Optimization of Extraction Conditions for Total Flavonoids from Fructus Aurantii Immaturus and Its Anti-UVB Radiation Activity
Xu CHANG,Yanhui WANG, Ximing LIU, Yunge MA, Yunhao LIU, Xiaobo CHEN, Mengyao SUN, Liyan LI

Abstract[Objectives]Optimum extraction conditions of total flavonoids from Fructus Aurantii Immaturus (TFFAI) and its resistance activity to ultraviolet radiation were investigated in present research. [Methods] The optimal extraction conditions of TFFAI were determined by single factor and orthogonal experiments, and the survival rate of TFFAI on HaCaT cells irradiated with UVB rays was investigated.Its antioxidant capacity was determined by ABTS. [Results] The results showed that the highest yield of TFFAI was obtained with 70% ethanol at a solid-to-liquid ratio of 1:50 (w/v) and 40 ℃ for 1.5 h by single-factor and orthogonal experiments. Total flavonoids (0.25-1.00 mg/ml) could significantly improve the survival rate of HaCaT cell line. Meanwhile, the maximum absorption peak of TFFAI was found at 283 nm, and in-vitro antioxidant experiment identified that TFFAI had a good clearance rate to ABTS.It suggestes that TFFAI could protect the cells from UVB damage by absorption of UVB rays and anti-oxidation. [Conclusions] TFFAI played a protective role on UVB irradiated cells through UVB ultraviolet absorption and antioxidant pathways.
Key wordsFructus Aurantss Immaturus; Total flavonoids; Optimization of extraction conditions; UVB radiation resistance
DOI:10.19759/j.cnki.2164-4993.2023.04.024
Bitter orange is the dried young fruit of lime and its cultivated varieties or sweet orange in the family Rutaceae. The main active components of bitter orange are phenolic acids or flavonoids, volatile oils and alkaloids[1-4], which have the effects of liver protection, antioxidation, antibacterial, antitumor, hypoglycemic and antianxiety[5-10]. Among them, fat-soluble citrus biofanones are unique polymethoxyflavonoids (PMFs) of citrus, which have stronger biological activity than general flavonoids.
More and more people are suffering from skin photodamage caused by excessive ultraviolet radiation due to the aggravation of ozone layer destruction[5], and sunscreens are the most effective way to prevent skin from photodamage. However, in view of distruction disadvantages of traditional sunscreens,it is urgent to develop plant-based sunscreens with no toxic side effects.Many researchers have reported about plant origined UV filters,such as Lonicera caerulea L, Bilberry fruit polyphenols, Codium fragile, green tea polyphenols, Sophora japonica L, blackberries, raspberries, Coffea Arabica, Ginkgo Biloba L, Vitis vinífera L, Amorphophallus,Litchi chinensis, Hylocereus polyrhizus, solanum nigrum, Guava-fruit, Perilla frutescens, etc.[11-23],but the anti-UV ability of TFFAI has not been reported.
In present research, the anti-UVB irradiation ability of TFFAI was investigated in human epidermal cell model.It is meaningful for developing plant origined products as UVB fiter for sunscreens.
Materials and Methods
Materials and Reagents
Fructus aurantii was purchased from the standardized demonstration area of ecological cultivation of Fructus aurantii in Hunan Province, and dried at 50-55 ℃, then crushed at 100-200 mesh. 98% hesperidin analytical standards were purchased from Aladdin. Human immortalized epidermal cell line HaCaT was purchased from Wuhan Procell Life Technology Co.,LTD.Penicillin-streptomycin DMEM medium, trypsin-EDTA, and others were purchased from Life Science Co.,LTD.Absolute ethanol and concentrated sulfuric acid were analytically pure.
Experimental Methods
Drawing of new hesperidin standard curve0.01 g of the new hesperidin standard was prepared with 60% ethanol solution to concentrations of 10, 20, 30, 40, 50 and 60 μg/ml, respectively. The absorbance of each standard solution was measured by a UV-VIS spectrophotometer at the wavelength of 283 nm. The regression equation was obtained by drawing a standard curve with absorbance as the ordinate and concentration as the abscission.
Determination of TFFAIAccording to the maximum absorption at 283 nm of neohesperidin and TFFAI extract, the content of TFFAI extract was determined by ultraviolet spectrophotometry with neohesperidin as the standard. The egression equation was C=30.91A-1.381 4 (correlation coefficient R2=0.999 9).
