Effects of Polygonatum sibiricum Polysaccharide (PSP) on Inflammatory Factors in Rats Fed on High-fat Diet
Mingzhe SU, Mingjun LI, Lingli BAI, Chenzhong JIN, Yihong HU, Yan WANG
Abstract[Objectives] This study was conducted to explore the effects of Polygonatum sibiricum polysaccharide (PSP) on chronic intestinal inflammation caused by high-fat diet. [Methods] Thirty five male healthy SD rats were randomly divided into a normal control group (NC, normal diet, n=10) and a high-fat diet group (HF, high-fat diet, n=25). After 8 weeks, an obesity model was established. The HF group was randomly divided into an HF group and a PSP treatment group [PSP, 300 mg/(kg·d)]. After 6 weeks of intervention with PSP, rat serum was collected, and the spleen and thymus were stripped, and weighed. Serum IgG, IgM, LPS and IL-1β and IL-6 contents were detected by ELISA, and HE staining was adopted to observe the pathological changes of the colon tissue. [Results] PSP reduced the level of LPS caused by high-fat diet and the levels of inflammatory factors IL-6 and TNF-α, increased the indexes of the thymus and spleen serving as immune organs, increased IgG and IgM contents, and alleviated pathological damage to the colon tissue caused by high-fat diet. [Conclusions] This study provides a theoretical basis and experimental basis for the development of drugs for treating metabolic diseases such as obesity and inflammation.
Key wordsPSP; High-fat diet; Obesity; SD rats
DOI:10.19759/j.cnki.2164-4993.2023.04.002
Obesity is a chronic disease that has become a global health problem. According to the World Health Organization, the total number of obese patients is four times that of 30 years ago[1], and they are becoming increasingly younger. Obesity is an important risk factor for type 2 diabetes, cardiovascular disease, liver disease, and even cancer. The mechanism of obesity and its intervention and treatment research are both hot topics in scholars research. In recent years, some scholars have put forward the "obesity inflammatory response theory", which believes that obesity leads to the increase and enlargement of the number of fat cells, the appearance of a large number of infiltrating macrophages in adipose tissue, and the increase of biomarkers of inflammatory response including interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) in the blood. More and more evidence shows that the incidence rate of inflammatory bowel disease caused by obesity is increasing year by year[2].
At present, several drugs such as VDZ and ustekinumab are used to treat intestinal inflammation. VDZ exerts selective anti-inflammatory effects in the intestine by suppressing α-4-β-7 integrin receptors. Ustekinumab is currently the only drug clinically used to inhibit IL-12 and IL-23. Corticosteroids are the main means to treat acute intestinal inflammation. Although they can alleviate inflammation in the short term, long-term use will lead to serious adverse reactions, increase body mass, and cause other metabolic syndrome, which limits their clinical application[3-4]. Therefore, there is an urgent need for natural active compounds that can prevent and alleviate intestinal inflammation. The natural polysaccharides of medicinal plants, microorganisms and marine organisms have attracted attention due to their unique biological activity and minimal side effects, as well as their protective effects on toxicity, inflammation, and tumors, as well as their ability to lower blood lipids[5-7].
Polygonatum sibiricum, a perennial herb of Liliaceae and Polygonatum, is rich in a variety of pharmaceutical active ingredients, including polysaccharides, saponins, flavonoids, quinones, etc. P. sibiricum polysaccharide (PSP) is the main bioactive component of P. sibiricum, which has antioxidant activity, anti-aging activity, anti-fatigue effect, immunity enhancing and antibacterial effect, anti-inflammatory effect, and blood fat-reducing, anti-atherosclerosis, diabetes treatment and anti-cancer effects[8]. Moreover, our previous research found that PSP can improve the colonic oxidative stress status of obese mice on a high-fat diet[9], effectively inhibit obesity in rats fed on high-fat diet, and enhance the antioxidant capacity of rats fed on high-fat diet. However, there are few reports on the regulatory effect of PSP on inflammatory responses induced by high-fat diet. Therefore, based on the previous experiments, we investigated the effects of PSP on serum inflammatory factors and observed changes in intestinal morphology of rats fed on high-fat diet, and explored whether PSP can improve inflammation caused by high-fat diet.
