Slurry ice as an alternative cooling medium for fish harvesting and transportation: Study of the effect on seabass flesh quality and shelf life
Athina Ntzimani, Rafael Angelakopoulos, Ioanna Semenoglou, Efimia Dermesonlouoglou,Theofania Tsironi,3,*, Katerina Moutou, Petros Taoukis
1 Laboratory of Food Chemistry and Technology, School of Chemical Engineering, National Technical University of Athens, Athens, 15780, Greece
2 Laboratory of Genetics, Evolutionary and Comparative Biology, Department of Biochemistry and Biotechnology, University of Thessaly, Biopolis, 41500, Larissa, Greece
3 Laboratory of Food Process Engineering, Department of Food Science and Human Nutrition, Agricultural University of Athens, Athens, 11855, Greece
Keywords:European sea bass Slurry ice Shelf life modelling Spoilage Flesh quality Proteolytic enzymes
ABSTRACT The objective of the study was to investigate the efficiency of slurry ice during harvesting and transportation of European sea bass (Dicentrarchus labrax) to retain flesh quality and extend shelf life, compared with conventional flake ice.Fish was slaughtered and transported in different mixtures of slurry ice and conventional flake ice (C:slaughtered and transported in 100% flake ice-Control samples, SC: slaughtered in 100% slurry ice and transported in 100% flake ice, S50: slaughtered and transported in 50% slurry ice-50% flake ice, S100: slaughtered and transported in 100% slurry ice) and subsequently stored under controlled isothermal conditions at 0 ◦C for shelf life modelling and flesh quality evaluation (proteolytic enzymes).The replacement of conventional flake ice with slurry ice as a slaughtering method led to improved quality stability during subsequent refrigerated storage and shelf life extension, in terms of microbial growth, flesh quality and sensory degradation of fish.Based on microbial growth, the shelf life of C samples was found to be 19 days, whereas the shelf life of S50/S100 and SC was 21 and 25 days, respectively, showing that the replacement of flake ice with slurry ice resulted in 2–6 days shelf life extension of whole sea bass stored at 0 ◦C.The use of slurry ice at slaughter and flake ice in transportation was accompanied by low activities and late peaks of all four enzymes that is expected to lead to delayed proteolytic degradation and extended freshness.
1.Introduction
Fish is highly susceptible to spoilage, which can be caused by both intrinsic chemical reactions and microbial growth.An estimated 25% of primary agricultural and fishery products are lost every year, mostly because of chemical deterioration and microbial spoilage (Baird-Parker,2000).The deterioration process is accelerated by increased temperatures, physical damage and contamination.Therefore, the key to fish preservation is the immediate chilling upon catch or harvest to a temperature slightly above the freezing point and maintaining this temperature throughout the cold chain (Kauffeld, Wang, Goldstein, & Kasza,2010).Among the various preservation methods, flake ice, refrigerated seawater and chilled sea water are commonly used for seafood preservation, but over the last two decades interest in using phase-change ice slurry coolants has grown significantly (Kauffeld et al., 2005).The use of slurry ice for preservation of aquatic products was first reported by Chapman (1990) and it is now referred as a promising technology that has been increasingly used immediately after capture, on-board handling, storage and transportation of fish.Slurry ice has also been reported to slow down microbial growth and provide a significantly increased shelf life for a broad variety of marine species, such as salmon,seabream, horse mackerel and pink shrimp (Huidobro, Mendes, &Nunes, 2001; Losada et al, 2004a, 2005; Rodríguez, Losada, Aubourg, &Barros-Vel´azquez, 2004).
Slurry ice, is a biphasic system consisting of small spherical ice particles surrounded by seawater at subzero temperature (Cakli, Kilinc,Cadun, & Tolasa, 2006).Its reported advantages over traditional fresh-water ice (such as flake, tube, and block ice) include its lower temperature, faster chilling (due to a more rapid heat exchange), and lower rate of physical damage (due to its spherical microscopic particles) (Bellas & Tassou, 2005; Kauffeld et al., 2010).The ability of adjusting the ice concentration up to 60% and the salt content in the range of 2–3% in the ice slurry ensures maximum preservation results without damage to delicate fish and avoids excessive salt uptake by the fish (Kauffeld et al., 2010).Additionally, complete coverage of the fish surface by the slurry ice mixture also protects sufficiently fish tissues from the action of oxygen and therefore from lipid oxidation and dehydration (Huidobro, Lopez-Caballero, & Mendes, 2002).