Formula for calculation of total flavonoid content:
P (%)=c×V1×nW×106×100
where c is the concentration of total flavonoids (μg/ml) calculated from the regression equation obtained from the new hesperidin standard curve; V1 is the total volume (ml) of immature orange extract; n is dilution factor; W is the feed amount of immature orange (g).
Optimization of extraction conditions for TFFAI
Single factor experimentThe basic conditions of single factor experiments were 60% of ethanol,solid-to-liquid ratio as 1:20 (w/v),60 ℃ and 1 h, and then the single variables such as ethanol concentration (50%, 60%, 70%, 80%, 90%), solid-to-liquid ratio (1:10, 1:20, 1:30, 1:40, 1:50 g/ml), temperature (40, 50, 60, 70, 80 ℃), and time (0.5, 1.0, 1.5, 2.0, 2.5 h). Four single factors were used as variables to investigate effect on the extraction rate of TFFAI.
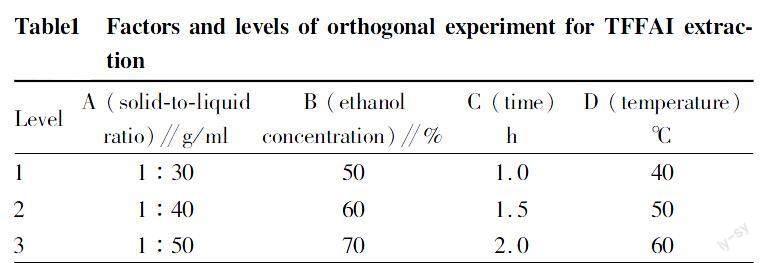
Orthogonal experimentAccording to the results of single factor experiments, solid-to-liquid ratio (A), ethanol concentration (B), time (C) and temperature (D) were used as the investigation levels (Table 1), and the extraction rate of TFFAI was used as the index to conduct an L9(34) orthogonal experiment (Table 2), and the results were verified by the experiment.
Qualitative analysis of the extract
The standard substance of TFFAI extract was scanned under UV using a UV-vis spectrophotometer in the wavelength range from 200 to 450 nm.
Cytotoxicity test of TFFAI on HaCaT cells
Human immortalized epidermal cells were cultured in MEM complete medium (37 ℃, 5% CO2) containing 10% fetal bovine serum to 80% confluence and seeded into 96-well plates at a density of 5×104 cells/ml. On the next day, the medium containing serial concentrations of TFFAI (0.125-4.000 mg/ml) were changed to continue to culture for 24 h. Then, the culture medium containing 10% CCK-8 was changed for another 0.5 h, and the OD450 value was measured. Cell survival rate was calculated according to Cell survival rate=(OD drug group /OD normal control group)×100%, and the cell survival rate of normal control group was set as 100%.
Protective effect of TFFAI on HaCaT cells irradiated by UVB
Cells grown to a confluence of 80% were seeded at a density of 5×104 cells/ml into a 96-well plate at 100 μl per well. The next day, the cells were divided into three groups: normal control group, UVB radiation group (30-120 mJ/cm2), UVB radiation and TFFAI group (0.125, 0.25, 0.5, 1 mg/ml). After the cells were incubated with the medium containing serial concentrations of TFFAI for 2 h, the medium was removed and replaced with 30 μl of MEM medium for cell infiltration. To prevent cell damage caused by the heat generated by UV irradiation, the culture plate was placed on ice. After exposure to different doses of UVB (30, 60, 90, 120 mJ/cm2) (UVB tube, 312 nm, irradiation intensity of 210 μw/cm2), fresh medium was added to 100 μl, and the cells were further cultured for 24 h. After 0.5 h of incubation with 10% CCK-8 medium, the cells were cultured for 24 h. The OD450 value was determined. Cell viability was calculated.
