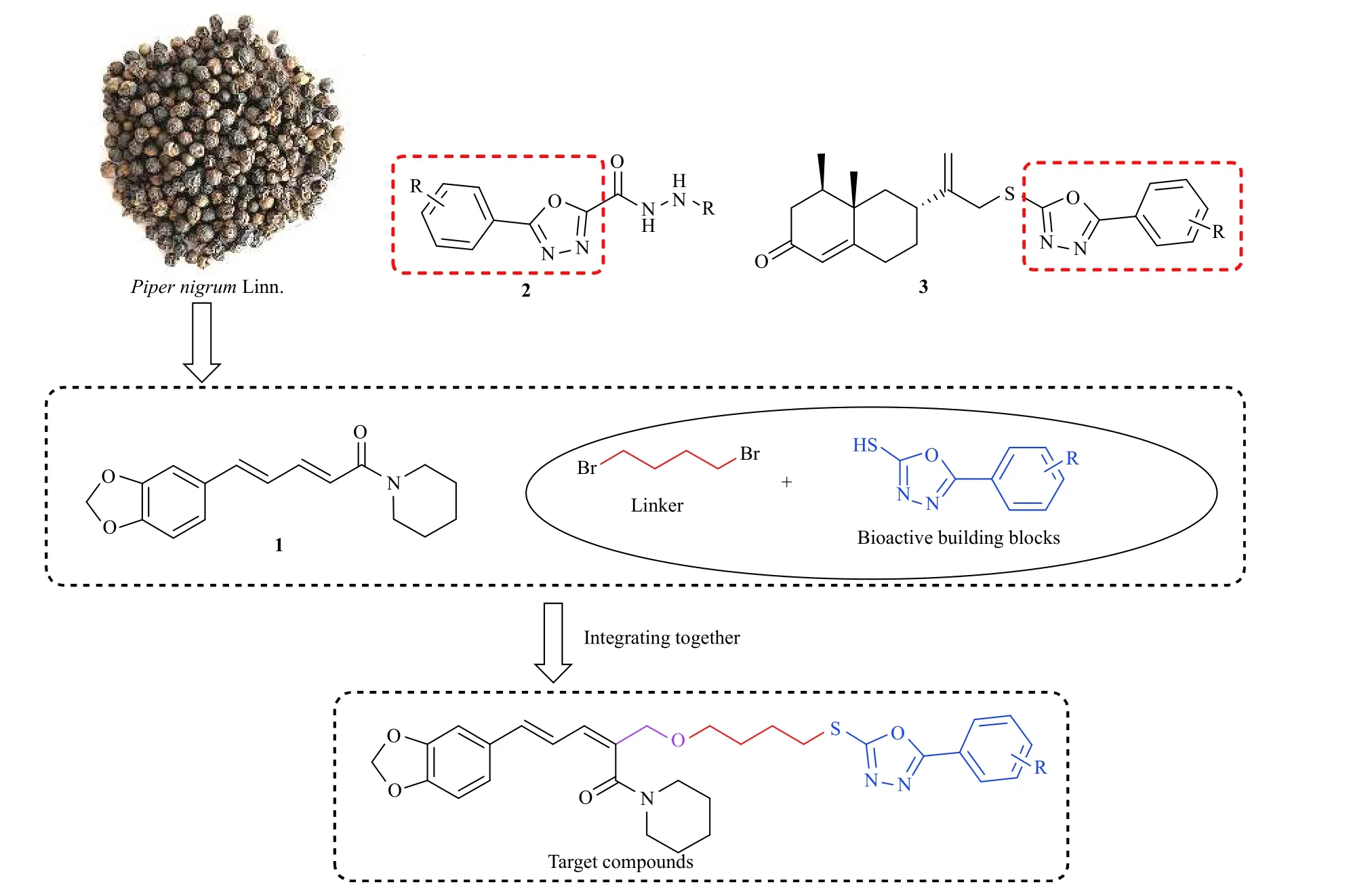
图式1 目标化合物的设计思路Scheme 1 Design strategy of target compounds
目标化合物的设计思路见图式1,合成路线见图式2。
1 材料与方法
1.1 仪器与试剂
Bruker Advance Ш 400/500MHz 核磁共振仪,德国Bruker 公司;Bruker TENSOR27 红外光谱仪,德国Bruker 公司;ZF-6 型三用紫外线分析仪,上海嘉鹏科技有限公司;RE-52 旋转蒸发器,上海嘉鹏科技有限公司;ALC-210.4 电子分析天平,上海精科天平仪器公司;DHG-9240A 型电热恒温鼓风干燥箱,上海精宏实验设备有限公司;国华88-1 大功率磁力搅拌器,常州国华电器有限公司;ZSA302 体视显微镜,重庆光电仪器有限公司;XT-415 型双目显微熔点测定仪,北京泰克仪器有限公司;Bruker SMART APEX II 晶体衍射仪,德国Bruker 公司。
200~300 目柱层析硅胶 (粒径54~74 μm) 和GF254薄层层析硅胶,青岛海洋化工厂;核磁共振光谱测试所用溶剂为氘代氯仿 (CDCl3),以四甲基硅烷 (TMS) 为内标物;所用试剂和溶剂均为分析纯。
对照药剂:98.32%螺螨酯 (spirodiclofen) 原药,购于陕西美邦农药有限公司。
1.2 化合物的合成
1.2.1 中间体4 的合成 参考文献方法[16]进行。取DMF (5 mmol) 于100 mL 圆底烧瓶中并置于0 ℃冰浴中搅拌,逐滴加入三氯氧磷 (5 mmol) 后继续搅拌30 min。将胡椒碱 (1 mmol) 溶于DMF(5 mL) 中,缓慢逐滴加到上述反应液中。滴加完毕后,将反应瓶置于油浴中继续搅拌,并逐渐升温至90 ℃。待薄层层析(TLC)监测 (V(石油醚,PE)/V(乙酸乙酯,EA) = 2 : 1) 反应完成后,将反应瓶重新置于冰浴中,用20%氢氧化钠水溶液调节pH 值至8~9,继续搅拌30 min。过滤,滤饼放置烘箱中干燥,然后用硅胶柱层析 (V(PE)/V(EA) =2 : 1) 分离提纯,得到化合物4。黄色固体,产率55%,熔点 (m.p.) 184~186 ℃; IR (KBr),ν/cm-1:3052, 2937, 2858, 2733, 1678, 1607, 1445, 1256,1188, 812, 707;1H NMR (500 MHz, CDCl3)δ: 9.49(s, 1H), 7.12 (d,J= 11.5 Hz, 1H), 6.97~7.03 (m, 3H),6.80~6.89 (m, 2H), 6.01 (s, 2H), 3.72~3.75 (m, 2H),3.24~3.26 (m, 2H), 1.64~1.69 (m, 4H), 1.50~1.57 (m,2H);13C NMR (125 MHz, CDCl3)δ: 189.74, 163.92,149.64, 149.47, 148.50, 144.61, 137.51, 129.96,124.45, 121.57, 108.71, 106.41, 101.69, 48.01, 42.59,26.66, 25.83, 24.52.HRMS [ESI]: C18H19NNaO4([M + Na]+), 理论值:336.1212;测定值:336.1194.
