Study on Changes of Nitrite Content in Two Kinds of Foods under Different Storage Conditions
Xiulan WU, Feitong ZHUO
Abstract[Objectives] This study was conducted to store and consume overnight vegetables more safely and reasonably. [Methods] Fried cabbage and barbecue was selected as raw materials, which were determine by Griess reagent colorimetry for the effects of storage time, storage temperature and storage method on the changes in nitrite content in the two foods. [Results] The nitrite content in fried cabbage and barbecue increased with the prolongation of storage time. Both types of food, whether stored in a sealed or open manner, had a nitrite content that increased with the storage temperature. When barbecue was stored in an open manner, its nitrite content was greater than that in sealed storage. The condition of stir-fried cabbage was the same as barbecue when stored at 10 ℃, but the situation was opposite when stored at 20 and 30 ℃. In this study, except for the slightly excessive nitrite content in stir-fried cabbage stored at 30 ℃ in open and sealed conditions for 72 h, the nitrite contents in both foods under other storage conditions all met the national standard limit. [Conclusions] This study provides a theoretical basis for the safe and reasonable storage and use of leftovers.
Key wordsNitrite; Storage; Stir-fried cabbage; Barbecue
DOI:10.19759/j.cnki.2164-4993.2023.04.010
Nitrite is the general name of salts containing nitrite ions. Its physical properties are similar to ordinary table salt. It is a white or yellowish powder, slightly salty in taste, and easily soluble in water[1-2]. However, the biggest difference between nitrite and table salt is that nitrite is a highly toxic substance, which can oxidize low-iron hemoglobin that normally carries oxygen in the blood into nitrosohemoglobin, making hemoglobin lose its oxygen carrying capacity, leading to histanoxia. The toxic dose is 0.3-0.5 g, and the lethal dose is 3 g. If the food with high nitrite content is ingested excessively for a long time, such as pickled products or smoked products, nitrite will combine with protein to produce nitrosamine, one of the four major food pollutants, and then induce gastric cancer, esophageal cancer, liver cancer and other cancers. The study of Lei et al.[3] found that nitrite had adverse effects on the reproductive capacity of female mice, such as decreased number of oocytes and damaged polocytes due to extrusion. However, nitrite is still widely used in cooked meat products due to its low price, good antisepsis and color protection. In order to reduce the harm of nitrite to people, Shen et al.[4] studied the coloring effect of monascorubin instead of sodium nitrite in meat products.
With the improvement of life quality, food quality and safety are extremely important, and detecting the content of nitrite common in food poisoning is particularly important. At present, the determination methods of nitrite in food include spectrophotometry (spectrophotometry and fading spectrophotometry), oscillographic polarography, fluorescence analysis method, dry chemical analysis method, colorimetric method, ion chromatography, resonance light scattering method with nuclear fast red, electrochemical sensing methods (potential-based sensors and current-based sensors), etc.[5-6].
In our daily life, we often eat overnight dishes, but overnight dishes contain a certain amount of nitrite. Therefore, in this study, fried cabbage and barbecue were used as raw materials to simulate the situation of storing overnight dishes in life, and the nitrite contents of these two kinds of vegetables were determined by Griess reagent colorimetry (visible spectrophotometry) at 10, 20 and 30 ℃ in both sealed and open conditions. This study provides a theoretical basis for safer and more reasonable storage and consumption of leftovers.
Materials and Methods
Experimental raw materials
Stir-fried cabbage and barbecue were acquired from the fourth canteen of Zhaoqing University.
Experimental methods
Establishment of standard curvesA standard curve was drawn referring to the method of Bai et al.[7].
After the solutions were prepared, they were stood in a dark place for 25 min. A 1.0 cm cuvette was used to perform zero setting with a solution without adding sodium nitrite. Absorbance values were measured at a wavelengthn of 538 nm, and the standard curve was drawn.