Greece is one of the leading European sea bass producers worldwide.European sea bass (Dicentrarchus labrax) is one of the finfish species preferred by the consumer mainly for its white flesh, mild flavor and low fat content (Body, Green, & LePors, 1992; Limbo, Sinelli, Torri, & Riva,2009).These attributes have made several bass species popular and high-valued around the world.Demand for chilled fish (in polystyrene boxes covered with melting ice) has increased significantly over the last decade.The objective of this study was to investigate the effect of slurry ice during harvesting and transportation of European sea bass (Dicentrarchus labrax) on the quality and shelf life, based on microbiological,physicochemical and sensory results, compared with traditional flake ice.
2.Materials and methods
2.1. Preparation of slurry ice
Slurry ice was prepared from filtered seawater (salinity: 3.5%) using a semi-industrial scale slurry ice machine (ZIEGRA, Germany).The temperature of the slurry ice mixture was - 3.2◦C.
2.2. Raw materials- European sea bass and treatments
Whole European sea bass (Dicentrarchus labrax) was taken from the net cages (fish weight: 200–400 g, 120 specimens were used for all tested parameters and replicate analyses) in Philosofish S.A.farming facilities (Larymna, Fthiotida, Greece) and within 24 h after slaughtering in slurry ice 0, 50 or 100% slurry ice (prepared from seawater), was transported to the Laboratory of Food Chemistry and Technology(NTUA) and the Laboratory of Genetics, Evolutionary and Comparative Biology (UTH) in polystyrene boxes.Four different mixtures of slurry ice and conventional flake ice were tested in the present study and were coded as C: slaughtered and transported in 100% flake ice-Control samples, SC: slaughtered in 100% slurry ice and transported in 100%flake ice, S50: slaughtered and transported in 50% slurry ice-50% flake ice, S100: slaughtered and transported in 100% slurry ice.The ratio of ice (slurry or flake) to fish (w/w) was 1:1 and the temperature of the slurry ice was - 3.2◦C.
Upon receipt at the laboratory, all fish samples (C, SC, S50, S100)were stored in high-precision low temperature incubators (Sanyo MIR 153, Sanyo Electric, Ora-Gun, Japan) under controlled isothermal conditions at (0 ±0.2◦C).The temperature in the incubators was monitored using miniature data-loggers (COX TRACER, Belmont, NC).Duplicate samples were taken in appropriate time (days 0, 4, 7, 13, 15, 18, 21, 24,27, 31 and 33) during a 33-day period to allow microbial, physicochemical, sensory and shelf life modelling.Eight specimens were used for the determination of the activity of proteolytic enzymes in the fillet at each time point.
2.3. Microbiological analysis
A representative sample of 10 g of fish muscle were dissected aseptically from chilled sea bass specimens, mixed with 90 mL sterilized Ringer’s solution (Ringer tablets, Merck, Darmstadt, Germany), and homogenized for 60 s with a Stomacher (BagMixer ® interscience,France).In all cases, serial dilutions from the microbial extracts were prepared in Ringer’s solution.The microorganisms tested in the present study were Total Viable Count (TVC),Pseudomonasspp.,Brochothrix thermosphacta, H2S-producing bacteria (e.g.Shewanellaspp.), Yeasts/Molds andEnterobacteriaceaespp.Microbial load was expressed as the average log CFU/g.Samples (0.1 mL) of 10-fold serial dilutions of fish homogenates were transferred into the appropriate media on Petri dishes for the enumeration of TVC andPseudomonasspp.TVC was enumerated on plate count agar (PCA, Merck, Darmstadt, Germany)after incubation at 25◦C for 72 h, whereasPseudomonasspp.were enumerated on Cetrimide agar (CFC, Merck, Darmstadt, Germany) after incubation at 25◦C for 48 h.For H2S-producing bacteria andEnterobacteriaceaespp.enumeration, the pour-plate method was used.H2Sproducing bacteria were enumerated on Iron Agar (Iron agar withLcysteine) followed by incubation at 25◦C for 48 h.ForEnterobacteriaceaespp.enumeration violet red bile glucose agar (VRBG, Merck, Darmstadt,Germany) was used, which was incubated at 37◦C for 18–24 h.Two replicates of at least three appropriate dilutions were enumerated.The microbial growth was modelled using the Baranyi Growth Model (Baranyi & Roberts, 1995).For curve fitting the program DMFit (IFR,Institute of Food Research, Reading, UK) was used (available at http://www.combase.cc/index.php/en/).Kinetic parameters such as the rate(k) and lag phase (λ) of microbial growth were estimated.