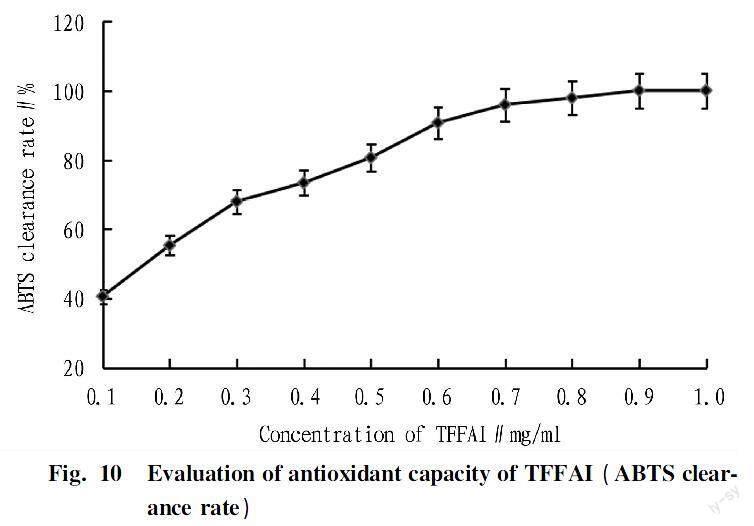
Evaluation of antioxidant capacity of TFFAI (ABTS) in vitro
According to the determination method of Wang Hui et al.[24], ABTS working solution was prepared with slight changes, and an appropriate amount of TFFAI was weighed. Sample solutions with concentrations of 0.1-1.0 mg/ml were prepared with 60% ethanol solution, and then placed in a test tube with ABTS working solution at the amount of 1:100. After mixing, the reaction was carried out at 30 ℃ for 10 min, and the absorbance at 734 nm was measured.
Statistical analysis
Experimental data were expressed as mean±SEM. ANOVA and T test were used for data analysis. P<0.05 was considered as significant difference, and P<0.01 was considered as extremely significant difference.
Results and Discussion
New hesperidin standard curve
The new hesperidin standard curve (Fig. 1) was drawn, and the regression equation was y=0.032 3x+0.044 8, R2=0.999 9.
Single factor experiments on extraction of TFFAI
Effect of ethanol concentration on extraction yield of TFFAIThe effect of ethanol concentration on the extraction yield of TFFAI was tested under the conditions of solid-to-liquid ratio as 1:20 (w/v), 50 ℃ and 1 h with only ethanol concentration changed, and the results are shown in Fig. 2. According to the figure, the extraction rate increased first and then decreased with the increase of ethanol concentration, and the extraction rate of TFFAI was the highest under the condition of ethanol concentration of 60%.
Effect of solid-to-liquid ratio on extraction yield of TFFAIThe effect of solid-to-liquid ratio on the extraction yield of TFFAI was tested at 60% ethanol concentration, 50 ℃ and 1 h, and the results are shown in Fig. 3. According to the figure, the extraction rate gradually increased with the increase of solid-to-liquid ratio and then decreased slightly. The increase of solvent amount is conducive to the full dissolution of the effective components, but too large solid-to-liquid ratio will cause the waste of solvent and energy, so the optimal solid-to-liquid ratio of 1:40 (w/v) was determined.
Effect of temperature on extraction yield of TFFAIThe effect of temperature on the extraction yield of TFFAI was tested under the conditions of ethanol concentration of 60%, solid-to-liquid ratio of 1:20 (w/v), and water bath time of 1 h. The results are shown in Fig. 4. According to the figure, with the increase of temperature, the extraction rate of TFFAI gradually increased first, then decreased and then increased, and the extraction rate of TFFAI was the highest at the temperature of 50 ℃.
Effect of extraction time on the extraction yield of TFFAIThe effect of extraction time on the extraction yield of TFFAI was tested at ethanol concentration of 60%, solid-to-liquid ratio of 1:20 (w/v), and temperature of 50 ℃, and the results are shown in Fig. 5. According to the figure, the extraction rate at 1.5 h (9.7%) decreased slightly, and the extraction rate at 2.5 h (9.93%) increased again, but the range was not large. The optimal extraction time was 1.5 h from the perspective of energy saving.
Optimization of extraction condition of TFFAI
Orthogonal experimental design and resultsThe L9 (34) orthogonal test was designed on the basis of the single factor test results, and the results and range analysis are shown in Table 2.
According to the R value in Table 2, the order of the effects of solid-to-liquid ratio, ethanol concentration, time and temperature on the extraction rate of TFFAI was A>C>D>B, that is, solid-to-liquid ratio > time > temperature > ethanol concentration. The optimal extraction condition of TFFAI was A3C2D1B3, which was extracting with 70% ethanol at a solid-to-liquid ratio of 1:50 (g/ml) at 40 ℃ for 1.5 h. The TFFAI extraction yield was 19.14%±1.56% under these conditions.
