Effects of Polygonatum sibiricum Polysaccharide on Antioxidant Capacity of the Liver in High-fat Diet Rats
Heyi GONG, Zhifeng LI, Chenzhong JIN, Yihong HU, Yan WANG
Abstract[Objectives] This study was conducted to investigate the effects of Polygonatum sibiricum polysaccharide (PSP) on antioxidant function in high-fat diet obese rats. [Methods] Thirty five healthy male SD rats were selected to establish an obesity model after feeding a high-fat for 8 weeks. They were then randomly divided into a normal group (NC), a high-fat diet group (HF), and an HF+P. sibiricum polysaccharide group [HF+PSP, 300 mg/(kg·d)]. After 6 weeks of PSP intervention, the serum and liver of rats were collected, and the activity of aspartate transaminase (AST) and alanine aminotransferase (ALT) in serum, the enzyme activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) in liver tissue were measured. The pathological changes of liver tissue were observed by HE staining. [Results] Compared with the HF group, PSP could effectively inhibit obesity caused by high-fat diet. It reduced body weight and serum AST and ALT levels, increased the contents of T-SOD, CAT and GSH-Px in the liver, and inhibited the accumulation of MDA content, thereby reducing damage to liver cells caused by a high-fat diet. It indicated that PSP could effectively inhibit obesity in high-fat diet rats and enhance their antioxidant capacity. [Conclusions] This study provides a reference for the study of the antioxidant capacity of PSP.
Key wordsPolygonatum sibiricum polysaccharide; High-fat diet; Obesity; SD rat
DOI:10.19759/j.cnki.2164-4993.2023.04.003
Obesity is a global epidemic caused by multiple factors. According to relevant reports, it is estimated that by 2025, more than half of global deaths will be caused by obesity and related diseases[1]. At present, the main methods for treating obesity include changing lifestyle habits, increasing exercise, medication treatment, and weight loss surgery, but the effects are not satisfactory, especially for Western medicine treatment, which can cause serious side effects[2]. Therefore, it is particularly important to develop safe and effective natural products to treat obesity. As a kind of natural product, the function of plant polysaccharides has become a research hotspot in recent years. Numerous literature studies have found that polysaccharides have antioxidant[3], anti-tumor[4], liver-protection[5] and anti-obesity effects[6]. Several kinds of natural polysaccharides have been proven to play important roles in clearing free radicals, preventing oxidative damage, and inhibiting obesity, and can serve as new potential antioxidants[7-9].
Polygonatum sibiricum polysaccharide (PSP) is one of the main active ingredients of P. sibiricum, which has been widely used in clinical treatment of diabetes[10], osteoporosis[11] and atherosclerosis[12]. It has functions such as immune-regulation, anti-tumor, anti-fatigue, and antioxidant[13-15]. At present, research on the antioxidant properties of PSP mainly focuses on in-vitro antioxidant activity[16-17] and in-vivo aging models[4,15], but there are few reports on the oxidative stress induced by obesity caused by high-fat diet. Therefore, in this study, with SD (sprague dawley) male rats with oxidative stress induced by obesity due to high-fat diet as the object of study, the effects of PSP on the activity of aspartate transaminase (AST) and alanine aminotransferase (ALT) in serum, the enzyme activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-P) and malondialdehyde (MDA) content in liver tissue and pathological sections of the liver, aiming to provide reference for the research on antioxidant capacity of PSP.
Materials and Methods
Animals
Thirty SPF male healthy SD rats (body weight: 250-280 g), purchased from Hunan SJA Laboratory Animal Co., Ltd. (license number: SCXK (Xiang) 2016-0002); ordinary feed, provided by Hunan SJA Laboratory Animal Co., Ltd. (10% kcal fat, 3.6 kcal/g); high-fat feed, provided by Beijing Botai Hongda Biotechnology Co., Ltd. (34% kcal fat, 4.6 kcal/g).
Drugs and reagents
P. sibiricum polysaccharide (PSP) was separated and purified from P. sibiricum. Adopting the alkali-extraction and alcohol-precipitation method, the PSP was obtained through Sevag separation and purification. PSP is mainly composed of five monosaccharides, namely galactose, arabinose, rhamnose, xylose and glucose. The total sugar content in the sample was measured to be about 51.66% by the phenol-sulfuric acid method[18].