2.4. Physicochemical analyses
2.4.1.Proximate analysis of fish flesh
Protein content in fish flesh was determined according to AOAC(AOAC, 1997), using a Kjeldhal distillation unit (Büchi 321 Distillation unit, Flawwil, Switzerland).Total lipids were determined based on the method reported by Smedes (1999).Moisture content was determined gravimetrically, by drying at 110◦C (WTB BINDER 7200, Type Е53,Tuttlingen, Germany) for 24 h.Ash was determined after ignition at 500◦C for 12 h.
2.5. pH measurement
The pH of all samples examined in the present study was measured using a pH-meter (pH-meter 338, AMEL Instruments, Milan, Italy).The pH-meter was calibrated using standard buffer solutions.10 g of each sea bass sample was diluted in 90 mL Ringer’s solution (1:10 dilution)and its pH was recorded.
2.5.1.Lipid oxidation
To evaluate lipid oxidation, 2-thiobarbituric acid reactive substances(TBARs) assay was performed according to the method of Loovas(1992).5 g of fish flesh was homogenized with 15 mL of distilled water.2 mL of an acid solution of TBA was added in 1 mL of the aquatic fish flesh homogenize followed by heating in a boiling water bath for 15 min,to obtain maximum color development.The absorbance was measured at 532 nm with a digital spectrophotometer (Unicam Helios; Spectronic Unicam EMEA, Cambridge, UK).The concentration of TBARs as calculated from a standard curve prepared by 1,1,3,3-tetraethoxypropane and expressed as mg malonaldehyde (MDA) per kg of fish muscle.
2.5.2.Color measurement
Color of all fish samples was measured on the dorsal part of the body with the color meter Minolta CR-200 (Minolta Company, Chuo-Ku,Osaka, Japan), using a CIE color scale (Commission International de l’Eclairage) Lab (CIE 1978).CIELAB is an opponent color system based on the earlier (1942) system of Richard Hunter called L*, a*, b*.CIELAB indicates these values with three axes: L*, a*, and b*.The central vertical axis represents lightness (signified as L*) and values run from 0 (black) to 100 (white).The color meter is calibrated before measurements using a white calibration plate (Calibration plate CR-200, L* =97.5, a* = - 0.31, b* = - 3.83).The color changes during storage are expressed as ΔE with the color of the creams measured on the first day of sampling as a reference sample.ΔE is the total color change calculated from:
2.5.3.Texture analysis
Texture parameters were defined using a texture analyzer with a load cell of 5 kg (TA-XT2i, Stable Micro Systems, Godalming, Surrey, United Kingdom).A flat-ended cylinder of 20 mm diameter was selected.Double compression was applied to construct the texture profile analysis(TPA) parameters of three different specimens.The probe approached the sample at the speed of 0.5 mm/s and distance of 2 mm into the fish flesh.Then the force was reduced and the sample was allowed to rebound 5 s before the second compression.Force-time curves were obtained and texture parameters (hardness, springiness, cohesiveness and adhesiveness) were determined using Exponent Software (Version 6.1.16, Stable Micro Systems Ltd) (Sigurgisladottir et al., 1999; Jin et al.,2014).
2.6. Sensory evaluation
The sensory attributes of raw and cooked fish were evaluated by a sensory panel of eight trained evaluators using descriptive tests with practice evaluation methods of determining spoilage characteristics in fish (Botta, 1995).European sea bass samples were cooked individually wrapped in aluminum foil, at 180◦C for 40 min, in a pre-heated oven, as described by Tsironi, Maltezou, Tsevdou, Katsaros, and Taoukis (2015).The sensory parameters (appearance, texture and odour of raw and cooked samples, and taste of cooked samples) were evaluated, and sensory scores were recorded in appropriate forms, reflecting the organoleptic evolution of quality deterioration.Additionally, panelists were asked to score the overall impression and acceptability.Rating was assigned separately for each parameter on a 1–9 scale (9 = like extremely and 1 =dislike extremely).A sensory score of 5 was taken as the average score for minimum acceptability.
2.7. Determination of proteolytic enzymes
For the measurement of enzymatic activity of calpain, collagenase,cathepsin B and L, a piece of white muscle (~200 mg) was extracted from the fillet at slaughter (day 0) and on days 1, 2, 4, 8 and 15 post slaughter.The samples were snap-frozen in liquid nitrogen and stored at- 80◦C until enzyme extraction.
2.7.1.Preparation of enzyme extracts
Crude enzyme extract for cathepsin determination was obtained according to Lakshmanan, Patterson, and Piggot (2005) with slight modifications.Briefly, minced sea bass muscle was homogenized with cold water (4◦C) at a ratio of 1:2.The homogenate was centrifuged for 20 min at 14600g (4◦C) and the supernatant was stored at - 80◦C until further analysis (Teixeira et al., 2013).