Materials and Methods
Experimental materials
P. sibiricum polysaccharideP. sibiricum polysaccharide (PSP) was separated and purified from P. sibiricum using alkali-extraction and alcohol-precipitation method by our research team in the early period, and the total sugar content in the sample was measured to be about 51.66% by the phenol-sulfuric acid method[9].
ReagentsRats with lipopolysaccharide (LPS) kit, rat immunoglobulin M (IgM) ELISA kit, rat immunoglobulin G (IgG) ELISA kit, rat tumor necrosis factor-α (TNF-α) ELISA kit, monocyte chemotactic protein 1 (MCP-1) kit, and rat interleukin 6 (IL-6) and -10 (IL-10) ELISA kit were all purchased from Shenzhen Mindray.
Experimental methods
Animal model preparation and groupingThirty five 8-week-old male SD rats, purchased from Hunan SJA Laboratory Animal Co., Ltd., were randomly divided into two groups: a normal group (NC) with 10 rats and a high-fat diet group (HF) with 25 rats. The NC group was fed on a normal diet and the HF group had a high-fat diet. After 8 weeks of feeding, 20 rats with an average body weight exceeding 20% of the average body weight of the NC group, were selected from the HF group. The selected rats were re-divided into an HF group and a PSP group, with 10 animals in each group. The HF group was still fed on a high-fat diet, while the PSP group was fed on a high-fat diet and a PSP solution at 300 mg/kg·d. Samples were taken after continuous feeding for 6 weeks.
Sample collectionAfter 6 weeks of administration, the body weights of rats in each group were recorded after 12 h on an empty stomach. Next, the rats were anesthetized with pentobarbital sodium, and blood was drawn from the abdominal aorta and stood for 30 min. The blood was centrifuged at 4 ℃ and 3 000 r/min for 15 min, and serum was separated. Dissection was performed to separate the spleen, thymus, and colon, which were cleaned with cold physiological saline. Next, the moisture on the surface was absorbed with absorbent paper, and the organs were weighed to calculate their organ coefficients, and then stored in a refrigerator at -80 ℃ for later use.
Measurement of animal weight and spleen and thymus coefficientsAfter the experiment was completed, rats in each group were weighed for their body weights. The immune organs of the spleen and thymus were then quickly separated and accurately weighed to calculate the spleen and thymus coefficients according to formula (1)[10].
Organ coefficient (%)=[Organ weight (g)/Body weight (g)]×100(1)
Determination of inflammation-associated cytokinesThe contents of LPS, IgM, IgG, TNF-α, MCP-1, IL-6 and IL-10 in serum were determined by enzyme-linked immunosorbent assay according to specific steps in the instructions of corresponding reagent kit.
Pathological analysis of colon tissueFirst, the colon tissue was fixed with formalin, and then subjected to gradient elution with 80%, 95%, and 100% ethanol, respectively. After being transparentized with xylene, it was immersed in melted paraffin for embedding. The embedded sample was cut into sections about 5 μm in thickness, and they were then dehydrated with ethanol, re-stained with an eosin solution, and transparentized with xylene. The staining conditions were observed under an optical microscope to evaluate the degree of inflammation.
Statistical methods
The statistical significance analysis of all data was conducted using SPSS 21.0 software, and the experimental results were presented in the form of (x±s). Independent-sample t tests were adopted for inter-group comparisons, with P<0.05 being significant and P<0.01 being extremely significant.