Preparation of total flavonoids from Fructus Aurantii Immaturus
According to the best extraction conditions obtained under "Orthogonal experimental design and results", the TFFAI were extracted, and the combined extraction supernatant was collected, distilled and concentrated under reduced pressure, and freeze-dried in vacuum to obtain the dry powder of TFFAI.
Qualitative analysis of extract
The full UV scanning patterns of standard and immature orange extract sample solutions are shown in Fig. 6 and Fig. 7.
There were obvious peak at 283nm in UV scanning specturum of standard(Fig.6) and the samplesolution (Fig.7), indicating that the extract contained neohesperidin.
Evaluation of cytotoxicity of TFFAI
The viability of HaCaT cells was determined by CCK-8 assay after incubation of cells with TFFAI for 24 h, and the results are shown in Fig. 8. According to the figure, the cytotoxicity of TFFAI increased following with the increased concentration, so the maximum concentration of TFFAI in subsequent experiments was 1 mg/ml.
Evaluation of anti-UV radiation activity of TFFAI
The effect of TFFAI on the survival rate of HaCaT cells under UVB irradiation was shown in Fig. 9. According to the figure, the cell survival rate decreased significantly with the increase of UVB irradiation dose, and the cell survival rate was only 41.83% at 120 mJ/cm2. However, pretreatment with TFFAI could significantly improve the cell survival rate, and the protective effect was more obvious with the increase of irradiation dose. 1mg/mL TFFAI could increase the cell survival rate from 46.42% to 69.23% in 90 mJ/cm2 group.In addition, from the results of cytotoxicity, 1 mg/ml of TFFAI was still poisonous to the cells, and the cell survival rate was only 35.12%, but the UVB radiation on cell survival and UV scanning results showed that the TFFAI had strong ultraviolet absorption ability in UVB segment.The treated epidermal cells preferentially absorbed UVB rays when receiving ultraviolet radiation, reduced the damage of ultraviolet radiation to cells, and greatly improved the survival rate of cells. Therefore, the anti-ultraviolet radiation activity of TFFAI was confirmed.
Evaluation of antioxidant capacity of TFFAI in vitro
The antioxidant capacity of TFFAI was determined by ABTS clearance rate, and the results are shown in Fig. 10. The clearance rate of ABTs was 97.85% when TFFAI concentration was 0.8 mg/ml, and 100% when TFFAI concentration was 0.9 mg/ml. These results indicated that TFFAI had strong antioxidant capacity.
In recent years, scholars have focused on extracting effective ingredients from natural resources that can reduce ultraviolet light damage and enhance skin protection, such as Lonicera caerulea L, Bilberry fruit polyphenols, Codium fragile, green tea polyphenols, Sophora japonica L, blackberries, raspberries, Coffea Arabica, Ginkgo Biloba L, Vitis vinífera L, Amorphophallus, Litchi chinensis, Hylocereus polyrhizus, solanum nigrum, Guava-fruit, Perilla frutescens, etc.[11-23]. Among them, polyphenols or flavonoids have excellent absorption ability to ultraviolet rays in the 280-320 nm band. Zhang et al. [25] also concluded that the maximum absorption value of UVB zone of 30 common Chinese herbal medicine chemicals is mostly between 280 and 320 nm. Li et al.[30] summarized the research on UV-resistant components from plants in the past five years and found that most of them are flavonoids. The maximum UV absorption peak of total flavonoids in the present study was also in this range, indicating that it had UV absorption ability. Studies on total flavonoids in passion fruit, leve, kiwi fruit, jujube, green tea and other components[26-29] have found that they have strong antioxidant capacity, thereby reducing intracellular oxidative damage caused by UVB radiation and showing anti-UV radiation activity. By in vitro antioxidant experiment shows the optimal ABTS clearance level was obtained with 0.9 mg/ml TFFAI in this study, at the same time by cytology have confirmed that its cells and dermis of UVB radiation has a good protective effect.These tips for health food of the code is sunscreen function development and application of Chinese medicine has a good potentiality.This study provides a certain research basis for the screening and development of plant sunscreens and clinical drug candidates for the treatment of skin photodamage. The specific mechanism of the protection of TFFAI against UV light damage needs to be further studied.