Superoxide dismutase (SOD) kit, catalase (CAT) kit, malondialdehyde (MDA) kit, glutathione peroxidase (GSH-Px) kit, alanine aminotransferase (ALT) kit, and aspartate transaminase (AST) kit were all purchased from Nanjing Jiancheng Bioengineering Institute.
Methods
Establishment of rat obesity modelThirty five male SD rats were randomly divided into two groups: a normal group (NC, n=10) and a high-fat diet group (HF, n=25). The NC group was fed on a normal diet and the HF group had a high-fat diet. After 8 weeks of feeding, 20 rats with an average body weight exceeding 20% of the average body weight of the NC group, were selected from the HF group. The selected rats were re-divided into an HF group and an HF+PSP group, with 10 animals in each group.
Drug administrationNext, the HF+PSP group was administered at a dose of 300 mg/kg[18]. For the NC and HF groups, corresponding volumes of normal saline were administered by gavage. After 6 weeks of administration, rats stayed overnight on an empty stomach, and all rats were anesthetized with pentobarbital sodium, and blood was extracted from the abdominal aorta to separate serum for centrifugation. The bodies were dissected to separate the liver and perirenal fat, which were stored in a refrigerator at -80 ℃ for later use.
Body weightBefore and after modeling, the weights of rats were measured once a week and recorded.
Effects on liver functionAccording to the instructions of the reagent kit, the levels of ALT and AST in serum were measured using an automatic biochemical analyzer.
Liver oxidation indexesFirstly, a liver tissue homogenate was prepared according to the ratio of liver:physiological saline=1:9. The obtained homogenate was centrifuged to get a supernatant, which was determined for SOD, CAT and GSH-Px enzyme activity and MDA content according to the instructions of the reagent kit.