Results and Analysis
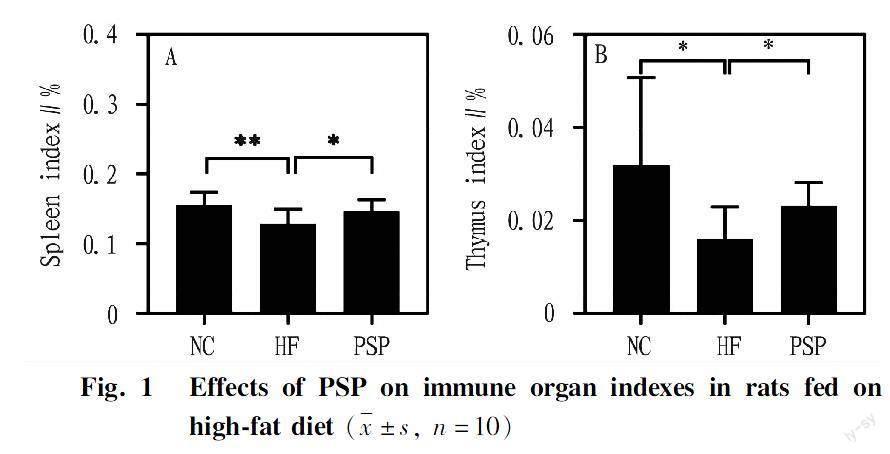
Effects of PSP on immune organ indexes in rats fed on high-fat diet
The spleen and thymus are important lymphatic organs in the body, and their increased mass often leads to lymphocyte proliferation, which is the most direct manifestation of the bodys immune response. Compared with the NC group, the spleen (P<0.05) and thymus indexes (P<0.01) in the HF group significantly decreased (Fig. 1). However, after intervention with PSP, the spleen (P<0.05) and thymus (P<0.05) indexes significantly increased, approaching the NC group (Fig. 1).
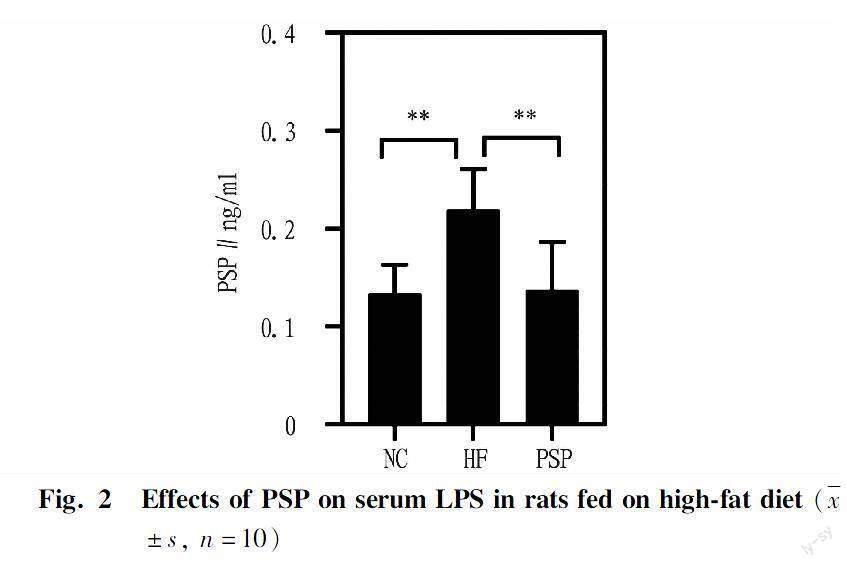
Effects of PSP on serum LPS in rats fed on high-fat diet
LPS is a type of endotoxin that can cause inflammation in the body and disrupt the intestinal barrier, resulting in metabolic disorders. Compared with the NC group, the LPS in the serum of rats in the HF group significantly increased (P<0.01), and after intervention with PSP, LPS significantly reduced (P<0.01).