Conclusion
In this study, the yield of TFFAI was the highest (19.14%) under the conditions of solid-to-liquid ratio 1:50 (w/v), 1.5 h, 40 ℃ and 70% ethanol concentration. Cytological experiments showed that TFFAI pretreatment could significantly improve the survival rate of UVB-irradiated cells, 1 mg/ml TFFAI could increase 22.81% of the survival rate on 90mJ/cm2 dose group. These results indicate that TFFAI has good anti-UVB radiation activity. Meanwhile,TFFAI had the strongest absorption peak at 283 nm, and has a good clearance ability to ABTS. Therefore, TFFAI may played a protective role on UVB-irradiated cells through both UVB ray absorption and antioxidant pathways.
References
[1] JABRI KAROUI I, MARZOUK B. Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activity[J]. BioMed Res Int, 2013(2013): 345415.
[2] GONZáLEZ-MAS MC, RAMBLA JL, LPEZ-GREASA MP, et al. Volatile compounds in citrus essential oils: A comprehensive review[J]. Front Plant Sci, 2019(10): 1-18.
[3] SUNTAR I, KHAN H, PATEL S, et al. An overview on Citrus aurantium L.: Its functions as food ingredient and therapeutic agent[J]. Oxid Med Cell Longev., 2018(2018): 7864269.
[4] SINGH B, SINGH JP, KAUR A, et al. Insights into the chemical composition and bioactivities of citrus peel essential oils[J]. Food Res Int, 2021(143): 110231.
[5]WU JZ, HUANG GR, Li YJ, et al. Flavonoids from Aurantii Fructus Immaturus and Aurantii Fructus: Promising phytomedicines for the treatment of liver diseases [J]. Chinese Medicine, 2020(15): 89-106.
[6] DIVYA PJ, JAMUNA P, JYOTHI LA. Antioxidant properties of fresh and processed Citrus aurantium fruit[J]. Cogent Food Agric, 2016(2):1184119.
[7] KARABIYIKLI S, DEˇGIRMENCI H, KARAPINAR M. Inhibitory effect of sour orange (Citrus aurantium) juice on Salmonella typhimurium and Listeria monocytogenes[J]. LWT Food Sci Technol, 2014(55): 421-425.
[8] LIM SW, LEE D R, CHOI B K, et al. Protective effects of a polymethoxy flavonoids-rich Citrus aurantium peel extract on liver fibrosis induced by bile duct ligation in mice[J]. Asian Pac J Trop Med, 2016(9): 1158-1164.
[9] KIM GS, PARK HJ, WOO JH, et al. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells[J]. BMC Complement Altern Med, 2012(12): 31.
[10] CHAVES NETO GC, BRAGA JEF, ALVES MF, et al. Anxiolytic effect of Citrus aurantium L. in Crack Users. Evid. based complement[J]. Evid Based Complement Alternat Med, 2017(2017): 7217619.
[11] LEE C, PARK GH, AHN EM, et al. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB /c mice[J]. Fitoterapia, 2013(86): 54-63.
[12] VOSTALOVA J, ZDARILOVA A, SVOBODOVA A. Prunella vulgaris extract and rosmarinic acid prevent UVB-induced DNA damage and oxidative stress in HaCaT keratinocytes[J]. Arch Dermatol Res, 2010(302): 171- 181.
[13] EIMETS CA, SINGH D, TUBESING K, et al. Cutaneous photoprotection from ultraviolet injury by green tea poly-phenols[J]. J Am Acad Dermatol, 2001(44): 425-432.
[14] LI LY, HUANG T, LAN C, et al. Protective effect of polysaccharide from Sophora japonica L. flower buds against UVB radiation in a human keratinocyte cell line (HaCaT cells)[J]. Journal of Photochemistry & Photo-biology, B: Biology, 2019(191): 135-142.
[15] CEFALI LC, FRANCO JG, NICOLINI GF, et al. In vitro antioxidant activity and solar protection factor of blackberry and raspberry extracts in topical formulation[J]. J Cosmet Dermatol, 2019, 18(2): 539-544.
[16 ] CHO YH, BAHUGUNA A, KIM HH, et al. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells[J]. J Photochem Photobiol B, 2017(174): 323-332.