1.2.2 中间体5 的合成 参考文献方法[17]进行。将中间体4 (2 mmol) 溶于甲醇 (5 mL) 中,冰浴条件下搅拌,温度降至0 ℃后,缓慢加入NaBH4(3 mmol) 继续搅拌,TLC (V(二氯甲烷,DCM)/V(EA) =4 : 1) 跟踪监测至反应完成;加入清水 (15 mL) 淬灭反应,乙酸乙酯 (30 mL × 3) 萃取,合并有机相,干燥,减压浓缩,硅胶柱层析 (V(DCM)/V(EA) =4 : 1) 分离,得到中间体5。淡黄色固体,产率89%, m.p.118~120 ℃; IR (KBr),ν/cm-1: 3393,2937, 2856, 1608, 1476, 1440, 1247, 1102, 1030, 806;1H NMR (500 MHz, CDCl3)δ: 6.88 (d,J= 1.0 Hz,1H), 6.78 (dd,J= 1.5, 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.49~6.56 (m, 2H), 6.30~6.37 (m, 1H), 5.96(s, 2H), 4.30 (s, 2H), 3.68~3.70 (m, 2H), 3.43~3.45(m, 2H), 1.63~1.66 (m, 4H), 1.50~1.53 (m, 2H);13C NMR (100 MHz, CDCl3)δ: 168.81, 148.14, 147.74,136.30, 135.20, 131.23, 127.98, 122.29, 121.92,108.46, 105.55, 101.23, 64.43, 47.92, 42.45, 26.73,25.86, 24.50; HRMS [ESI]: C18H21NNaO4([M +Na]+), 理论值:338.1368;测定值:338.1353.
1.2.3 中间体6 的合成 参考文献方法[18]进行。将1,4-二溴丁烷 (3 mmol) 和NaH (3 mmol) 溶于DMF (5 mL) 中,冰浴条件下搅拌。再将中间体5(1.5 mmol) 分批缓慢加入到反应中,继续搅拌至TLC (V(PE)/V(EA) = 1 : 1) 监测反应完全。然后加入乙酸乙酯 (40 mL) 稀释反应体系,用0.1 mol/L稀盐酸 (20 mL × 3) 洗涤,有机相用无水硫酸钠干燥,减压浓缩,硅胶柱层析 (V(PE)/V(EA) = 1:1)分离,得到中间体6,淡黄色固体,产率74%,m.p.64~66 ℃; IR (KBr),ν/cm-1: 3016, 2933, 2856,1622, 1487, 1442, 1251, 1090, 1027, 609;1H NMR(500 MHz, CDCl3)δ: 6.89 (s, 1H), 6.81 (dd,J= 1.5, 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H),6.50~6.57 (m, 2H), 6.24~6.31 (m, 1H), 5.96 (s, 2H),4.17 (s, 2H), 3.69~3.71 (m, 2H), 3.49 (t, J = 6.0 Hz,2H), 3.42~3.45 (m, 4H), 1.91~1.98 (m, 2H),1.70~1.75 (m, 2H), 1.63~1.66 (m, 4H), 1.50~1.52 (m,2H);13C NMR (125 MHz, CDCl3)δ: 168.21, 148.17,147.80, 135.27, 134.20, 131.23, 128.83, 122.39,121.95, 108.48, 105.55, 101.26, 72.90, 70.01, 47.83,42.40, 33.80, 29.73, 28.32, 26.64, 25.92, 24.58;HRMS [ESI]: C22H28BrNNaO4([M + Na]+), 理论值:472.1099;测定值:472.1100.
1.2.4 目标化合物8a~8z 的合成 参考文献方法[19]进行,将中间体6 (0.2 mmol),Cs2CO3(0.3 mmol) 和化合物7a~7z (0.3 mmol) (制备按照参考文献[15]) 溶于乙腈 (5 mL) 中,置于40 ℃油浴中搅拌。TLC (V(PE)/V(EA) = 1 : 1 或V(DCM)/V(丙酮,AT) = 6 : 1) 跟踪监测,反应完全后,加入清水 (15 mL),乙酸乙酯 (30 mL × 3) 萃取,合并有机相,并用无水硫酸钠干燥,减压浓缩,制备薄层层析 (V(PE)/V(EA) = 1 : 1 或V(DCM)/V(AT) =6:1) 分离,得到目标化合物8a~8z,产率38%~77%。
(2Z,4E)-2-((5-苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8a):产率59%,淡黄色液体; IR (KBr),ν/cm-1:3417, 2934, 2858, 1713, 1623, 1477, 1444, 1247, 1105, 1034,699;1H NMR (500 MHz, CDCl3)δ: 7.99 (dd,J= 2.0, 8.5 Hz,2H), 7.46~7.53 (m, 3H), 6.89 (d,J= 1.0 Hz, 1H), 6.80 (dd,J=1.5, 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.49~6.56 (m, 2H),6.25~6.32 (m, 1H), 5.95 (s, 2H), 4.18 (s, 2H), 3.68~3.70 (m,2H), 3.52 (t,J= 6.5 Hz, 2H), 3.42 (t,J= 5.5 Hz, 2H), 3.32 (t,J= 7.5 Hz, 2H), 1.92~1.97 (m, 2H), 1.74~1.79 (m, 2H),1.62~1.65 (m, 4H), 1.48~1.51 (m, 2H); HRMS [ESI]:C30H34N3O5S ([M + H]+), 理论值:548.2219;测定值:548.2209.
(2Z,4E)-2-((5-(2-氟)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8b):产率64%,黄色液体; IR (KBr),ν/cm-1: 3417, 2933, 2857, 1741, 1623, 1477, 1357, 1246, 1036,768;1H NMR (500 MHz, CDCl3)δ: 7.98 (t,J= 8.0 Hz, 1H),7.49~7.53 (m, 1H), 7.27~7.29 (m, 1H), 7.21~7.25 (m, 1H),6.89 (s, 1H), 6.80 (dd,J= 1.5, 8.0 Hz, 1H), 6.73 (d,J= 8.0 Hz,1H), 6.49~6.57 (m, 2H), 6.25~6.32 (m, 1H), 5.95 (s, 2H), 4.19(s, 2H), 3.69-3.71 (m, 2H), 3.52 (t,J= 6.0 Hz, 2H), 3.43 (t,J=5.0 Hz, 2H), 3.32 (t,J= 7.5 Hz, 2H), 1.92~1.98 (m, 2H),1.74~1.79 (m, 2H), 1.63~1.65 (m, 4H), 1.49~1.52 (m, 2H);HRMS [ESI]: C30H32FN3NaO5S ([M + Na]+), 理论值:588.1944;测定值:588.1929.
(2Z,4E)-2-((5-(3-氟)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8c):产率58%,黄色液体; IR (KBr),ν/cm-1: 3449, 2934, 2858, 1739, 1624, 1475, 1456, 1220, 1106,1037, 867, 796;1H NMR (500 MHz, CDCl3)δ: 7.80 (d,J= 7.5 Hz, 1H), 7.68 (d,J= 9.0 Hz, 1H), 7.44~7.49 (m, 1H),7.19~7.23 (m, 1H), 6.89 (s, 1H), 6.80 (dd,J= 1.0, 8.0 Hz, 1H),6.73 (d,J= 8.0 Hz, 1H), 6.49~6.57 (m, 2H), 6.25~6.32 (m,1H), 5.95 (s, 2H), 4.19 (s, 2H), 3.69~3.71 (m, 2H), 3.52 (t,J=6.0 Hz, 2H), 3.43 (t,J= 5.0 Hz, 2H), 3.33 (t,J= 7.5 Hz, 2H),1.92~1.98 (m, 2H), 1.74~1.80 (m, 2H), 1.62~1.65 (m, 4H),1.49~1.52 (m, 2H); HRMS [ESI]: C30H32FN3NaO5S ([M +Na]+), 理论值:588.1944;测定值:588.1923.
(2Z,4E)-2-((5-(4-氟)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8d):产率50%, 黄色固体, m.p.77~79 ℃;IR (KBr),ν/cm-1: 3418, 2930, 2858, 1736, 1626, 1438, 1220,1109, 1042, 801;1H NMR (500 MHz, CDCl3)δ: 7.98~8.01 (m,2H), 7.16 (t,J= 8.5 Hz, 2H), 6.88 (s, 1H), 6.80 (d,J= 7.0 Hz,1H), 6.73 (d,J= 8.0 Hz, 1H), 6.49~6.56 (m, 2H), 6.25~6.32(m, 1H), 5.95 (s, 2H), 4.18 (s, 2H), 3.68~3.70 (m, 2H), 3.52 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.0 Hz, 2H), 3.32 (t,J= 7.5 Hz,2H), 1.91~1.97 (m, 2H), 1.74~1.79 (m, 2H), 1.62~1.65 (m,4H), 1.49~1.52 (m, 2H); HRMS [ESI]: C30H33FN3O5S ([M +H]+), 理论值:566.2125;测定值:566.2114.