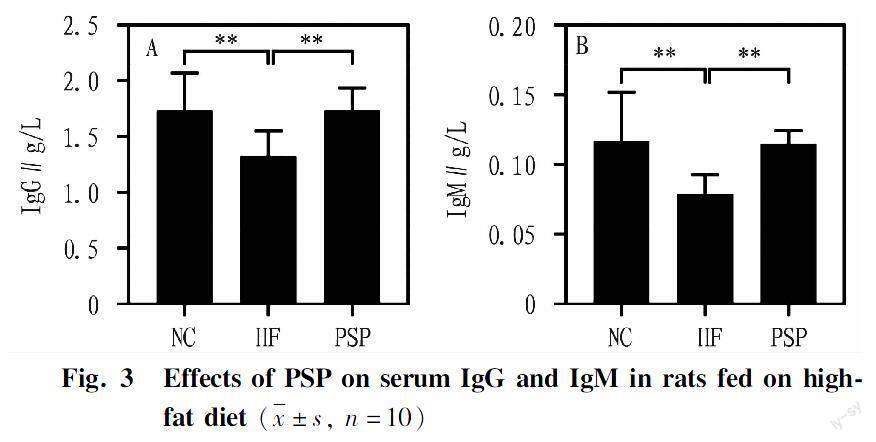
Effects of PSP on serum immunoglobulins in rats fed on high-fat diet
Compared with the control group, the mass concentrations of IgG and IgM in the HF group were significantly reduced (P<0.01, Fig. 3). Compared with the model group, after intervention with PSP, the mass concentrations of IgG and IgM significantly increased (P<0.01, Fig. 3), indicating that PSP could enhance the immune function of the body.
Effects of PSP on inflammation-associated cytokines
Compared with the NC group, the contents of pro-inflammatory factors TNF-α, IL-6 and MCP-1 in the serum of rats in the HF group significantly increased (P<0.01, Fig. 4A, C, D), while the content of anti-inflammatory factor IL-10 significantly decreased (P<0.01, Fig. 4B). Compared with the HF group, the contents of TNF-α, IL-6 and MCP-1 of the PSP group significantly decreased (P<0.01, Fig. 4A, C, D), while the content of IL-10 significantly increased (P<0.05, Fig. 4B).
Agricultural Biotechnology2023Effects of PSP on colon morphology of rats fed on high-fat die
The NC group had intact colonic mucosal structure, normal glandular structure, and no obvious inflammatory cells or edema (Fig. 5). Compared with the NC group, the mucosal epithelium of rats in the HF group was damaged, and the arrangement of mucosal villi and glands was disordered and the structure was unclear (Fig. 5). After 4 weeks of intervention with PSP, the colon structure of rats fed on high-fat diet was improved, with distinct layers of mucosal tissue and reduced distortion and relatively neat arrangement of mucosal villi and glands (Fig. 5), indicating that PSP alleviated colon damage caused by high-fat diet.
Conclusions and Discussion
Obesity is a chronic disease, usually defined as excessive body fat, which can cause harm to the bodys health. It has become one of the major global public health issues in the 21st century. Research has shown that obesity can affect the occurrence and development of chronic inflammation and other autoimmune diseases in the body[11], leading to significantly-elevated markers of obesity inflammatory factors IL-6 and TNF-α and activated inflammatory signals in the body[12]. In addition, obese patients showed significantly-increased levels of LPS and MCP-1 in their blood[13] and decreased immune function.
The contents of pro-inflammatory factors IL-6 and TNF-α and LPS in the serum of obese rats induced by high-fat diet in this study significantly increased, and the level of anti-inflammatory factor IL-10 significantly decreased, and the villi and glands of the colonic mucosa showed distortion, suggesting that a high-fat diet may cause inflammation in rats. After treatment with PSP, it inhibited the secretion of pro-inflammatory factors IL-6 and TNF-α, reduced the load of LPS, increased the content of anti-inflammatory factor IL-10, and improved the structure of colonic mucosa and muscularis mucosae, indicating that PSP had a certain inhibitory effect on inflammation caused by high-fat diet. It is consistent with the result that PSP lowers LPS level, reduces the release of inflammatory factors IL-6 and TNF-α and improves chronic intestinal inflammation[14]. In addition, PSP, as the main active ingredient of medicinal and edible Polygonatum sibiricum, can not only improve the immune system of MFC gastric cancer bearing mice, but also inhibit tumor growth[15]. In this study, PSP could enhance the immune function of rats fed on high-fat diet by increasing the indexes of the spleen and thymus serving as immune organs, and it promoted the secretion of immunoglobulins IgG and IgM and enhanced the immune function of rats.
To sum up, PSP could inhibit intestinal inflammation caused by high-fat diet by enhancing the immune function of rats fed on high-fat diet, inhibiting the secretion of proinflammatory factors and promoting anti-inflammatory factors, which provides a theoretical basis and experimental basis for the development of drugs for treating metabolic diseases such as obesity and inflammation.