Pathological analysis of liver tissueFirst, the liver tissue was fixed with formalin, and then eluted with gradient ethanol. Next, the tissue was transparentized with xylene and embedded with paraffin. The embedded paraffin blocks were cut into pieces approximately 5 μm thick. Finally, the pieces were dehydrated with ethanol solutions of different concentrations, and re-stained with an eosin solution and transparentized with xylene. The prepared sections were observed under an optical microscope for the staining conditions, and their degree of lipid accumulation was evaluated.
Statistical methods
SPSS 21.0 software was used for significance analysis, and the experimental results were presented in the form of (x±s). The comparison of the mean values between the two groups of samples was conducted by an independent-samples t test, with P<0.05 being significant and P<0.01 being extremely significant.
Results and Analysis
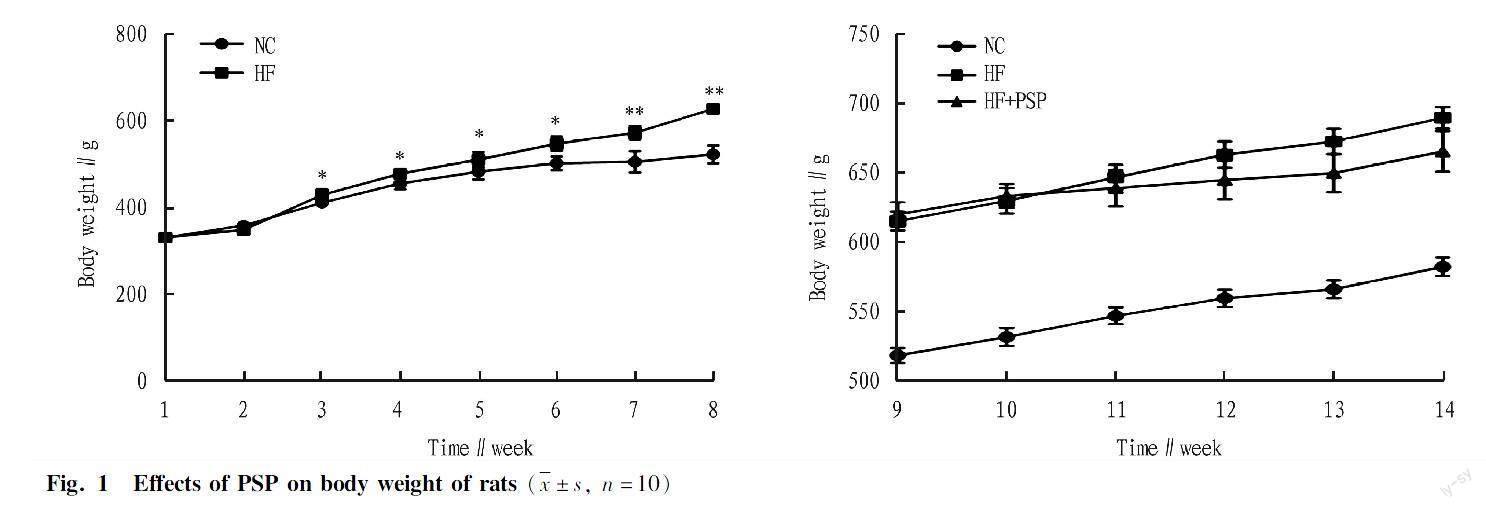
Effects of PSP on body weight of rats fed a high-fat diet
As shown in Fig. 1, after 8 weeks of high-fat diet, the weight of rats in the HF group was (620.3±7.32) g, significantly higher than that in rats of the NC group by 20.23%. After 6 weeks of treatment with PSP, the weight gain of rats in the HF+PSP group significantly decreased (P<0.01). It indicated that PSP could inhibit the formation of obese rats with a high-fat diet.
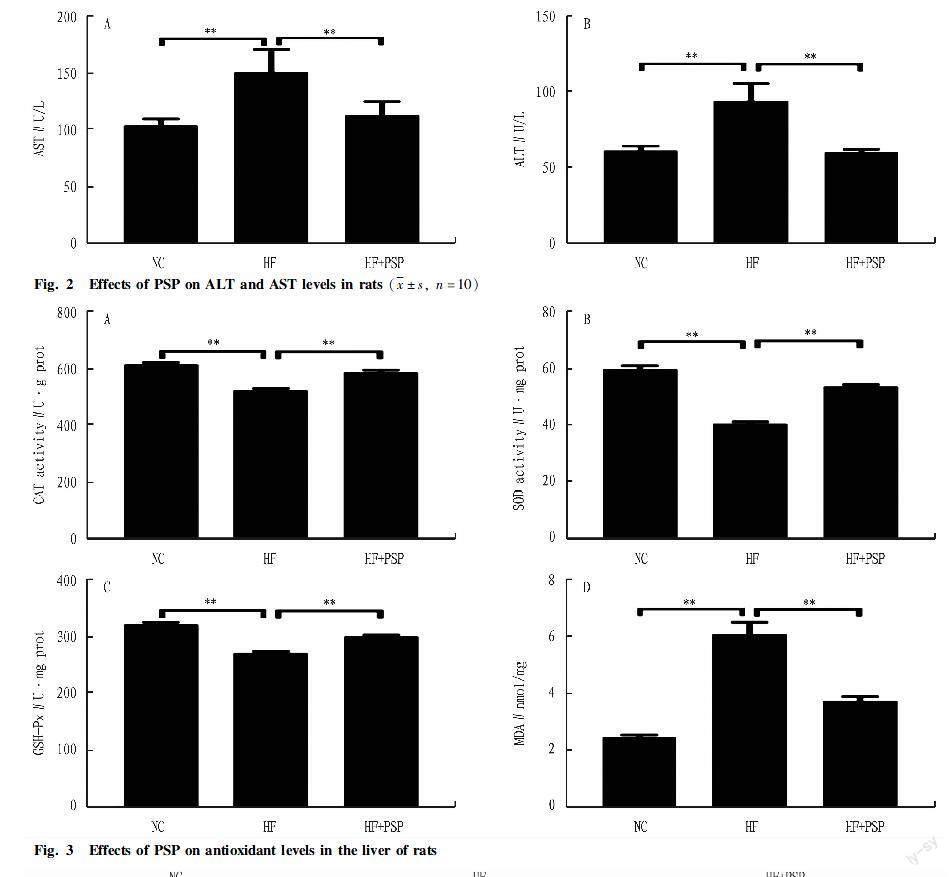
Effects of PSP on liver function enzymes of rats fed a high-fat diet
Fig. 2 shows that a high-fat diet significantly induced an increase in ALT and AST levels in rats of the HF group (P<0.01), and after 6 weeks of intervention with PSP, the increase in serum ALT and AST levels caused by a high-fat diet could be significantly reduced (P<0.01). It indicated that PSP alleviated liver function damage caused by a high-fat diet.