(2Z,4E)-2-((5-(2-氯)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8e):产率59%,黄色液体; IR (KBr),ν/cm-1: 3442, 2933, 2857, 1739, 1624, 1446, 1361, 1245, 1096,1035, 769, 728;1H NMR (400 MHz, CDCl3)δ: 7.93 (dd,J=2.0, 8.0 Hz, 1H), 7.52 (dd,J= 0.8, 7.6 Hz, 1H), 7.43~7.47 (m,1H), 7.37~7.41 (m, 1H), 6.89 (d,J= 1.6 Hz, 1H), 6.80 (dd,J=1.2, 8.0 Hz, 1H), 6.74 (d,J= 8.4 Hz, 1H), 6.49~6.58 (m, 2H),6.24~6.33 (m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.68~3.70 (m,2H), 3.51 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.6 Hz, 2H), 3.32 (t,J= 7.2 Hz, 2H), 1.92~1.99 (m, 2H), 1.73~1.80 (m, 2H),1.62~1.65 (m, 4H), 1.48~1.52 (m, 2H);13C NMR (100 MHz,CDCl3)δ: 168.21, 165.16, 164.00, 148.14, 147.76, 135.26,134.15, 132.96, 132.32, 131.24, 130.93, 128.88, 127.09,122.97, 122.39, 121.93, 108.45, 105.55, 101.22, 72.88, 70.20,47.83, 42.40, 32.47, 28.63, 26.63, 26.37, 25.91, 24.55; HRMS[ESI]: C30H33ClN3O5S ([M + H]+), 理论值: 582.1829;测定值:582.1805.
(2Z,4E)-2-((5-(3-氯)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8f):产率56%,淡黄色液体; IR (KBr),ν/cm-1: 3448, 2933, 2857, 1723, 1624, 1450, 1359, 1246, 1107,1034, 784;1H NMR (400 MHz, CDCl3)δ: 7.98 (s, 1H), 7.88(d,J= 7.6 Hz, 1H), 7.41~7.50 (m, 2H), 6.89 (s, 1H), 6.80 (d,J= 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.48~6.57 (m, 2H),6.24~6.33 (m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.68~3.70 (m,2H), 3.51 (t,J= 6.4 Hz, 2H), 3.42 (t,J= 5.2 Hz, 2H), 3.33 (t,J= 7.2 Hz, 2H), 1.92~1.98 (m, 2H), 1.74~1.80 (m, 2H),1.61~1.66 (m, 4H), 1.48~1.52 (m, 2H);13C NMR (100 MHz,CDCl3)δ: 168.18, 165.03, 164.51, 148.14, 147.76, 135.27,135.17, 134.14, 131.63, 131.23, 130.43, 128.86, 126.58,125.28, 124.70, 122.37, 121.93, 108.45, 105.54, 101.23, 72.87,70.16, 47.82, 42.38, 32.46, 28.60, 26.64, 26.24, 25.91, 24.55;HRMS [ESI]: C30H32ClN3NaO5S ([M + Na]+), 理论值:604.1649;测定值:604.1605.
(2Z,4E)-2-((5-(4-氯)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8g):产率67%, 淡黄色固体, m.p.101~103 ℃; IR (KBr),ν/cm-1: 3418, 2929, 2858, 1715, 1620, 1438,1365, 1225, 1101, 1050, 805;1H NMR (400 MHz, CDCl3)δ:7.92 (d,J= 8.8 Hz, 2H), 7.45 (d,J= 8.8 Hz, 2H), 6.88 (d,J=1.2 Hz, 1H), 6.80 (dd,J= 1.6, 8.4 Hz, 1H), 6.74 (d,J= 8.0 Hz,1H), 6.48~6.57 (m, 2H), 6.24~6.33 (m, 1H), 5.96 (s, 2H), 4.18(s, 2H), 3.68~3.70 (m, 2H), 3.51 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.6 Hz, 2H), 3.32 (t,J= 7.2 Hz, 2H), 1.91~1.98 (m, 2H),1.73~1.80 (m, 2H), 1.62~1.66 (m, 4H), 1.48~1.52 (m, 2H);13C NMR (100 MHz, CDCl3)δ: 168.19, 164.87, 164.76, 148.14,147.77, 137.84, 135.27, 134.14, 131.23, 129.44, 128.86,127.90, 122.37, 122.15, 121.92, 108.46, 105.55, 101.23, 72.87,70.17, 47.82, 42.39, 32.47, 28.60, 26.64, 26.24, 25.91, 24.55;HRMS [ESI]: C30H32ClN3NaO5S ([M + Na]+), 理论值:604.1649;测定值:604.1608.
(2Z,4E)-2-((5-(2-溴)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8h):产率58%,黄色液体; IR (KBr),ν/cm-1: 3450, 2932, 2856, 1711, 1622, 1445, 1354, 1248, 1031,767, 530;1H NMR (500 MHz, CDCl3)δ: 7.87 (dd,J= 1.6, 7.6 Hz, 1H), 7.72 (dd,J= 0.8, 8.0 Hz, 1H), 7.42~7.46 (m, 1H),7.34~7.39 (m, 1H), 6.89 (d,J= 1.2, 1H), 6.80 (dd,J= 1.6, 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.49~6.58 (m, 2H),6.24~6.33 (m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.68~3.70 (m,2H), 3.51 (t,J= 6.4 Hz, 2H), 3.42 (t,J= 5.2 Hz, 2H), 3.32 (t,J= 7.2 Hz, 2H), 1.92~1.99 (m, 2H), 1.73-1.80 (m, 2H),1.62~1.66 (m, 4H), 1.48~1.52 (m, 2H);13C NMR (100 MHz,CDCl3)δ: 168.19, 165.20, 164.51, 148.14, 147.76, 135.25,134.56, 134.17, 132.43, 131.39, 131.25, 128.86, 127.61,125.06, 122.40, 121.94, 121.46, 108.46, 105.55, 101.22, 72.88,70.20, 47.82, 42.39, 32.49, 28.64, 26.64, 26.39, 25.92, 24.56;HRMS [ESI]: C30H32BrN3NaO5S ([M + Na]+), 理论值:648.1144;测定值:648.1110.
(2Z,4E)-2-((5-(3-溴)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8i):产率50%,黄色液体; IR (KBr),ν/cm-1: 3442, 2931, 2856, 1715, 1624, 1451, 1245, 1105, 1034,795, 529;1H NMR (400 MHz, CDCl3)δ: 8.14 (t,J= 2.0 Hz,1H), 7.93 (d,J= 8.0 Hz, 1H), 7.63 (d,J= 8.0 Hz, 1H), 7.34 (t,J= 8.0 Hz, 1H), 6.89 (d,J= 2.0 Hz, 1H), 6.80 (dd,J= 1.2, 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.48~6.58 (m, 2H),6.24~6.33 (m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.68~3.70 (m,2H), 3.51 (t,J= 6.0 Hz, 2H), 3.43 (t,J= 5.2 Hz, 2H), 3.33 (t,J= 7.2 Hz, 2H), 1.91~1.98 (m, 2H), 1.73~1.80 (m, 2H),1.62~1.65 (m, 4H), 1.48-1.52 (m, 2H);13C NMR (100 MHz,CDCl3)δ: 168.17, 165.04, 164.36, 148.13, 147.76, 135.25,134.54, 134.15, 131.23, 130.63, 129.44, 128.84, 125.49,125.14, 123.07, 122.38, 121.92, 108.45, 105.54, 101.22, 72.87,70.16, 47.81, 42.37, 32.46, 28.60, 26.63, 26.23, 25.90, 24.55;HRMS [ESI]: C30H32BrN3NaO5S ([M + Na]+), 理论值:648.1144;测定值:648.1124.