[17 ] CEFALI LC, ATAIDE JA, FERNANDES AR, et al. Evaluation of in vitro solar protection factor (SPF), antioxidant activity, and cell viability of mixed vegetable extracts from Dirmophandra mollis Benth, Ginkgo Biloba L, Ruta Graveolens L, and Vitis vinífera L[J]. Plants, 2019, 8(11): 453-465.
[18] CEFALI LC, ATAIDE JA, SOUSA IMO. In vitro solar protection factor, antioxidant activity, and stability of a topical formulation containing Benitaka grape (Vitis vinifera L.) peel extract[J].Nat Prod Res, 2020, 34(18): 2677-2682.
[19] THIESEN LC, BRETZKE PE, BITTENCOURT CMDS, et al. Litchi chinensis leaf extract provides high in vitro photoprotection associated to a natural mineral clay[J]. Photodermatol Photoimmunol Photomed, 2020, 36(1): 61-62.
[20] VIJAYAKUMAR R, ABB GANI SS, ZAIDAN UH, et al. Exploring the potential use of Hylocereus polyrhizus peels as a source of cosmeceutical sunscreen agent for its antioxidant and photoprotective properties[J]. Evid Based Complement Alternat Med, 2020(2020): 7520736.
[21] SALEEM MA, NAZIR A, NAZIR F, et al. Comparison of UV protection properties of cotton fabrics treated with aqueous and methanolic extracts of Solanum nigrum and Amaranthus viridis plants[J]. Photodermatol Photoimmunol Photomed, 2019, 35(2): 93-99.
[22] MOTA MD, COSTA RYS, GUEDES AAS, et al. Guava-fruit extract can improve the UV-protection efficiency of synthetic filters in sun cream formulations[J]. J Photochem Photobiol B, 2019(201): 111639.
[23] CHOI HJ, SONG BR, KIM JE, et al. Therapeutic effects of cold-pressed perilla oil mainly consisting of linolenic acid, oleic acid and linoleic acid on UV-induced photoaging in NHDF cells and SKH-1 hairless mice[J]. Molecules, 2020(25): 989-1007.
[24] WANG H, ZHOU Y. The method of ABTS assay for screening and evaluating antioxidant[J]. Guangzhou Chemical Industry, 2012, 40(22): 41-43.
[25] ZHANG H, ZHANG G, CHEN J. Study on the sunproof potential for thirty kinds of chemical constituents from Chinese herb medicine[J]. China Pharmacist, 2010, 13(7): 935-938. (in Chinese).
[26] ZHANG XL, LI SY, SUN JN, el al. Optimization of extraction process and antioxidant activity of total flavonoids from passion fruit peel by response surface methodology[J/OL]. Feed Research, 2022(16): 83-88. (in Chinese).
[27] HU XH, LONG JF, YANG SZ, et al. Study on toal flavonoids content and antioxidant activity of Allium tuberosum in three regions of southeast Guizhou[J]. Journal of Kaili University, 2022, 40(3): 67-74. (in Chinese).
[28] LI JH, WENG GY, ZHU M, et al. Microwave-assisted extraction and antioxidant activities of total flavonoids from kiwi fruit residues[J]. Food Research and Development, 2022, 43(12): 79-85. (in Chinese).
[29] ZAHRA ABOTORABIL, MOHSEN KHORASHADIZADEH, MINA ARAB, et al. Jujube and green tea extracts protect human fibroblast cells against UVB-mediated photo damage and MMP-2 and MMP-9 production[J]. Avicenna Journal of Dhytpmedicine, 2020, 10(3): 287-296.
[30] LI LY, LAN C, HUANG T, et al. Natural products and extracts from plants as natural UV filters for sunscreens: A review [J]. Animal Model and Experiment Medicine, 2022(00): 1-13.
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Research on Maize Seed Classification Method Based on Convolutional Neural Network
- Common Species Distribution Models in Biodiversity Analysis and Their Challenges and Prospects in Application
- Vegetable Tunnel House Technology in Tropical Island Countries
- Purification and Characterization of Hyaluronate Lyases Produced by Two Types of Bacteria
- Renewable Energy Seawater Desalination Technology and Application Analysis
- Impact of Water Quality Sampling Process on Environmental Monitoring Results