Sample treatment and determiantion
Sample treatmentSamples were treated referring to the method of Bai et al.[7]. In specific, a 5 g of sample was ground thoroughly with a mortar and transferred to a beaker, into which 40 ml of distilled water and 8 ml of 20 g/L sodium hydroxide solution were added. After mixing well, the pH of the solution was adjusted to 8 with dilute hydrochloric acid. Next, 6 ml of 0.42 mol/L zinc sulfate solution was added, and the resultant solution was stirred to mix well, and heated in a water bath at 55 ℃ for 15 min. After cooling to room temperature, the solution was diluted with distilled water to 100 ml. After standing for 30 min, filtration was preformed, and 20 ml of the initial filtrate was discarded, while the remaining filtrate was collected for later use and record as the sample treatment solution. Meanwhile, a blank control was set by replacing the sample with 5 g of distilled water.
Sample determinationSample determination referred to the method of Cai[8]. In specific, 10 ml of the above sample treatment solution was added into a 25 ml test tube, into which 4.5 ml of ammonia ammonium chloride buffer (pH=8), 2.5 ml of 60% acetic acid, 2.5 ml of 1 g/L sulfanilic acid solution, 2.5 ml of 10 g/L N-1-naphthylethylenediamin dihydrochloride solution were added. Next, the obtained solution was diluted with water to constant volume, and stood in a dark place for 25 min after mixing well. Next, zero setting was performed with a blank solusion, and the absorbance was measured at 538 nm. The measured absorbance was converted to nitrite mass concentration through the standard curve.
Experimental conditionsIn this experiment, sealed and open storage methods were selected under three temperature conditions: 10, 20, and 30 ℃. Each sample was divided into 6 parts, which were stored in sealed and open conditions at 10, 20 at 30 ℃, respectively. The nitrite content was determined at 0, 24, 48 and 72 h, with 3 repetitions.
Results and Analysis
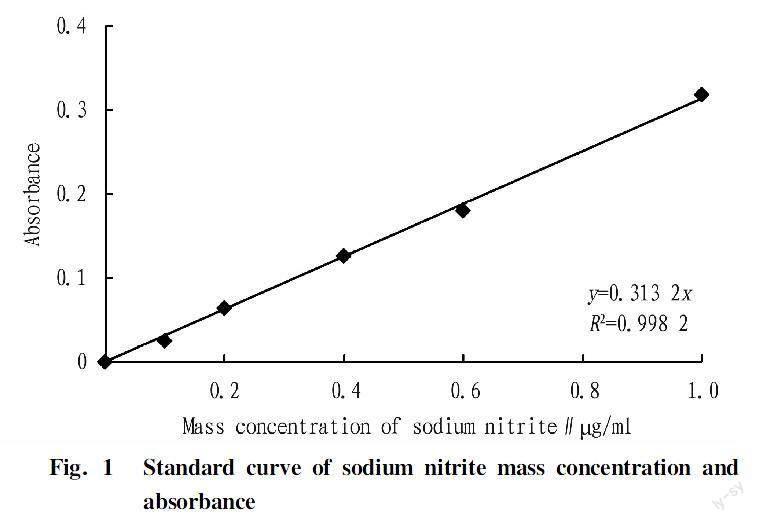
Standard curve of sodium nitrite
The standard curve of sodium nitrite is shown in Fig. 1. Its regression equation was y=0.313 2x, R2=0.998 2, indicating that there was a good linear relationship between the mass concentration of sodium nitrite and the absorbance, which can be used for subsequent experimental analysis.