Crude enzyme extracts for calpain and collagenase determination were prepared according to Ch´eret, Delbarre-Ladrat, Lamballerie-Anton,and Verrez-Bagnis (2007) with minor modifications.Minced sea bass muscle was homogenized in 500 mM Tris-HCl (pH 7.5), 10 mM β-mercaptoethanol, 1 mM EDTA at a ratio of 1:3.The homogenate was centrifuged at 10000g for 40min (10◦C) and the supernatant was transferred and stored at - 80◦C until enzymatic analysis.
2.7.2.Determination of proteolytic enzyme activity
The activity of cathepsin B and L was assayed by the Barrett and Kirschke (1981) method with minor changes, using Z-arginine-arginine-7-amido-4-methylcoumarin hydrochloride and Z-phenylalanine-arginine-7-amido-4-methylcoumarin hydrochloride as substrates, respectively.The enzyme extract was mixed with substrate solution 3.125 mM in 100 mmol/L Tris-HCl, 20 mmol/L EDTA, 4 mmol/L DTT, pH 6.5.
The activities of calpain and collagenase were both assayed according to the Barrett and Kirschke (1981) method.6.250 mM L-methionine-AMC trifluoroacetic salt in DMSO and 3.125 mM Suc-Gly-Pro-Leu-Gly-Pro-AMC in DMSO were used as substrates for calpain and collagenases, respectively.Enzyme extracts were mixed with substrate solution in 100 mM bis-Tris, 5 mM CaCl2pH 6.5.
The fluorescence of 7-amino-4-methylcoumarin released was measured for all the enzymes (Ex = 360 nm, Em = 460 nm) using a spectrofluorometer (Varioskan™ LUX multimode microplate reader,Thermofisher).Enzymatic activity was expressed as fluorescence units(FU) change per minute per mg protein.Two replicates per sample were performed.
The protein content of enzyme extracts was quantified with the Bradford (1976) method using bovine serum albumin as a standard.Two replicates per sample were performed.
The statistical analysis was conducted using R packages in RStudio,including one-way and two-way ANOVA models, Tukey’s HSD test and a Pearson correlation coefficient.
3.Results and discussion
3.1. Microbiological, sensory and shelf life evaluation
In Fig.1(a–c), the growth curves of TVC,Pseudomonasspp.and H2Sproducing bacteria during isothermal storage at 0οC are depicted.The experimental data were fitted to the Baranyi growth model.Data showed that the microbial counts increased with storage time, in contrast to the counts ofBrochothrix thermosphacta, Yeasts/Molds (<2.0 log CFU/g) andEnterobacteriaceae(<1.0 log CFU/g) which remained below the detection limit during the 33 day storage period (data not shown).TVC,Pseudomonasspp.and H2S-producing bacteria had a similar growth pattern, with the two latter being the dominant bacteria responsible for the deterioration of whole sea bass.Initial counts of the aforementioned microorganisms were low (i.e.2.0 ±0.2, 2.0 ±0.1 and 1.0 ± 0.1 log CFU/g for TVC,Pseudomonasspp.and H2S-producing bacteria, respectively) and comparable with those reported in the literature for fresh fish stored aerobically (Dalgaard, 1995; Erkan &¨Ozden, 2006; Gram & Huss, 1996; Koutsoumanis & Nychas, 2000;Paleologos, Savvaidis, & Kontominas, 2004; Papaharisis, Tsironi,Dimitroglou, Taoukis, & Pavlidis, 2019; Tsironi et al., 2019).
It was observed that microorganisms in all samples tested showed similar growth curves at 0◦C.For the C, SC, S50 and S100 sea bass samples, TVC reached 8.0, 7.5, 7.9 and 7.6 log CFU/g, respectively after 33 days of storage at 0◦C (Fig.1a).The significant inhibitory effect of slurry ice was observed for the total viable microbial growth due to a lag phase increase in samples treated with the different mixtures of slurry ice (p <0.05) (e.g., 7.5 d for the SC samples compared to 5.6 d for the C samples stored at 0◦C).The rates of TVC growth were lower for the SC and S100 samples (slaughtered in 100% slurry), compared to the C and S50 samples.As far as counts ofPseudomonasspp.(Fig.1b) are concerned, they remained at a level 2.0 log CFU/g, for a period of approximately 4 and 7 days, for the C and slurry ice treated samples,respectively.Pseudomonasspp.growth rates were significantly lower for SC and S100 samples compared to C samples stored at 0◦C (p <0.05).Similar results were obtained for H2S-producing bacteria (Fig.1c).It was concluded that 100% slurry ice delayed the growth of bothPseudomonasspp.and H2S-producing bacteria therefore, slurry ice as an alternative cooling medium for slaughtering and transportation led to better control of microbial growth as compared to conventional ice.Results were in agreement with data from the literature, where it is reported that the rapid cooling of muscle by slurry ice may significantly delay (or inhibit)the growth of decay-producing microorganisms and their metabolites(Kauffeld et al., 2010), and therefore extend the shelf life of fish.