(2Z,4E)-2-((5-(4-溴)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8j):产率53%,黄色固体, m.p.88~90 ℃;IR (KBr),ν/cm-1: 3450, 2928, 2857, 1736, 1437, 1224, 1107,1055, 799, 530;1H NMR (400 MHz, CDCl3)δ: 7.85 (d,J= 8.4 Hz, 2H), 7.62 (d,J= 8.4 Hz, 2H), 6.88 (s, 1H), 6.80 (d,J= 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.48~6.57 (m, 2H),6.24~6.32 (m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.68-3.70 (m,2H), 3.51 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.5 Hz, 2H), 3.32 (t,J= 7.2 Hz, 2H), 1.91~1.98 (m, 2H), 1.73~1.80 (m, 2H),1.61~1.66 (m, 4H), 1.48~1.52 (m, 2H);13C NMR (100 MHz,CDCl3)δ: 168.17, 164.96, 164.80, 148.14, 147.77, 135.25,134.16, 132.40, 131.23, 128.84, 128.03, 126.25, 122.58,122.38, 121.92, 108.46, 105.55, 101.23, 72.87, 70.16, 47.81,42.37, 32.47, 28.60, 26.64, 26.24, 25.91, 24.55; HRMS [ESI]:C30H32BrN3NaO5S ([M + Na]+), 理论值:648.1144;测定值:648.1136.
(2Z,4E)-2-((5-(2-三氟甲基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8k):产率40%,黄色液体; IR(KBr),ν/cm-1: 3454, 2935, 1734, 1625, 1476, 1450, 1250,1131, 1035, 724;1H NMR (500 MHz, CDCl3)δ: 8.19 (d,J=6.5 Hz, 1H), 7.84 (d,J= 7.0 Hz, 1H), 7.65~7.71 (m, 2H), 6.89(s, 1H), 6.80 (dd,J= 1.5, 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H),6.50~6.57 (m, 2H), 6.25~6.32 (m, 1H), 5.95 (s, 2H), 4.18 (s,2H), 3.68~3.71 (m, 2H), 3.52 (t,J= 6.0 Hz, 2H), 3.43 (t,J=5.0 Hz, 2H), 3.30 (t,J= 7.5 Hz, 2H), 1.91~1.97 (m, 2H),1.74~1.79 (m, 2H), 1.62~1.65 (m, 4H), 1.48~1.52 (m, 2H);HRMS [ESI]: C31H32F3N3NaO5S ([M + Na]+), 理论值:638.1912;测定值:638.1865.
(2Z,4E)-2-((5-(3-三氟甲基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8l):产率51%,黄色液体; IR(KBr),ν/cm-1: 3455, 2935, 2858, 1738, 1625, 1476, 1445,1352, 1247, 1034, 806, 695;1H NMR (500 MHz, CDCl3)δ:8.25 (s, 1H), 8.19 (d,J= 8.0 Hz, 1H), 7.76 (d,J= 7.5 Hz, 1H),7.72 (t,J= 8.0 Hz, 1H), 6.88 (s, 1H), 6.80 (dd,J= 1.0, 8.0 Hz,1H), 6.73 (d,J= 8.0 Hz, 1H), 6.49~6.57 (m, 2H), 6.25~6.32(m, 1H), 5.95 (s, 2H), 4.19 (s, 2H), 3.68~3.70 (m, 2H), 3.52 (t,J= 6.5 Hz, 2H), 3.43 (t,J= 5.0 Hz, 2H), 3.35 (t,J= 7.0 Hz,2H), 1.93~1.99 (m, 2H), 1.75~1.80 (m, 2H), 1.62~1.66 (m,4H), 1.49~1.53 (m, 2H); HRMS [ESI]: C31H32F3N3NaO5S([M + Na]+), 理论值:638.1912;测定值:638.1886.
(2Z,4E)-2-((5-(4-三氟甲基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8m):产率51%,黄色固体, m.p.67~69 ℃; IR (KBr),ν/cm-1: 3447, 2937, 2859, 1737, 1629,1444, 1221, 1115, 1025, 793;1H NMR (500 MHz, CDCl3)δ:8.11 (d,J= 8.0 Hz, 2H), 7.74 (d,J= 8.5 Hz, 2H), 6.88 (s, 1H),6.80 (dd,J= 1.0, 8.0 Hz, 1H), 6.73 (d,J= 8.0 Hz, 1H), 6.49-6.56 (m, 2H), 6.25~6.32 (m, 1H), 5.95 (s, 2H), 4.18 (s, 2H),3.68~3.70 (m, 2H), 3.52 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.0 Hz,2H), 3.35 (t,J= 7.0 Hz, 2H), 1.93~1.99 (m, 2H), 1.75~1.80(m, 2H), 1.62~1.65 (m, 4H), 1.49~1.52 (m, 2H); HRMS [ESI]:C31H32F3N3NaO5S ([M + Na]+), 理论值: 638.1912;测定值:638.1888.
(2Z,4E)-2-((5-(2-硝基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8n):产率56%,淡黄色液体; IR (KBr),ν/cm-1: 3425, 2933, 2857, 1714, 1621, 1476, 1449, 1249, 1104,1037, 854, 724;1H NMR (500 MHz, CDCl3)δ: 8.00 (d,J= 7.5 Hz, 1H), 7.94 (dd,J= 1.0, 7.5 Hz, 1H), 7.71~7.77 (m, 2H),6.89 (s, 1H), 6.81 (d,J= 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H),6.50~6.57 (m, 2H), 6.25~6.33 (m, 1H), 5.96 (s, 2H), 4.18 (s,2H), 3.68~3.70 (m, 2H), 3.52 (t,J= 6.0 Hz, 2H), 3.43-3.45 (m,2H), 3.29 (t,J= 7.0 Hz, 2H), 1.90-1.96 (m, 2H), 1.73~1.79 (m,2H), 1.62~1.66 (m, 4H), 1.48-1.53 (m, 2H); HRMS [ESI]:C30H32N4NaO7S ([M + Na]+), 理论值:615.1889;测定值:615.1865.
(2Z,4E)-2-((5-(3-硝基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8o):产率65%,淡黄色液体; IR (KBr),ν/cm-1: 3454, 2933, 2858, 1738, 1623, 1445, 1225, 1106, 1040,856, 802, 701;1H NMR (500 MHz, CDCl3)δ: 8.81 (s, 1H),8.34~8.37 (m, 2H), 7.69 (t,J= 8.0 Hz, 1H), 6.87 (s, 1H), 6.79(dd,J= 1.5, 8.0 Hz, 1H), 6.73 (d,J= 8.0 Hz, 1H), 6.49~6.56(m, 2H), 6.25~6.32 (m, 1H), 5.95 (s, 2H), 4.19 (s, 2H),3.68~3.70 (m, 2H), 3.53 (t,J= 6.0 Hz, 2H), 3.43 (t,J= 5.5 Hz,2H), 3.36 (t,J= 7.5 Hz, 2H), 1.94~2.00 (m, 2H), 1.75~1.81(m, 2H), 1.62~1.67 (m, 4H), 1.50-1.52 (m, 2H); HRMS [ESI]:C30H32N4NaO7S ([M + Na]+), 理论值:615.1889;测定值:615.1868.