Sample determination
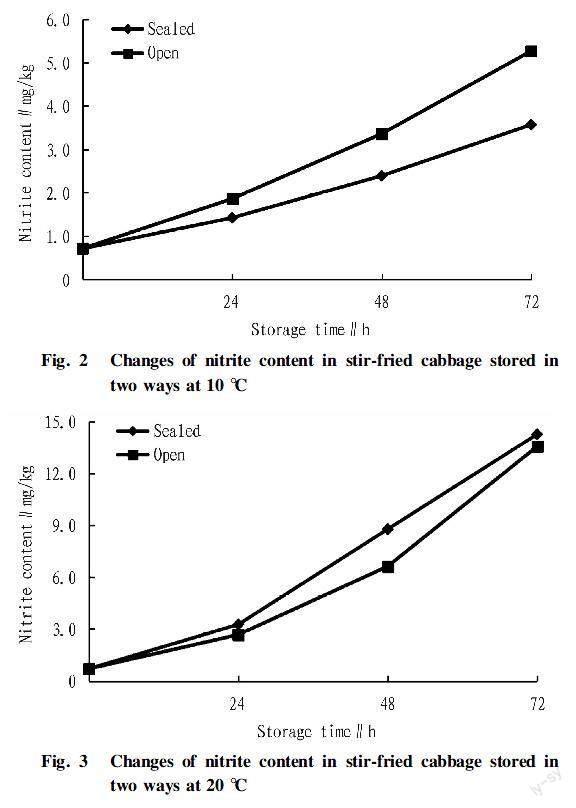
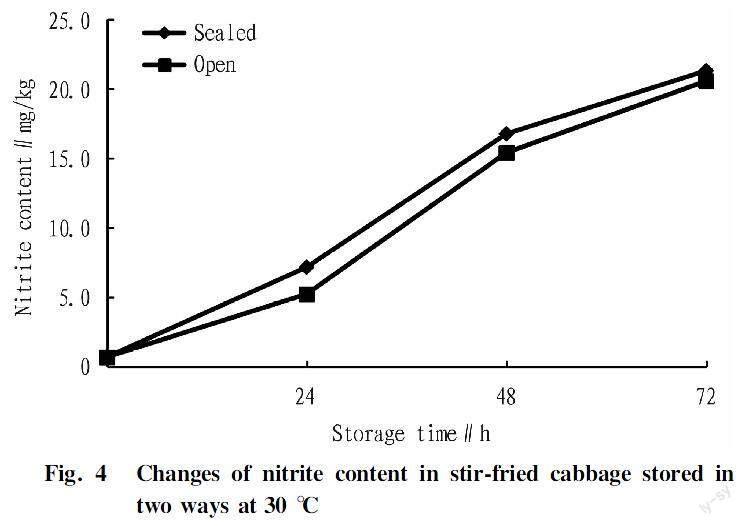
Relation between storage time and nitrite content of stir-fried cabbageIt can be seen from Fig. 2 to Fig. 4 that within 72 h, the nitrite contents of stir-fried cabbage stored in both sealed or open conditions increased with the storage time at all three temperatures of 10, 20 and 30 ℃.
It can be seen from Fig. 2 that under the storage condition of 10 ℃, the maximum nitrite content measured under open storage was 5.27 mg/kg, and the maximum nitrite content measured under sealed storage was 3.57 mg/kg. Both did not exceed the national standard limit of 20 mg/kg[9].
It can be seen from Fig. 3 that under the storage condition of 20 ℃, the maximum nitrite content measured in the open storage condition was 13.56 mg/kg, and the maximum nitrite content measured in the sealed storage condition at 20 ℃ was 14.26 mg/kg. Both did not exceed the national standard limit of 20 mg/kg.
As shown in Fig. 4, the maximum nitrite content measured under open storage at 30 ℃ was 20.53 mg/kg, and the maximum nitrite content measured under sealed storage at 30 ℃ was 21.27 mg/kg. Both the values exceeded the national standard limit of 20 mg/kg.
The reason for the above phenomenon might be that the nitrogen fertilizer used in the planting process of Chinese cabbage was converted into nitrate in its body. During the storage process of fried cabbage, microorganisms might be generated due to pollution and other reasons, and the number of microorganisms was also constantly increasing with the extension of storage time. These microorganisms transformed nitrate contained in the cabbage itself into nitrite.
Relation between storage temperature and nitrite content of stir-fried cabbageIt can be seen from Fig. 2 to Fig. 4 that when the storage temperature was 10, 20 and 30 ℃, the nitrite contents of stir-fried cabbage stored in sealed or open conditions increased with the increase of storage temperature at the same storage time. The maximum nitrite content was found in stir-fried cabbage stored at 30 ℃, and the nitrite content in stir-fried cabbage under sealed storage was 21.27 mg/kg, and the nitrite content in stir-fried cabbage under open storage was 20.53 mg/kg. Both the values exceeded the national standard limit of 20 mg/kg. It could be seen that the higher the storage temperature was, the more unfavorable it was for the storage of fried cabbage.