Data obtained from the present study, revealed thatPseudomonasspp.and H2S-producing bacteria were the dominant spoilage microorganisms in all samples tested (Fig.1b and c).In fact, at the end of the storage period,Pseudomonasspp.and H2S-producing bacteria reached populations as high as 7.9 and 7.1 log cfu/g respectively, while counts ofB.thermosphacta, yeasts/molds and LAB, which were also tested,remained below the detection limit, with Enterobacteriaceae reaching final counts of 3.2 log CFU/g.Results of the present study were in accordance with relevant data for Mediterranean fish from other researchers (Paleologos et al., 2004; Papadopoulos, Chouliara, Badeka,Savvaidis, & Kontominas, 2003).
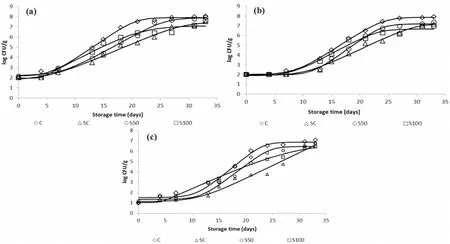
Fig.1.Growth of (a) total viable counts (TVC), (b) Pseudomonas spp.and (c) H2S-producing bacteria in sea bass samples during storage at 0 ◦C (⋄ C, △ SC, ○S50,□ S100).
The results of the sensory analysis are shown in Fig.2(a–d).The scorings for freshness (appearance, odour and taste) for all samples were high for approximately 4–7 days, in contrast to the SC samples for which the respective scores remained high for approximately 13 days, when stored at 0◦C.Fresh fish had a sharply sea weedy smell, with brightly red and without odour gills; whereas the sensory spoilage characteristics were sour, fish and putrid off flavour, with a grey-yellowish color and intense ammonia odour of the gills.The slurry ice-treated samples showed significantly lower rates of sensory degradation compared to Control fillets (P < 0.05).A score of 5 for overall acceptability was considered as the limit of acceptability equivalent to a slight off odour and off taste development.At all tested conditions, the time of sensory rejection coincided with aPseudomonasspp.and H2S-producing bacteria level of 106CFU/g.This value was similar with the respective rejection limits reported in the literature for chilled Mediterranean, whole fish(Koutsoumanis & Nychas, 2000; Rodriguezet al., 2006; Papaharisis et al., 2019; Tsironi et al., 2015).Based on this limit, the shelf life of C samples was found to be 19 days, whereas the shelf life of S50/S100 and SC was 21 and 25 days, respectively, showing that the replacement of flake ice with slurry ice resulted in 2–6 days shelf life extension of whole sea bass stored at 0◦C.Results also indicated that the combination of slurry ice and flake ice controlled effectively the initial temperature of the cooling medium and prevented potential surface damage to fish,usually caused by long term exposure at sub-zero temperatures.Results of the present study were in accordance with Campos, Rodruqiez, Losada, Aubourg, and Barros-Vel´azquez (2005), who reported an approximately 7-day shelf life extension of sardine when slurry ice (15 d) was applied in comparison with flake ice (8d), showing a similar inhibitory effect on quality loss mechanisms, since rapid and robust cooling of fish with the use of slurry ice proved to be effective in slowing down the postharvest deterioration.This preservative effect of slurry ice may be attributed to the surface wash caused by the liquid phase of the slurry ice together with the subzero temperature achieved with this advanced storage system (Rodríguez, Losada, Aubourg, & Barros-Vel´azquez,2005).In the present study, slurry ice did not show a significant preservative effect during transportation of whole sea bass, as an alternative cooling medium.This was in accordance with Cakli et al.(2006) who reported that slurry ice during transportation did not extend the shelf life of farmed sea bass stored at 4◦C, compared with transportation with conventional flake ice.
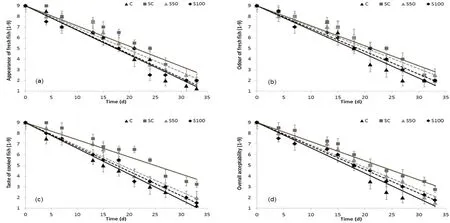
Fig.2.Scores of (a) appearance and (b) odour of raw samples, (c) taste of cooked samples and (d) overall acceptability of sea bass samples, during isothermal storage at 0 ◦C (▴C, ■SC, ▴S50, ◆S100).