(2Z,4E)-2-((5-(4-硝基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8p):产率58%, 淡黄色固体, m.p.79~81 ℃; IR (KBr),ν/cm-1: 3418, 2931, 1623, 1450, 1247, 1110,853, 798;1H NMR (500 MHz, CDCl3)δ: 8.34 (d,J= 9.0 Hz,2H), 8.18 (d,J= 9.0 Hz, 2H), 6.87 (s, 1H), 6.80 (dd,J= 1.0,8.0 Hz, 1H), 6.73 (dd,J= 1.5, 8.0 Hz, 1H), 6.49~6.56 (m, 2H),6.25~6.32 (m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.68-3.70 (m,2H), 3.53 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.0 Hz, 2H), 3.36 (t,J= 7.5 Hz, 2H), 1.94~2.00 (m, 2H), 1.75~1.80 (m, 2H),1.63~1.66 (m, 4H), 1.48~1.52 (m, 2H); HRMS [ESI]:C30H32N4NaO7S ([M + Na]+), 理论值:615.1889;测定值:615.1881.
(2Z,4E)-2-((5-(2-甲基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8q):产率62%,无色液体; IR (KBr),ν/cm-1: 3452, 2931, 2857, 1725, 1624, 1478, 1248, 1106, 1037,726;1H NMR (500 MHz, CDCl3)δ: 7.86 (d,J= 8.0 Hz, 1H),7.38~7.41 (m, 1H), 7.29~7.33 (m, 2H), 6.89 (d,J= 1.0 Hz,1H), 6.80 (dd,J= 1.5, 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H),6.49-6.56 (m, 2H), 6.25~6.32 (m, 1H), 5.96 (s, 2H), 4.18 (s,2H), 3.68~3.70 (m, 2H), 3.52 (t,J= 6.5 Hz, 2H), 3.42 (t,J=5.5 Hz, 2H), 3.32 (t,J= 7.5 Hz, 2H), 2.68 (s, 3H), 1.93~1.99(m, 2H), 1.74~1.80 (m, 2H), 1.62~1.65 (m, 4H), 1.48~1.52 (m,2H); HRMS [ESI]: C31H35N3NaO5S ([M + Na]+), 理论值:584.2195;测定值:584.2181.
(2Z,4E)-2-((5-(3-甲基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8r):产率67%,无色液体; IR (KBr),
ν/cm-1: 3449, 2931, 2857, 1625, 1476, 1249, 1098, 1035, 851,798, 684;1H NMR (500 MHz, CDCl3)δ: 7.82 (s, 1H), 7.78 (d,J= 8.0 Hz, 1H), 7.37 (d,J= 8.0 Hz, 1H), 7.31 (d,J= 7.5 Hz,1H), 6.89 (d,J= 1.5 Hz, 1H), 6.80 (dd,J= 1.0, 8.0 Hz, 1H),6.74 (d,J= 8.0 Hz, 1H), 6.49~6.56 (m, 2H), 6.25~6.32 (m,1H), 5.95 (s, 2H), 4.18 (s, 2H), 3.68~3.70 (m, 2H), 3.52 (t,J=6.0 Hz, 2H), 3.42 (t,J= 5.5 Hz, 2H), 3.32 (t,J= 7.0 Hz, 2H),2.42 (s, 3H), 1.91~1.97 (m, 2H), 1.74~1.79 (m, 2H), 1.61~1.65(m, 4H), 1.48~1.51 (m, 2H); HRMS [ESI]: C31H35N3NaO5S([M + Na]+), 理论值:584.2195;测定值:584.2163.
(2Z,4E)-2-((5-(4-甲基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8s):产率60%,无色液体; IR (KBr),ν/cm-1: 3450, 2932, 2857, 1622, 1476, 1249, 1107, 1034, 814;1H NMR (500 MHz, CDCl3)δ: 7.87 (d,J= 8.0 Hz, 2H), 7.27(d,J= 8.0 Hz, 2H), 6.88 (d,J= 1.5 Hz, 1H), 6.80 (dd,J= 1.5,8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.49~6.56 (m, 2H),6.24~6.31 (m, 1H), 5.95 (s, 2H), 4.18 (s, 2H), 3.68~3.70 (m,2H), 3.51 (t,J= 6.5 Hz, 2H), 3.42 (t,J= 5.5 Hz, 2H), 3.31 (t,J= 7.0 Hz, 2H), 2.41 (s, 3H), 1.91~1.97 (m, 2H), 1.73~1.76(m, 2H), 1.61~1.65 (m, 4H), 1.48~1.51 (m, 2H); HRMS [ESI]:C31H36N3O5S ([M + H]+), 理论值:562.2376;测定值:562.2359.
(2Z,4E)-2-((5-(2-甲氧基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8t):产率56%,淡黄色液体; IR(KBr),ν/cm-1: 3454, 2933, 2855, 1729, 1618, 1478, 1444,1254, 1115, 1031, 733;1H NMR (400 MHz, CDCl3)δ: 7.56 (d,J= 1.6, 7.6 Hz, 1H), 7.47~7.51 (m, 1H), 7.03~7.07 (m, 2H),6.89 (d,J= 1.2 Hz, 1H), 6.80 (dd,J= 1.2, 8.0 Hz, 1H), 6.74(d,J= 8.4 Hz, 1H), 6.48~6.57 (m, 2H), 6.24~6.33 (m, 1H),5.96 (s, 2H), 4.18 (s, 2H), 3.95 (s, 3H), 3.68~3.70 (m, 2H),3.51 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.2 Hz, 2H), 3.30 (t,J=7.2 Hz, 2H), 1.90~1.98 (m, 2H), 1.73~1.80 (m, 2H), 1.61~1.65(m, 4H), 1.48-1.52 (m, 2H);13C NMR (125 MHz, CDCl3)δ:168.22, 164.44, 164.07, 157.72, 148.16, 147.77, 135.24,134.22, 133.01, 131.28, 130.26, 128.84, 122.43, 121.95,120.74, 112.87, 111.92, 108.48, 105.58, 101.24, 72.89, 70.27,56.00, 47.85, 42.40, 32.44, 28.65, 26.65, 26.35, 25.94, 24.57;HRMS [ESI]: C31H35N3NaO6S ([M + Na]+), 理论值:600.2144;测定值:600.2133.
(2Z,4E)-2-((5-(3-甲氧基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8u):产率55%,淡黄色液体; IR(KBr),ν/cm-1: 3451, 2932, 2855, 1733, 1623, 1477, 1443,1244, 1103, 1036, 851, 795, 682;1H NMR (500 MHz, CDCl3)δ: 7.85 (dd,J= 8.0 Hz, 1H), 7.52 (s, 1H), 7.37 (t,J= 8.0 Hz,1H), 7.04 (dd,J= 1.5, 8.0 Hz, 1H), 6.89 (s, 1H), 6.80 (d,J=8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.49~6.56 (m, 2H),6.24~6.32 (m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.87 (s, 3H),3.68~3.70 (m, 2H), 3.52 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.5 Hz,2H), 3.32 (t,J= 7.5 Hz, 2H), 1.91~1.97 (m, 2H), 1.74~1.79(m, 2H), 1.62~1.65 (m, 4H), 1.48~1.52 (m, 2H);13C NMR(100 MHz, CDCl3)δ: 168.19, 165.64, 164.47, 159.93, 148.14,147.76, 135.24, 134.18, 131.25, 130.20, 128.84, 124.77,122.40, 121.92, 119.05, 118.13, 111.20, 108.45, 105.55,101.22, 72.88, 70.20, 55.52, 47.82, 42.38, 32.45, 28.62, 26.63,26.28, 25.91, 24.55; HRMS [ESI]: C31H35N3NaO6S ([M +Na]+), 理论值: 600.2144;测定值:600.2123.
