6-OHDA诱导的PD模型小鼠的运动及焦虑症状
陈凤华 石丽敏 谢俊霞
[摘要]目的探討6-羟基多巴胺(6-OHDA)诱导的单侧帕金森病(PD)模型小鼠的运动及焦虑症状。方法7周龄雄性C57BL/6小鼠20只,随机分为对照组及模型组,每组10只。模型组小鼠通过左侧纹状体立体定位注入6-OHDA(2 g/L,2 μL)制备PD模型,对照组小鼠注入等量的生理盐水。2周后进行旷场实验检测小鼠的移动总距离和中心区探索时间,采用酪氨酸羟化酶(TH)免疫荧光染色检测黑质区多巴胺能神经元数目。结果旷场实验结果显示,与对照组相比,模型组小鼠移动总距离明显减少,中心区探索时间明显增加,差异具有统计学意义(t=2.201、2.576,P<0.01)。免疫荧光染色结果显示,与对照组相比,模型组小鼠黑质区TH阳性神经元的数目明显减少,差异有统计学意义(t=17.570,P<0.001)。结论6-OHDA诱导的单侧PD模型小鼠黑质-纹状体系统功能受损,出现运动障碍但没有产生焦虑。
[关键词]帕金森病;羟多巴胺;纹状体;小鼠;症状评估
[中图分类号]R338.2[文献标志码]A[文章编号]2096-5532(2023)03-0321-04
doi:10.11712/jms.2096-5532.2023.59.076[开放科学(资源服务)标识码(OSID)]
[网络出版]https://kns.cnki.net/kcms2/detail/37.1517.R.20230719.1611.001.html;2023-07-2013:27:08
MOTOR AND ANXIETY SYMPTOMS IN A MOUSE MODEL OF 6-HYDROXYDOPAMINE-INDUCED PARKINSONS DISEASE CHEN Fenghua, SHI Limin, XIE Junxia (Department of Physiology and Pathophysiology, School of Basic Medicine, Qing-dao University, Institute of Brain Sciences and Diseases, Qingdao 266071, China)
[ABSTRACT]ObjectiveTo investigate the motor and anxiety symptoms in a mouse model of 6-hydroxydopamine (6-OHDA)-induced unilateral Parkinsons disease (PD). MethodsA total of 20 male C57BL/6 mice, aged 7 weeks, were randomly divided into control group and model group, with 10 mice in each group. The mice in the model group were given stereotactic injection of 2 μL 6-OHDA (2 g/L) into the left corpus striatum to establish a model of PD, and those in the control group were gi-ven injection of an equal volume of normal saline. Two weeks later, the open field test was used to measure total moving distance and time spent in the center of the open field, and tyrosine hydroxylase (TH) immunofluorescent staining was used to measure the number of dopaminergic neurons in the substantia nigra. ResultsThe open field test showed that compared with the control group, the model group had a significant reduction in total moving distance and a significant increase in time spent in the center of the open field (t=2.201,2.576;P<0.01). Immunofluorescent staining showed that compared with the control group, the model group had a significant reduction in the number of TH-positive neurons in the substantia nigra (t=17.570,P<0.001). ConclusionImpaired function of the substantia nigra-corpus striatum system is observed in a mouse model of 6-OHDA-induced unilateral PD, with the presence of movement disorders, but without the presence of anxiety.