3.2. Physicochemical analysis
Initial concentration of fish flesh was 20.5 ± 2.1% proteins, 73.2 ±3.5% moisture, 6.0 ±1.4% lipids and 0.8 ±0.2% ash.These values are in agreement with previous data reported for fish flesh composition of European sea bass (Kotzamanis et al., 2020).
pH values of fish muscle were determined during the shelf life test.pH for all tested conditions increased during isothermal storage at 0◦C.pH values for SC samples were remained almost constant (6.542–6.590),no significant changes were noted until day 15, with these values being lower compared to the respective values of both control and treated samples (data not shown).pH increased until day 15 and then decreased to final values 6.37–6.51 at the end of storage period.The pH of live fish muscle is close to the value 7.0.However post-mortem pH can vary from 6.0 to 7.1 depending on the season, the species, and other factors(Simeonidou, Govaris, & Vareltzis, 1998), which was in accordance with the values noted in the present study.
TBARs index is a widely used indicator for the assessment of the degree of lipid oxidation (Nishimoto et al., 1985), therefore TBARs values indicate levels of secondary oxidation products formed, such as malonaldehyde (MDA).Initial values (day 0) of TBARs were 0.064,0.040 and 0.068 mg MDA/kg for C, SC/S100 and S50, respectively being significant lower than the values reported for gilthead seabream and seabass (Goulas & Kontominas, 2007; Masniyom, Benjakul, & Visessanguan, 2005).TBARs values of all samples remained well below the acceptability level of 1 mg MDA/kg during the 33 days storage, indicating that lipid oxidation was not an adequate quality index for whole sea bass during isothermal storage at 0◦C (Tsironi & Taoukis, 2017).Color parameters of both control and slurry treated samples were determined in the present study.At the beginning of the storage period averageL*(Lightness) value of C, SC, S50 and S100 were determined as 69.72, 71.83, 66.45 and 66.16, respectively (Fig.3).When comparingL*values of all sea bass samples at the last day of storage at 0◦C (day 33), it was observed that SC samples had similar values to those determined for C samples, whereas respective values for S50 and S100 samples, were lower.S50 samples showed the lowestL*values of all samples tested.L*values on day 33 were 66.48, 66.66, 56.26 and 62.52 for the C, SC, S50 and S100 samples, respectively.Therefore, the combination of slurry ice-as a slaughtering method- and flake ice-as a transportation method-managed to retain the lightness of the fish samples.

Fig.3. L* values of sea bass samples, during storage at 0 ◦C (■C, ■SC,■S50, □S100).
Texture attributes such as hardness, which is related to denaturation of the protein, is often used as freshness indicator for fish (Bourne,2002).Texture parameters were also affected by slurry ice treatment.As shown in Fig.4, hardness of all samples decreased significantly (P <0.05) after storage at 0◦C for 33 days, demonstrating that fish muscle might have softened quickly, which might be attributed to myofibrillar protein denaturation and gel formation (Cheftel & Culioli, 1997;Ohshima, Suzuki, & Koizumi, 1993).The tenderization of the fish muscle during chilled/cold storage may be caused due to the actions of endogenous enzymes and microbial activity (Okpala, 2014).The increasing microbial activity contributes in the breakdown of the proteins.In the present study, SC samples compared to C, S50 and S100, had significant higher values of hardness on day 0 until day 4 of storage,indicating a combined effect of 100% slurry ice and flake ice during transportation against endogenous enzymes activity and spoilage microorganisms, showing that this combination might have a positive effect on the texture of the fish.Since microbial growth occurred during storage of the samples at 0◦C, the values of hardness seem to be connected with the microbial growth rate.Feng, Jiang, Wang, and Li (2012)also observed that the texture measurements of black sea bream were in accordance to the microbial growth noted for the samples.Springiness in treated samples remained almost stable during preservation at 0◦C.
3.3. Proteolytic enzymes
In addition to the microbial spoilage, biochemical changes occurpost mortemduring storage and transportation, and they can be either metabolic or structural (e.g.changes in the myofibrillar and transversal structure, sarcolemma anchorage and changes in the extracellular matrix) all triggered by the action of proteases (Delbarre-Ladrat et al.,2006; Singh & Benjakul, 2018).The main proteases in action in the fish fillet are calpains, cathepsins and collagenases (Ahmed, Donkor, Street,& Vasiljevic, 2015; Bao et al., 2020).Calpains are intracellular endopeptidases, responsible for the initiation of the proteolytic degradation of myofibrils (Ch´eret, Hern´andez-Andr´es, Delbarre-Ladrat, de Lamballerie, & Verrez-Bagnis, 2006).Cathepsins are lysosomal cysteine proteases with a wide range of functions, including intracellular protein degradation and turnover (Yeh & Klesius, 2009).Cathepsins are divided into three subgroups according to the amino acid present in the active site: cysteine protease (i.e.B and L) aspartic protease (i.e.D), and serine protease (Liaudet-Coopman et al., 2006).The fish fillet consists of myotomes held together by connective tissue called myocommata,which are in turn surrounded by collagenous fibrils.Collagenases are the matrix metalloproteases (Pedersen, Vuong, Rønning, & Kolset, 2015)that act to degrade the collagenous fibrils producing the characteristic gaps of fish fillet kept under chilled storage (Singh & Benjakul, 2018).