The reason for this phenomenon might be that the storage of stir-fried cabbage at 10 ℃ was unfavorable to the growth and reproduction of microorganisms in it. Therefore, the nitrite content of stir-fried cabbage at 10 ℃ had little change with time. With the increase of storage temperature, it was more and more beneficial to the growth of microorganisms in fried cabbage. Therefore, the nitrite content in fried cabbage stored at 30 ℃ was the largest, and the change with time was also the largest.
Relation between storage way and nitrite content of stir-fried cabbageAs shown in Fig. 2, at 10 ℃, the nitrite content in fried cabbage stored in the open condition was greater than that in fried cabbage stored in the sealed condition. It can be seen from Fig. 3 and Fig. 4 that at both 20 and 30 ℃, the nitrite content in the fried cabbage stored in sealed conditions was greater than that in the fried cabbage stored in open conditions. Therefore, when it was stored in an environment above 10 ℃, stir-fried cabbage should be stored open. For an environment below 10 ℃, it should be sealed for storage.
The reason for this phenomenon might be that the condition of 10 ℃ was not conducive to the growth and reproduction of microorganisms, and it was also difficult for external microorganisms to invade under the sealed storage condition. Hence, the nitrate contained in the food itself was not easy to be converted into nitrite. Consequently, the nitrite content in stir-fried cabbage stored in the open condition at 10 ℃ was higher than that stored in the sealed condition. With the increase of storage temperature, the growth and reproduction of microorganisms in food gradually accelerated, and the microorganisms that convert nitrate into nitrite are anaerobic microorganisms, so when the stir-fried cabbage was stored at 20 and 30 ℃, the nitrite content in stir-fried cabbage under sealed conditions was greater than the nitrite content in stir-fried cabbage under open conditions.
Relation between storage time and nitrite content of barbecue
It can be seen from Fig. 5 to Fig. 7 that within 72 h, the nitrite content of barbecue stored in both sealed or open conditions increased with the extension of storage time at 10, 20 and 30 ℃, all reaching a maximum value at the 72 h.
The reason for this phenomenon might be that with the increase of storage time, the number of microorganisms produced in barbecue was also more, and these microorganisms would convert nitrate in the barbecue into nitrite. Among them, the maximum nitrite content for barbecue stored in an open manner at 30 ℃ was 6.38 mg/kg, which did not exceed the national standard limit of 30 mg/kg[10], and was lower than the nitrite content in fried cabbage stored at the same temperature.
Relation between storage temperature and nitrite content of barbecueAs shown in Fig. 5-Fig. 7, when the storage temperature was 10, 20 and 30 ℃, the nitrite content of barbecue stored in sealed or open conditions increased with the increase of storage temperature at the same storage time. The maximum nitrite content of barbecue stored under open strage at 30 ℃ for 72 h was 6.38 mg/kg, which did not exceed the national standard limit of 30 mg/kg. It could be seen that the higher the temperature was during storage, the more unfavorable it was for the storage of barbecue.
The reason for this phenomenon might be that for the storage temperature at 10, 20 or 30 ℃, a higher storage temperature was more suitable for the growth and reproduction of microorganisms in barbecue, and more microorganisms were produced during the storage process. These microorganisms would reduce the original nitrate in the barbecue to nitrite[11].
Relation between storage mode of barbecue and nitrite contentIt can be seen from Fig. 5 to Fig. 7 that the nitrite contents in the barbecue under the three temperature conditions were lower in sealed storage conditions than in open storage conditions, so barbecue should be stored in a sealed manner under any conditions.
The reason for this phenomenon might be that under 10 ℃ condition, it was not conducive to the growth and reproduction of microorganisms, and in sealed storage, it was also difficult for external microorganisms to invade. Therefore, the nitrite content of barbecue stored in the open condition at 10 ℃ was higher than that in the sealed condition. As the storage temperature increased, the growth and reproduction rate of microorganisms in food gradually accelerated. During the process of open storage, external microorganisms would enter the barbecue, and the barbecue contained more proteins and other substances, which were beneficial for the reproduction of microorganisms. Therefore, when barbecue was stored at 20 and 30 ℃, the nitrite content in barbecue stored in sealed conditions was less than the nitrate content in barbecue stored in open conditions.