References
[1] MISHRA AK, DUBEY V, GHOSH AR. Obesity: An overview of possible role(s) of gut hormones, lipid sensing and gut microbiota[J]. Metabolism 2016, 65(1): 48-65.
[2] KREUTER R, WANKELL M, AHLENSTIEL G, et al. The role of obesity in inflammatory bowel disease[J]. Biochim Biophys Acta Mol Basis Dis 2019, 1865(1): 63-72.
[3] HUANG C. Research progress on the relationship between inflammatory bowel disease and obesity[J]. Hainan Medical Journal, 2023, 34(5): 750-752.
[4] JOHNSON AM, LOFTUS EV. Obesity in inflammatory bowel disease: A review of its role in the pathogenesis, natural history, and treatment of IBD[J]. Saudi J Gastroenterol, 2021, 27(4): 183-190.
[5] XIN YF, YOU ZQ, GAO HY, et al. Protective effect of Lycium barbarum polysaccharides against doxorubicin-induced testicular toxicity in rats[J]. Phytother Res, 2012, 26(5): 716-721.
[6] YANG XM, XU HY, LIU SQ, et al. Research progress of edible fungus polysaccharide in regulating inflammatory bowel disease[J]. Food and Machinery, 2021, 37(9): 211-217. (in Chinese).
[7] LI YH, LIU JL, WANG TT, et al. Research progress in anti-tumor immune mechanism of Ganoderma lucidum polysaccharides[J]. Chinese Journal of Immunology, 2021, 37(4): 511-514. (in Chinese).
[8] CUI X, WANG S, CAO H, et al. A Review: The bioactivities and pharmacological applications of Polygonatum sibiricum polysaccharides[J]. Molecules, 2018, 23(5): 1170. (in Chinese).
[9] WANG Y, DONG P, JIN CZ, et al. Analysis on composition of Polygonatum sibiricum polysaccharide and its anti-oxidant activity[J]. Genomics and Applied Biology, 2019, 38(5): 2191-2199. (in Chinese).
[10] ALEXEEV V, UITTO J, IGOUCHEVA O. Gene expression signatures of mouse bone marrow-derived mesenchymal stem cells in the cutaneous environment and therapeutic implications for blistering skin disorder[J]. Cytotherapy, 2011, 13(1): 30-45.
[11] HARPER JW. Zisman TL: Interaction of obesity and inflammatory bowel disease[J]. World J Gastroenterol, 2016, 22(35): 7868-7881.
[12] WEI XX, LI NC, LI X, et al. Research progress on the effect of exercise on inflammatory factors related to obesity in the body[J]. Human Movement Science, 2018, 2(8): 15-17. (in Chinese).
[13] WANG JL, ZHANG C, XU YJ, et al. Regulation and mechanism of oxycodone on leukocytes inflammation in obese patients undergoing bariatric surgery[J]. Jinagsu Medical Journal, 2023, 49(2): 115-120. (in Chinese).
[14] ZHANG JN, YUAN H, MA CL, et al. Astragalus polysaccharide inhibits intestinal inflammatory response in mice fed on high-fat diet by regulating gut microbiota[J]. Journal of Food Science and Biotechnology, 2022, 41(4): 23-24. (in Chinese).
[15] LYU PT, DUAN XB. Inhibitory and immunomodulatory effects of Polygonatum sibiricum polysaccharide on MFC gastric cancer bearing mice[J]. 2020, 42 (8): 2169-2172. (in Chinese).
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Optimization of Extraction Conditions for Total Flavonoids from Fructus Aurantii Immaturus and Its Anti-UVB Radiation Activity
- Research on Maize Seed Classification Method Based on Convolutional Neural Network
- Common Species Distribution Models in Biodiversity Analysis and Their Challenges and Prospects in Application
- Vegetable Tunnel House Technology in Tropical Island Countries
- Purification and Characterization of Hyaluronate Lyases Produced by Two Types of Bacteria
- Renewable Energy Seawater Desalination Technology and Application Analysis