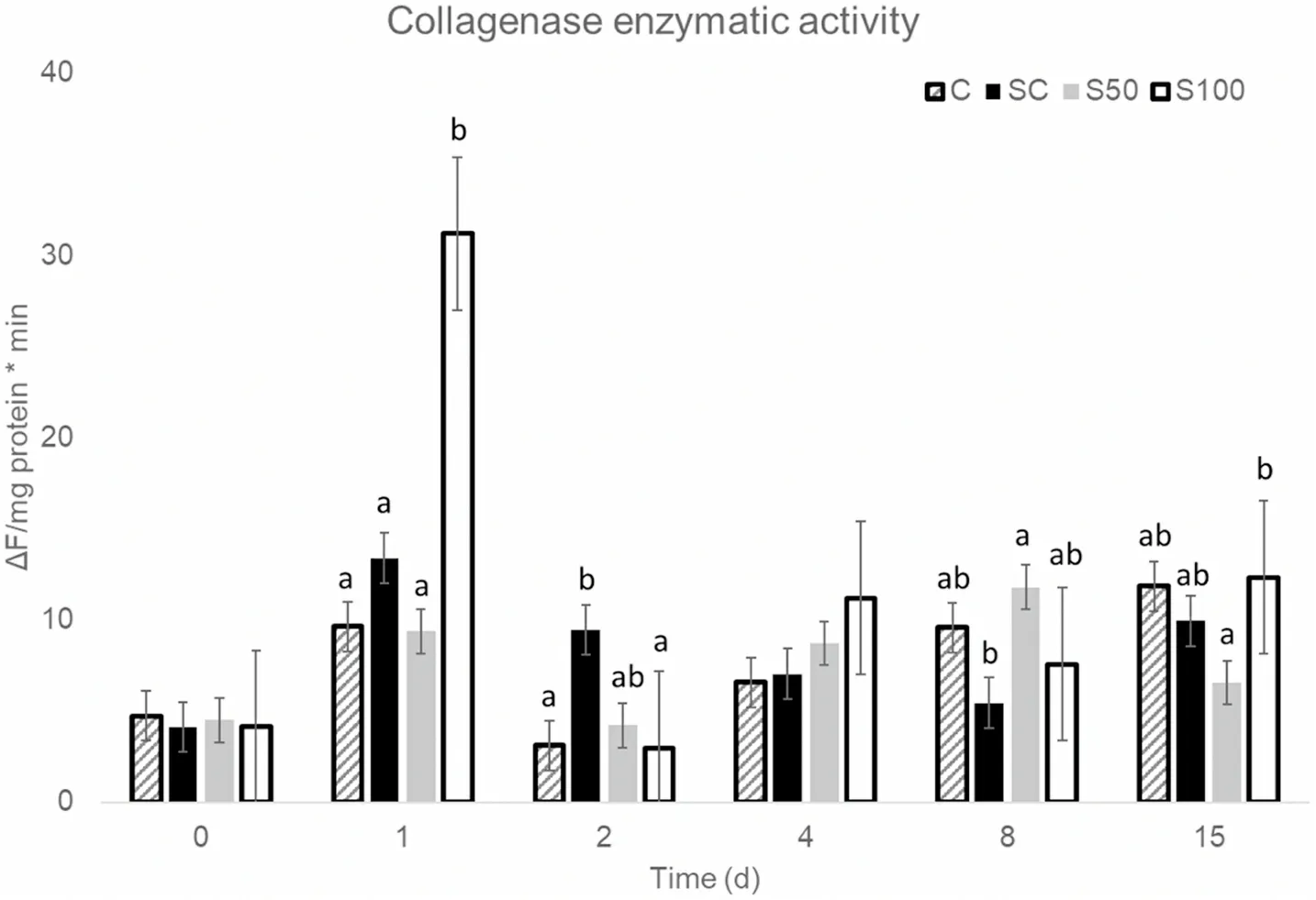
Calpains exhibited the highest levels of activity from all the proteolytic enzymes measured (Fig.5).Its activity did not differentiate between treatments on any day but day 1 post-harvest, when it was significantly higher in fish slaughtered and transported in 100% slurry ice (S100).Calpain is generally activated early post-mortem, leading to the degradation of the Z-disk (Bao et al., 2020; Giyatmi & Irianto, 2017).Calpain activity in C, SC and S100 samples was the highest on day 1,whereas in S50 samples it kept increasing up to day 8.