(2Z,4E)-2-((5-(4-甲氧基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基) 胡椒碱 (8v):产率77%,淡黄色液体; IR(KBr),ν/cm-1: 3450, 2933, 2855, 1711, 1619, 1489, 1444,1253, 1107, 1030, 730;1H NMR (500 MHz, CDCl3)δ: 7.92 (d,J= 9.0 Hz, 2H), 6.97 (d,J= 9.0 Hz, 2H), 6.88 (s, 1H), 6.80 (d,J= 8.0 Hz, 1H), 6.73 (d,J= 8.0 Hz, 1H), 6.49~6.56 (m, 2H),6.24~6.31 (m, 1H), 5.95 (s, 2H), 4.18 (s, 2H), 3.86 (s, 3H),3.68~3.70 (m, 2H), 3.51 (t,J= 6.5 Hz, 2H), 3.42 (t,J= 5.0 Hz,2H), 3.32 (t,J= 7.0 Hz, 2H), 1.90~1.96 (m, 2H), 1.73~1.79(m, 2H), 1.62~1.65 (m, 4H), 1.49~1.52 (m, 2H);13C NMR(100 MHz, CDCl3)δ: 168.18, 165.65, 163.62, 162.22, 148.13,147.75, 135.22, 134.19, 131.24, 128.82, 128.40, 122.39,121.91, 116.20, 114.46, 108.44, 105.54, 101.22, 72.86, 70.20,55.46, 47.81, 42.37, 32.46, 28.61, 26.62, 26.30, 25.91, 24.54;HRMS [ESI]: C31H35N3NaO6S ([M + Na]+), 理论值:600.2144;测定值:600.2115.
(2Z,4E)-2-((5-(2-乙基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8w):产率38%,淡黄色液体; IR (KBr),ν/cm-1: 3424, 2931, 2858, 1717, 1626, 1478, 1442, 1247, 1106,1037, 711;1H NMR (400 MHz, CDCl3)δ: 7.83 (d,J= 7.6 Hz,1H), 7.42~7.46 (m, 1H), 7.35 (d,J= 7.6 Hz, 1H), 7.29 (t,J=7.2 Hz, 1H), 6.89 (d,J= 1.6 Hz, 1H), 6.80 (dd,J= 1.6, 8.0 Hz,1H), 6.74 (d,J= 8.0 Hz, 1H), 6.49-6.57 (m, 2H), 6.24~6.33(m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.68-3.71 (m, 2H), 3.52 (t,J= 6.0 Hz, 2H), 3.42 (t,J= 5.6 Hz, 2H), 3.31 (t,J= 7.6 Hz,2H), 3.06 (q,J= 7.6 Hz, 2H), 1.92~2.00 (m, 2H), 1.73~1.80(m, 2H), 161~1.65 (m, 4H), 1.47~1.52 (m, 2H), 1.24~1.28 (m,3H);13C NMR (125 MHz, CDCl3)δ: 168.21, 165.68, 164.13,148.16, 147.78, 144.46, 135.26, 134.21, 131.41, 131.27,130.19, 129.09, 128.86, 126.16, 122.42, 122.14, 121.96,108.48, 105.57, 101.25, 72.91, 70.24, 47.85, 42.40, 32.42,28.69, 27.64, 26.66, 26.36, 25.94, 24.58, 15.30; HRMS [ESI]:C32H37N3NaO5S ([M + Na]+), 理论值:598.2352;测定值:598.2324.
(2Z,4E)-2-((5-(4-乙基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8x):产率49%,淡黄色液体; IR (KBr),ν/cm-1: 3421, 2932, 2859, 1725, 1623, 1477, 1445, 1247, 1107,1035, 841;1H NMR (500 MHz, CDCl3)δ: 7.90 (d,J= 8.0 Hz,2H), 7.30 (d,J= 8.0 Hz, 2H), 6.89 (s, 1H), 6.80 (d,J= 8.0 Hz,1H), 6.73 (d,J= 8.0 Hz, 1H), 6.50~6.56 (m, 2H), 6.25~6.32(m, 1H), 5.95 (s, 2H), 4.20 (s, 2H), 3.69-3.72 (m, 2H), 3.52 (t,J= 6.0 Hz, 2H), 3.43 (t,J= 5.0 Hz, 2H), 3.31 (t,J= 7.5 Hz,2H), 2.68~2.73 (m, 2H), 1.91~1.97 (m, 2H), 1.74~1.79 (m,2H), 1.62~1.65 (m, 4H), 1.49~1.52 (m, 2H), 1.24~1.27 (m,3H); HRMS [ESI]: C32H37N3NaO5S ([M + Na]+), 理论值:598.2352;测定值:598.2340.
(2Z,4E)-2-((5-(3-氰基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8y):产率52%,淡黄色液体; IR (KBr),ν/cm-1: 3421, 2933, 2857, 2231, 1732, 1624, 1454, 1245, 1103,1035, 805, 675;1H NMR (500 MHz, CDCl3)δ:8.27 (s, 1H),8.24 (d,J= 8.0 Hz, 1H), 7.79 (d,J= 7.5 Hz, 1H), 7.62 (t,J=8.0 Hz, 1H), 6.88 (s, 1H), 6.80~6.82 (m, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.49~6.57 (m, 2H), 6.25~6.32 (m, 1H), 5.96 (s, 2H),4.18 (s, 2H), 3.68~3.71 (m, 2H), 3.52 (t,J= 6.5 Hz, 2H), 3.43(t,J= 5.5 Hz, 2H), 3.35 (t,J= 7.0 Hz, 2H), 1.93~1.99 (m, 2H),1.75~1.80 (m, 2H), 1.63~1.66 (m, 4H), 1.49-1.52 (m, 2H);HRMS [ESI]: C31H32N4NaO5S ([M + Na]+), 理论值:595.1991;测定值:595.1980.
(2Z,4E)-2-((5-(4-氰基)苯基-1,3,4-噁二唑-2-基)-硫基-丁氧基甲基)胡椒碱 (8z):产率64%,淡黄色液体; IR (KBr),ν/cm-1: 3426, 2932, 2856, 2227, 1731, 1624, 1455, 1245, 1106,1033, 847, 803;1H NMR (500 MHz, CDCl3)δ: 8.10 (d,J= 8.0 Hz, 2H), 7.78 (d,J= 8.0 Hz, 2H), 6.88 (s, 1H), 6.80 (d,J= 8.0 Hz, 1H), 6.74 (d,J= 8.0 Hz, 1H), 6.49~6.58 (m, 2H),6.25~6.32 (m, 1H), 5.96 (s, 2H), 4.18 (s, 2H), 3.68-3.70 (m,2H), 3.52 (t,J= 6.5 Hz, 2H), 3.43 (t,J= 5.5 Hz, 2H), 3.35 (t,J= 7.5 Hz, 2H), 1.93~1.97 (m, 2H), 1.74~1.80 (m, 2H),1.62~1.66 (m, 4H), 1.49~1.52 (m, 2H); HRMS [ESI]:C31H32N4NaO5S ([M + Na]+), 理论值:595.1991;测定值:595.1972.