Conclusions and Discussion
In this study, the Griess reagent method was used to determine and explore the changes of nitrite content in two kinds of foods under different storage conditions. From the results of this study, the nitrite content of stir-fried cabbage reached a maximum value when it was stored at 30 ℃ for 72 h. The maximum nitrite content measured under open storage conditions was 20.53 mg/kg, and the maximum nitrite content measured under sealed storage conditions was 21.27 mg/kg, so both the values exceeded the national standard limit of 20 mg/kg. In addition, the nitrite contents of stir-fried cabbage stored at 10 and 20 ℃ for 3 d did not exceed the national standard limit in both ways of sealing and open. The nitrite content of barbecue stored at 10, 20 and 30 ℃ for 3 d also did not exceed the national standard limit in both sealed and open conditions. Therefore, overnight fried cabbage and barbecue should be stored at low temperatures (below 10 ℃) and sealed conditions as much as possible, and the storage time of fried cabbage should not exceed 3 d. Both kinds of foods can be consumed within 3 d without spoilage. With a lower temperature during storage, nitrite is less likely to produce in fried cabbage and barbecue. This conclusion also applies to foods of the same type as stir-fried cabbage or barbecue in daily life, providing a certain basis for food storage.
References
[1] DU LW, YANG HL. A food poisoning event caused by eating sodium nitrite by mistake[J]. Chinese Journal of Urban and Rural Industrial Hygiene, 2009(2): 26. (in Chinese).
[2] HE JK, WANG DD. Clinical analysis of nitrite poisoning[J]. Chinese Community Doctors (medical specialty), 2013, 15(1): 176. (in Chinese).
[3] GE L, HAN Z, GAO YQ, et al. Sodium nitrite negatively affects reproductive ability and offspring survival in female mice[J]. Toxicology, 2019, 427(C): 125-137.
[4] SHENG QL, ZHONG XH, JIN P, et al. Study on nitrite substitutes in meat products[J]. Standard & Quality of Light Industry, 2021(5): 122-123. (in Chinese).
[5] LI SY. Discussion on detection technology of nitrite in food[J]. China Food Safety Magazine, 2021(30): 140-141. (in Chinese).
[6] XI M, ZHANG MY, WANG FK, et al. Research progress on detection methods of nitrite in food[J]. Shanxi Chemical Industry, 2021, 41(3): 29-31. (in Chinese).
[7] BAI XJ, XU SZ. Determination of nitrite using Griess reagent colorimetric method in meat products[J]. Meat Industry, 2008(7): 46-48. (in Chinese).
[8] CAI JN. Study on the change of nitrite content in four kinds of fresh vegetables under different storage conditions[J]. China Food Safety Magazine, 2018(12): 74-75. (in Chinese).
[9] GB 2762-2017. National food safety standards: Quantity of pollutants in food[S]. Beijing: National Health and Family Planning Commission of Peoples Republic of China, China Food and Drug Administration, 2017. (in Chinese).
[10] GB 2760-2014. National food safety standards: Standard for use of food additives[S]. Beijing: National Health and Family Planning Commission of Peoples Republic of China, China Food and Drug Administration, 2014. (in Chinese).
[11] ZHANG N, LIU T, ZHANG Z, et al. Discussion on the detection of meat products without adding nitrite[J]. Meat Industry, 2020(5): 36-37. (in Chinese).
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Optimization of Extraction Conditions for Total Flavonoids from Fructus Aurantii Immaturus and Its Anti-UVB Radiation Activity
- Research on Maize Seed Classification Method Based on Convolutional Neural Network
- Common Species Distribution Models in Biodiversity Analysis and Their Challenges and Prospects in Application
- Vegetable Tunnel House Technology in Tropical Island Countries
- Purification and Characterization of Hyaluronate Lyases Produced by Two Types of Bacteria
- Renewable Energy Seawater Desalination Technology and Application Analysis