Effects of PSP on activity of antioxidant enzymes in the liver of rats fed a high-fat diet
As shown in Fig. 3, compared with the NC group, the activity of antioxidant enzyme (CAT, SOD, and GSH-Px) of the HF group decreased significantly (P<0.01), while the MDA content significantly increased (P<0.01). After 6 weeks of intervention with PSP, the activity of antioxidant enzymes in high-fat diet rats significantly increased (P<0.01), and the MDA content in the liver decreased (P<0.01). It indicated that PSP enhanced the antioxidant capacity of rats fed a high-fat diet.
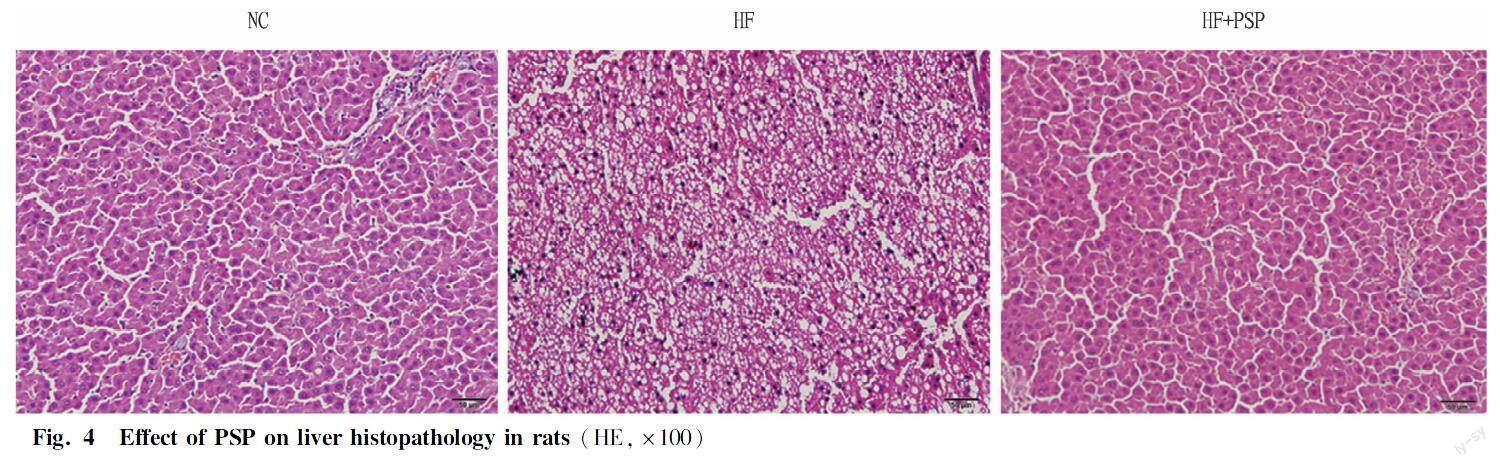
Effects of PSP on liver pathological sections of obese rats fed a high-fat diet
Fig. 4 shows that compared with the normal group, the liver sections of rats in the model group showed significant vesicular degeneration and a significant increase in lipid droplets. With the increase of intervention dose of PSP, vesicular degeneration was improved, and lipid droplets in the liver of rats were reduced, and cell arrangement became more orderly. It further showed that PSP alleviated liver function damage caused by high-fat diet.
Agricultural Biotechnology2023
Discussion and Conclusions
As a chronic metabolic disease, obesity is prone to cause oxidative stress, which eventually induces various metabolic disorders such as diabetes, cardiovascular disease and cancer. Some studies have shown that long-term high-fat diet will lead to the increase of reactive oxygen species in the body, causing a series of metabolic disorders, and the pathological mechanisms of these metabolic disorders can be understood as being caused by oxidative stress injury induced by high-fat diets[19]. The results of this study further prove that a high-fat diet is easy to cause oxidative damage. At present, the research on PSP mainly focuses on their extraction, separation and purification, and blood sugar- and lipid-lowering, antioxidant, immune-regulation and anti-tumor effects[3-6]. However, more theoretical basis is needed for the study of their effects on liver oxidative stress caused by obesity induced by high-fat diet. The results of this study indicated that PSP had a significant improvement effect on oxidative damage caused by high-fat diet.