1.3 杀螨活性测定
1.3.1 供试螨 朱砂叶螨T.cinnabarinus雌成螨,由西北农林科技大学植物保护学院农药设计与合成实验室提供。
1.3.2 杀螨活性测定 参考联合国粮食及农业组织(FAO)推荐的玻片浸渍法[20]并加以改进:以0.1‰吐温-80 溶液为空白对照,螺螨酯为阳性对照,用0.1‰吐温-80 溶液将待测样品及对照药物配制成质量浓度为0.5 mg/mL 的溶液备用。将宽度为1 cm 的双面胶粘在载玻片的一端,用0 号毛笔挑选螨龄一致、健康且活泼的朱砂叶螨雌成螨,小心整齐地将其背部粘在双面胶上,每行粘约15~20 头,每个玻片粘2 行。一块玻片为一个重复,每待测药液处理重复3 次。将载玻片放入垫有浸湿海绵的托盘中保湿,托盘放入温度为(26 ± 1) ℃、相对湿度为 (60~80)%、光照/黑暗周期为14 h/10 h 的人工气候箱内。4 h 后,用体视显微镜检查,剔除死亡和不活泼的个体,记录每块载玻片上的活螨数量。然后,将载玻片 (粘有螨的一端) 浸渍在供试药液中振荡,5s 后取出,用滤纸条吸去多余药液,放回托盘,再次置于人工培养箱中。浸药处理后,每隔24 h 用体视显微镜观察并记录螨的死亡情况,检查时用毛笔尖轻触螨体,以其螯肢不动者为死亡。按文献[20]方法计算48 h 和72 h 的死亡率和校正死亡率。
根据杀螨初筛结果,选取具有较好触杀活性的化合物,设置5~7 个浓度梯度,按照上述方法分别测定其校正死亡率。由SPSS 23.0 软件对所得数据进行分析,以Finney 机率值分析法求得毒力回归方程、LC50值及95%置信区间。
1.3.3 温室盆栽试验 参考文献方法[21]进行。以0.1%吐温-80 溶液为空白对照,将阳性对照螺螨酯、先导化合物胡椒碱及待测目标化合物用0.1%吐温-80 制成质量浓度为0.3 mg/mL 的溶液备用。在提前准备好的供试植株豇豆苗上接螨龄一致、健康、活泼的朱砂叶螨雌成螨,每株豇豆苗接50 头螨,每盆3 株为一个重复,每个待测化合物3 个重复。4 h 后,将10 mL 供试药液均匀喷至3 盆植株叶片上。每天观察植株危害情况,记录植株上活螨数,按文献[21]方法计算施药后第1、3、5 天螨口减退率及防治效果。
2 结果与分析
2.1 化合物的合成及表征
本文以胡椒碱为先导化合物,通过维尔斯麦尔哈克 (Vilsmeier-Haack) 反应得到中间体4,其在硼氢化钠条件下还原得到中间体5;然后与1,4-二溴丁烷反应得中间体6;最后,中间体6 与一系列不同取代的2-巯基-5-苯基-1,3,4-噁二唑 (7a~7z) 反应得到26 个目标化合物8a~8z。通过文献检索,所合成的化合物均未见报道,其结构通过核磁共振氢谱 (或碳谱)、红外光谱和高分辨质谱进行表征。如图1 所示,通过化合物8g (CCDC: 2168547)的X-射线单晶衍射确定了其空间构型,发现其C2 位双键的构型为顺式,即通过在胡椒碱羰基的邻位区域选择性地引入醛基后,使其C2 位双键的构型发生了变化。
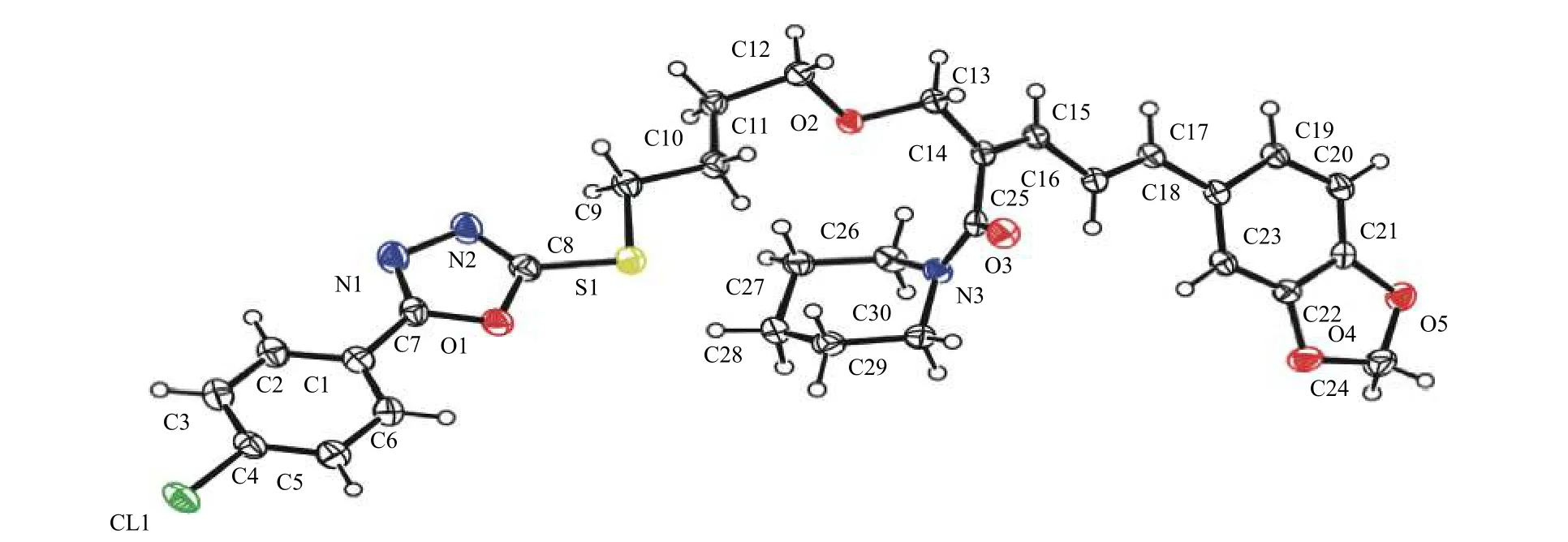
图1 化合物8g 的X-射线衍射单晶结构Fig.1 X-ray crystal structure of compound 8g
2.2 杀螨活性
通过玻片浸渍法测定化合物1、4~6、8a~8z及阳性对照螺螨酯在0.5 mg/mL 质量浓度下对朱砂叶螨雌成螨的48 和72 h 杀螨活性。结果如表1 所示,胡椒碱 (1) 及中间体4~6 在72 h 时的校正死亡率 (corrected mortality rates,CMRs) 分别为21.2%、28.4%、12.0%和23.8%。说明在胡椒碱C2 位引入羟基,不利于杀螨活性;羟基成醚后,其杀螨活性得到提高。继续引入1,3,4-噁二唑活性基团,部分目标化合物的杀螨活性得到显著提高,并且其活性与取代基R 的位置和电子效应相关。