Calpain activity was significantly and positively correlated with collagenase activity in all treatments (0.39 Fig.4.Values of (a) hardness and (b) springiness of sea bass samples, during storage at 0 ◦C (■C, ■SC, ■S50, □ S100). Fig.5.Enzymatic activity of calpain in all slaughter and storage methods.Superscripts indicate statistically significant differences (p <0.05) between treatments on each sampling day. No significant differences were observed in cathepsin B activity between treatments on any sampling day (data not shown).Cathepsin L on the other hand, significantly differentiated between treatments on days 2, 8 and 17 after slaughter (Fig.7).Slaughter and transportation in 100% slurry ice (S100) kept cathepsin L activity at low levels on all sampling days.Slaughter and transportation in 50% slurry ice-50% flake ice (S50) resulted in a gradual increase of cathepsin L between day 2 and 8, when it was the highest of all treatments.Slaughter in 100% slurry ice and transportation in 100% flake ice (SC) kept cathepsin L levels low on all sampling days to increase only on day 15. Proteolytic enzymes play a crucial role to the firmness and hardness of the fish fillet (Ahmed et al., 2015).The activation of these proteases or their synergistic effects leads in autolysis of myofibrils of the fish and the consequent softening of fish musclepost mortem(Delbarre-Ladrat et al.,2006).No such a link between hardness and proteolytic activity was observed in the current study. Two different means of slaughtering and transportation were used in this study, slurry ice and flake ice, to investigate their potential to affect quality and extend the shelf life of farmed sea bass.The replacement of conventional flake ice with slurry ice during slaughtering led to improved quality and microbial stability during refrigerated storage,resulting in 2–6 days shelf life extension of whole sea bass stored at 0◦C based on microbial growth, proteolytic enzyme activities and sensory evaluation, whereas it did not show additional preservative effects during transportation.Conclusively, the use of slurry ice as the slaughtering method for farmed European sea bass showed significant benefits for the overall quality of treated fish during the conventional cold chain. CRediT authorship contribution statement Athina Ntzimani:Methodology, Formal analysis, Investigation,Writing - original draft, Writing - review & editing, Visualization.Rafael Angelakopoulos:Formal analysis, Investigation, Writing - original draft, Supervision, Visualization.Ioanna Semenoglou:Formal analysis,Investigation, Supervision, Visualization.Efimia Dermesonlouoglou:Supervision, Supervision, Project administration, Visualization.Theofania Tsironi:Conceptualization, Methodology, Formal analysis,Writing - review & editing, Project administration.Katerina Moutou:Conceptualization, Methodology, Resources, Writing - review & editing,Funding acquisition.Petros Taoukis:Conceptualization, Methodology,Resources, Writing - review & editing, Funding acquisition. Fig.6.Enzymatic activity of collagenase in all slaughter and storage methods.Superscripts indicate statistically significant differences (p < 0.05) between treatments on each sampling day. Fig.7.Enzymatic activity of cathepsin L in all slaughter and storage methods.Superscripts indicate statistically significant differences (p <0.05) between treatments on each sampling day. Acknowledgment This research was funded by the Greek Operational Programme for Fisheries, Priority Axis “Innovation in Aquaculture”, Project title:“Development and application of novel methods for fish harvesting and processing for quality improvement and shelf life extension”(2018–2021) website: slurryfish.chemeng.ntua.gr.

4.Conclusions

 Aquaculture and Fisheries2023年4期
Aquaculture and Fisheries2023年4期
- Aquaculture and Fisheries的其它文章
- A framework for risk analysis of the shellfish aquaculture: The case of the Mediterranean mussel farming in Greece
- Application of hurdle technology for the shelf life extension of European eel(Anguilla anguilla) fillets
- Physicochemical properties of silver carp (Hypophthalmichthys molitrix)mince sausages as influenced by washing and frozen storage
- Bacterial community in response to packaging conditions in farmed gilthead seabream
- Effective algorithmic operational framework for fish texture evaluation in industry: Achieving maturity
- Seasonal variation in the biochemical composition, condition index, and meat yield of the non-indigenous pearl oyster Pinctada imbricata radiata(Leach, 1814) from the West of the Aegean Sea, Greece