Oxidative damage refers to the biomembrane lipid peroxidation reactions induced by excessive accumulation of ROS in the body caused by imbalance between the production and elimination of reactive oxygen species, which is closely related to the occurrence and development of various diseases. MDA is an important indicator to measure the degree of lipid peroxidation, which can reflect the degree of membrane lipid peroxidation in organisms. The liver is the main organ attacked by reactive oxygen species. In the process of oxidative damage to the liver, lipid peroxidation is produced due to the action of free radicals, and MDA leads to the disorder of biomacromolecules, including nucleic acids, proteins, phospholipids, etc. SOD and CAT are essential enzymes scavenging oxygen free radical in the body, which further convert superoxide anion free radical O2- into H2O2 and O2, and H2O2 was then decomposed into water by CAT or GSH-Px, preventing oxidative stress damage[20-21]. Moreover, the activity of AST and ALT can sensitively reflect the degree of liver cell damage. In this study, obesity of rats induced by high-fat diet resulted in a significant increase in liver MDA content and AST and ALT activity, while SOD, CAT and GSH-Px activity significantly decreased, indicating that high-fat diet caused oxidative damage to the liver of rats, but intervention with PSP significantly reduced MDA content and AST and ALT activity and increased the activity of antioxidant enzyme systems SOD, CAT, and GSH-Px, indicating that PSP could alleviate oxidative damage to the liver caused by high-fat diet, which is similar to the study by Deng et al.[22] and Liang et al.[23] on the anti-liver injury effect of polysaccharides. It might be due to the fact that polysaccharides mainly play an anti-liver injury role by resisting free radical damage, combating liver cell calcium overload and regulating mitochondrial function.
In addition, HE staining revealed vesicular degeneration in the liver of rats in the high-fat diet group, accompanied by a significant increase in lipid droplets. After intervention with PSP, the deposition of fat in the liver of rats was reduced, resulting in a more orderly arrangement of cells.
Overall, it indicated that PSP could improve liver oxidative damage, enhance antioxidant capacity and reduce fat deposition in the liver by reducing AST and ALT levels, thereby inhibiting the formation of obesity in rats fed a high-fat diet. In the future, the molecular mechanism by which PSP can alleviate liver oxidative damage will be further clarified.
References
[1] OLGA C, ALBERT G, YONG-MOON P, et al. The gut microbiome profilein obesity a systematic review[J]. International journal of endocrinology, 2018: 1-9.
[2] ERNI RG, YU HZ, LI HF, et al. A brief introduction to the research progress of obesity and therapeutic drugs[J]. World Latest Medicine, 2021, 21(95): 89-90. (in Chinese).
[3] MOHAMMADI G, HAFEZIEH M, KARIMI AA, et al. The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila[J]. Fish Shellfish Immunol, 2022(120): 304-313.
[4] ZHENG S. Protective effect of Polygonatum sibiricum polysaccharide on D-galactose-induced aging rats model[J]. Sci Rep, 2020, 10(1): 2246.
[5] ZHAO P, ZHAO C, LI X, et al. The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology[J]. J Ethnopharmacol, 2018(214): 274-291.
[6] SONG J, LIU Q, HAO M, et al. Effects of neutral polysaccharide from Platycodon grandiflorum on high-fat diet-induced obesity via the regulation of gut microbiota and metabolites[J]. Front Endocrinol (Lausanne) 2023(14): 1078593.
[7] SHANG HM, ZHOU HZ, LI R, et al. Extraction optimization and influences of drying methods on antioxidant activities of polysaccharide from cup plant (Silphium perfoliatum L.)[J]. PLoS One 2017, 12(8): e0183001.
[8] CHU L, YANG L, LIN L, et al. Chemical composition, antioxidant activities of polysaccharide from pine needle (Pinus massoniana) and hypolipidemic effect in high-fat diet-induced mice[J]. Int J Biol Macromol, 2019(125): 445-452.