当苯环上无取代基时,化合物8a (R = H) 72 h的CMR 为34.8%;当苯环上引入氟原子时,其对位取代化合物的杀螨活性得到显著提高,如化合物8d (72 h CMR:41.3%),而邻位或间位取代化合物的活性均降低,如化合物8b 与8c 的72 h CMRs分别为30.5% 与18.0%;当苯环上引入氯原子、溴原子、乙基或腈基时,其杀螨活性均降低,且在引入溴原子或乙基时活性显著降低,如化合物8h (R = 2-Br;72 h CMR:8.5%) 和化合物8w (R =2-CH2CH3;72 h CMR:8.9%);当苯环上引入甲基或甲氧基时,除化合物8t (R = 2-OCH3;72 h CMR:24.3%) 外,其杀螨活性均得到提高,尤其是间位取代化合物,如化合物8r (R = 3-CH3;72 h CMR:64.4%) 和化合物8u (R = 3-OCH3;72 h CMR:67.4%),其杀螨活性均大于60%。此外,取代基R 还具有明显的位置效应。例如,当R 为卤素原子或三氟甲基时,对位取代化合物的杀螨活性明显高于邻位和间位取代化合物的;然而,当R 为硝基时则正好相反,即对位取代化合物的杀螨活性显著低于邻位和间位取代化合物,如化合物8p(R = 4-NO2;72 h CMR:12.0%)的杀螨活性显著低于化合物8n (R = 2-NO2;72 h CMR:40.9%) 和8o (R = 3-NO2;72 h CMR:41.1%)。
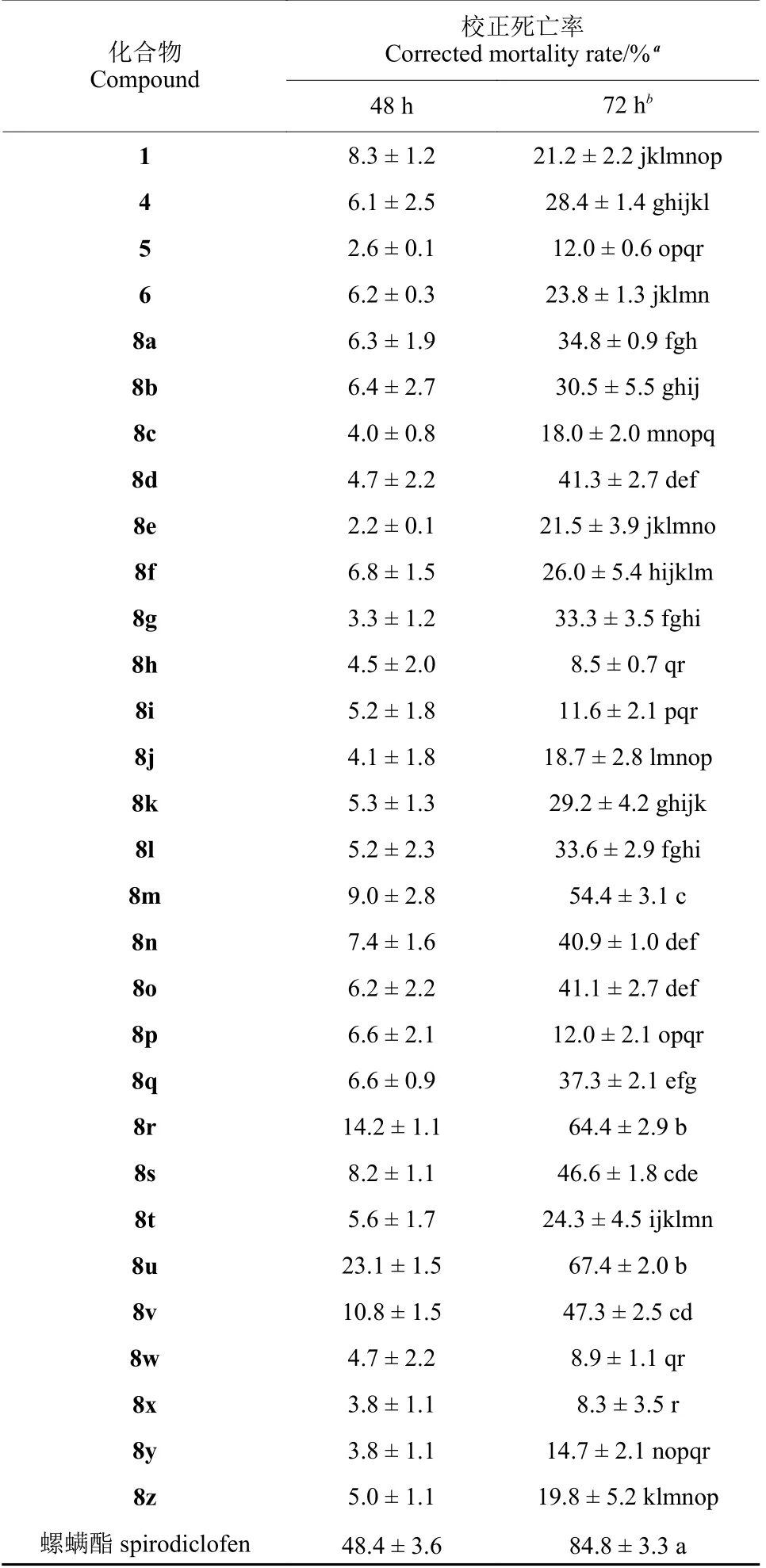
表1 化合物1、4~6、8a~8z 和螺螨酯在0.5 mg/mL质量浓度下对朱砂叶螨雌成螨的杀螨活性Table 1 Acaricidal activities of 1, 4-6, 8a-8z, and spirodiclofen against female adults of T.cinnabarinus at 0.5 mg/mL
如表2 所示,化合物1、8m、8r、8u 和螺螨酯对朱砂叶螨72 h LC50值分别为15.64、0.41、0.36、0.32 和0.12 mg/mL,即所测化合物的杀螨活性是胡椒碱的38.1~48.9 倍。另外,由图2 可以看出,喷雾法测定的化合物1、8r、8u 和螺螨酯在0.3 mg/mL 下对朱砂叶螨第5 天的盆栽防治效果分别为20.0%、56.2%、61.8%和76.5%。化合物1、8r 和8u 处理豇豆苗后5 d,空白对照组和胡椒碱处理组叶片均有大量被朱砂叶螨侵害的黄色斑点,而8r 和8u 处理组叶片黄斑不明显,表现出较好的防治效果 (图3)。今后我们将对化合物8m、8r 及8u 进行进一步结构优化,通过改变连接臂的长短、硫醚氧化为亚砜和砜等,来发现杀螨活性更好的化合物。
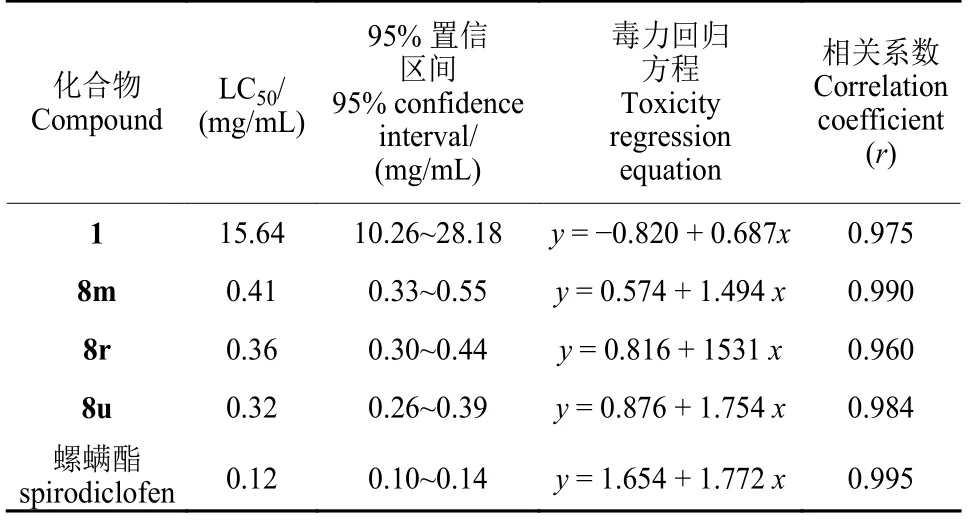
表2 化合物1、8m、8r、8u 和螺螨酯对朱砂叶螨72 h 的LC50 值Table 2 LC50 values of 1, 8m, 8r, 8u and spirodiclofen at 72 h against T.cinnabarinus

图2 化合物1、8r、8u 和螺螨酯在0.3 mg/mL 下对朱砂叶螨的室内防治效果Fig.2 Control efficacies of 1, 8r, 8u and spirodiclofen against T.cinnabarinus in the greenhouse tests at 0.3 mg/mL

图3 喷施化合物1、8r 和8u 后第5 天豇豆苗 (被朱砂叶螨雌成螨侵染) 照片 (CK 为空白对照)Fig.3 The pictures of asparagus bean seedlings (infested with the female adults of T.cinnabarinus) after spraying with 1, 8r and 8u on the 5th day(CK: the blank control group)
3 结论
本文以胡椒碱为先导化合物,设计并合成了一系列结构新颖的含1,3,4-噁二唑杂环的胡椒碱类衍生物8a~8z。部分化合物在0.5 mg/mL 质量浓度下对朱砂叶螨具有较好的杀螨活性,尤其是化合物8m、8r 及8u 表现出良好的杀螨活性,其活性是胡椒碱的38.1~48.9 倍。化合物8r 及8u 在0.3 mg/mL 质量浓度下对朱砂叶螨具有较好的室内防效。构效关系结果表明,在胡椒碱C2 位引入1,3,4-噁二唑杂环有利于提高其杀螨活性,且杀螨活性与噁二唑苯环上的取代基密切相关。化合物8m、8r 及8u 可作为先导化合物进一步进行结构优化来研究其杀螨活性,本研究结果为胡椒碱类新农药的研发提供了一定的理论依据。