[KEY WORDS]Parkinson disease; oxidopamine; corpus striatum; mice; symptom assessment
帕金森病(PD)是仅次于阿尔茨海默病的第二大神经退行性疾病,其病理学特征为黑质致密带多巴胺能神经元选择性丢失和纹状体轴突末梢多巴胺含量减少[1-3]。其运动症状主要有静止性震颤、肌僵直、运动迟缓和姿势不稳等,非运动症状有嗅觉障碍、睡眠障碍、认知障碍、焦虑和疲劳等。动物模型在探究PD发病机制和寻找潜在治疗靶点的过程中发挥着重要作用[4-6]。6-羟基多巴胺(6-OHDA)是一种儿茶酚胺选择性神经毒素,脑内纹状体注射6-OHDA会引起相应的黑质-纹状体多巴胺系统进行性和部分受损,可用于制备稳定有效的大鼠PD模型[7-9]。尽管6-OHDA单侧损伤大鼠模型是PD研究中最常用的模型之一,但随着光遗传和化学遗传技术的发展,6-OHDA制备PD模型也逐步应用于小鼠[10-14]。目前尚缺乏6-OHDA注射诱导的PD模型小鼠的系统性研究。本实验通过单侧纹状体立体定位注射6-OHDA制备小鼠PD模型,观察其运动及焦虑症状,以期为PD模型小鼠的基础研究提供实验证据。
322青岛大学学报(医学版)59卷
1材料與方法
1.1动物及主要试剂
SPF级雄性C57BL/6小鼠,7周龄,体质量为(22±2)g,购自北京维通利华公司。小鼠饲养于25 ℃、12 h昼夜循环光照的SPF级清洁环境中,可自由饮水、摄食、活动,适应环境1周后开始实验。6-OHDA购于中国Absin公司,L-Ascorbic acid以及地昔帕明购于美国Sigma公司,酪氨酸羟化酶(TH)抗体购于美国Millipore公司,其他试剂均为国产分析纯。
1.2动物分组及处理
将小鼠随机分为对照组和模型组,每组10只。术前30 min小鼠腹腔注射地昔帕明25 mg/kg。利用瑞沃德公司的呼吸麻醉机将小鼠麻醉后,固定在立体定位仪上。用耳杆适配器将小鼠固定好,调整高度使颅骨保持水平。剃除小鼠头部毛发,用碘附擦拭消毒,剪开头皮暴露颅骨的前囟和后囟。以前囟为零点,前囟前0.4 mm、旁开1.8 mm、深度-3.5 mm定位坐标。模型组将2 μL溶于2 g/L抗坏血酸的6-OHDA(2 g/L)按立体定位坐标注入左侧纹状体,流量6 nL/s,注射完成后停针10 min;对照组则以等量生理盐水代替6-OHDA。在整个手术过程中,用异氟烷麻醉小鼠并用加热垫维持体温。
1.3旷场实验
实验前小鼠置于测试环境中适应至少半小时。将小鼠放在一个27 cm×27 cm×35 cm大小不透明测试盒的中央,摄像机放于盒子的正上方。利用Smart v3.0系统记录小鼠10 min的活动情况。每只小鼠检测结束后,用体积分数0.75的乙醇清理旷场区域,并在测试时保持干燥。分析在10 min的旷场实验中小鼠的移动总距离和中心区探索时间,评估小鼠的运动行为和焦虑程度。
1.4脑组织切片及TH免疫荧光染色
行为学检测结束后,腹腔注射阿佛丁(20 mL/kg)麻醉小鼠。经心灌注9 g/L NaCl和多聚甲醛溶液(用0.1 mol/L PBS配制,pH值为7.2~7.4),小心取出鼠脑。将鼠脑置于多聚甲醛溶液中,4 ℃固定6 h,然后分别用200、300 g/L的蔗糖溶液(用0.1 mol/L PBS配制)进行梯度脱水。用冷冻切片机(Leica, CM1950)进行冠状面连续切片。参照小鼠脑图谱,确定黑质区域。进行厚度为20 μm的冠状面连续切片,每组10张,共4组。