[9] CHEN J, JIN X, ZHANG L, et al. A study on the antioxidant effect of Coriolus versicolor polysaccharide in rat brain tissues[J]. Afr J Tradit Complement Altern Med., 2013, 10(6): 481-484.
[10] KONG X, LIU JJ, LI H, et al. Effect of polysaccharides from Polygonatum sibiricum on lipid-metabolism related mRNA and protein expression in hyperlipidemic mice[J]. Zhongguo Zhong Yao Za Zhi, 2018, 43(18): 3740-3747.
[11] ZENG GF, ZHANG ZY, LU L, et al. Protective effects of Polygonatum sibiricum polysaccharide on ovariectomy-induced bone loss in rats[J]. J Ethnopharmacol, 2011, 136(1): 224-229.
[12] YE G, ZHAO Y, ZHU J, et al. Synergistic effect of polydatin and Polygonatum sibiricum polysaccharides in combating atherosclerosis via suppressing TLR4-Mediated NF-kappaB activation in ApoE-deficient mice[J]. Evid Based Complement Alternat Med, 2022(2022): 3885153.
[13] LUO T, LUO D, GAN L. Research progress on modern pharmacological effects of Polygonatum sibiricum polysaccharide[J]. Chinese Journal of Clinical Rational Drug Use, 2023, 16(2): 177-180. (in Chinese).
[14] CUI X, WANG S, CAO H, et al. A review: The bioactivities and pharmacological applications of Polygonatum sibiricum polysaccharides[J]. Molecules, 2018, 23(5): 1170.
[15] MA W, WEI S, PENG W, et al. Antioxidant effect of Polygonatum sibiricum polysaccharides in D-galactose-induced heart aging mice[J]. Biomed Res Int, 2021(2021): 6688855.
[16] KREMER FS, ESLABAO MR, DELLAGOSTIN OA, et al. Genix: A new online automated pipeline for bacterial genome annotation[J]. FEMS Microbiol Lett, 2016, 363(23).
[17] LI Q, ZENG J, GONG P, et al. Effect of steaming process on the structural characteristics and antioxidant activities of polysaccharides from Polygonatum sibiricum rhizomes[J]. Glycoconj J 2021, 38(5): 561-572.
[18] LIANG X, CHEN X, LI C, et al. Metabolic and transcriptional alternations for defense by interfering OsWRKY62 and OsWRKY76 transcriptions in rice[J]. Sci Rep, 2017, 7(1): 2474.
[19] SCHEUER H, GWINNER W, HOHBACH J, et al. Oxidant stress in hyperlipidemia-induced renal damage[J]. Am J Physiol Renal Physiol, 2000, 278(1): 63-74.
[20] LI S, TAN HY, WANG N, et al. The role of oxidative stress and antioxidants in liver diseases[J]. Int J Mol Sci, 2015, 16(11): 26087-26124.
[21] CUI MY, YIN YF, ZHU FM, et al. Effects of FVGL on levels of SOD, MDA and GSH-Px in rats with oxidative damage induced by D-galactose[J]. Acta Chinese Medicine and Pharmacology, 2021(12): 47-51. (in Chinese).
[22] DENG QF, ZHOU X, CHEN HG. Advance in study on hepatoprotective effects and its mechanism of polysaccharides[J]. China Journal of Chinese Materia Medica, 2016, 41(16): 2958-2967. (in Chinese).
[23] LIANG YF, YOU QX, GU QY, et al. Anti-vascular oxidative stress effect of corn silk polysaccharide on hypertensive rats induced by high salt[J]. Chinese Journal of Gerontology, 2022, 42(12): 3037-3040. (in Chinese).
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Optimization of Extraction Conditions for Total Flavonoids from Fructus Aurantii Immaturus and Its Anti-UVB Radiation Activity
- Research on Maize Seed Classification Method Based on Convolutional Neural Network
- Common Species Distribution Models in Biodiversity Analysis and Their Challenges and Prospects in Application
- Vegetable Tunnel House Technology in Tropical Island Countries
- Purification and Characterization of Hyaluronate Lyases Produced by Two Types of Bacteria
- Renewable Energy Seawater Desalination Technology and Application Analysis