取一组完整脑片进行TH免疫荧光染色。将脑片置于多聚甲醛溶液中固定10 min,用0.01 mol/L PBS漂洗3次,每次10 min。用含有体积分数0.05驴血清(Jackson)的PBST缓冲液室温封闭1 h,然后置于用PBST配制的一抗稀释液中4 ℃摇床孵育过夜。次日,用0.01 mol/L PBS漂洗3次,每次10 min。将脑片放于用PBST配制的荧光二抗稀释液中室温孵育2 h,之后用0.01 mol/L PBS漂洗3次,每次10 min。将脑片平铺于载玻片上,避光保存。免疫荧光染色实验中用到的一抗为anti-tyrosine hydroxylase(1∶2 000,rabbit),二抗为donkey anti-rabbit 555(稀释比为1∶500)。使用数字病理切片扫描系统(OLYMPUS,Tokyo,Japan,VS120)拍摄成像,应用OlyVIA软件对TH阳性神经元进行计数。
1.5统计学分析
应用GraphPad Prism 6软件进行统计学处理。实验所得计量资料结果以±s形式表示,两组比较采用t检验。P<0.05表示差异有统计学意义。
2结果
2.16-OHDA对小鼠运动行为的影响
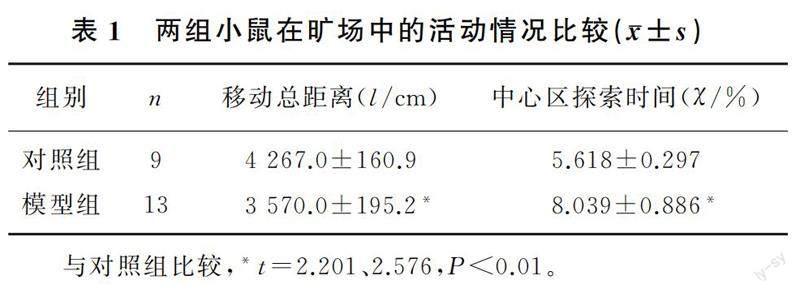
旷场实验结果显示,与对照组小鼠相比,模型组小鼠移动总距离明显减少,中心区探索时间明显增加,差异有统计学意义(t=2.201、2.576,P<0.01)。见表1。
2.26-OHDA对小鼠黑质TH阳性神经元的影响
免疫荧光染色结果显示,对照组和模型组小鼠黑质区TH阳性神经元的数目分别为10 852.0±209.8和6 072.0±173.3(n=10),与对照组相比,模型组小鼠黑质区TH阳性神经元的数目明显减少,差异有统计学意义(t=17.570,P<0.001)。
3讨论
PD是常发生于中老年人的第二大神经退行性疾病,其主要病理改变为黑质多巴胺能神经元进行性丢失,其临床表现除肌僵直、运动迟缓等运动症状外,还有嗅觉障碍、焦虑和抑郁等非运动症状。由于PD的病因病理尚未完全阐明,目前该病的治疗主要是对症治疗[5,15-16]。为了阐明PD的发病机制和寻找潜在治疗靶点,已经开发了许多动物模型[17-19]。6-OHDA可被黑质内含单胺氧化酶的多巴胺能神经元特异性摄取,并在单胺氧化酶的作用下转化成自由基损伤神经元,故被广泛应用于损伤黑质-纹状体多巴胺能系统制备PD模型[10,20-21]。长期以来6-OHDA多用于大鼠PD模型的制备,近年来随着光遗传学、化学遗传学的发展以及各种Cre小鼠的应用,6-OHDA也逐渐用于小鼠PD模型的制备。6-OHDA参与氧化应激反应,通过和多巴胺竞争,可与高亲和力的多巴胺转运体结合进入黑质-纹状体多巴胺能神经元,并迅速被氧化,生成大量的活性氧(ROS),发挥毒性作用损伤细胞。还有研究结果表明,6-OHDA可以抑制线粒体呼吸链的功能,从而引起神经毒性[10,22-24]。由于6-OHDA不能通过血-脑脊液屏障,因此必须通过立体定位技术将它直接注射到黑质、内侧前脑束或纹状体中。研究表明,6-OHDA单侧纹状体注射具有较大的优势:首先,注射到纹状体引起的多巴胺能神经元进行性丢失和区域性的病变与PD病理进展最为相似;其此,小鼠脑内纹状体是一个较大的区域,为立体定位注射减轻了难度[2,25-27]。
TH是多巴胺合成的限速酶,其功能缺失或表达不足直接影响多巴胺的合成与分泌。因此,检测模型动物TH免疫阳性细胞的数目不仅可以反映多巴胺能神经元的数目和功能状态,同时还可评估模型多巴胺水平[28-30]。本实验采用单侧纹状体注射4 μg 6-OHDA的方法制备PD模型,结果显示,单侧纹状体注射2周后,损伤侧黑质TH阳性神经元减少了约44%,提示多巴胺能神经元丢失;同时模型小鼠出现运动缺陷,在旷场实验中的运动总距离减少。但是本实验中PD模型小鼠在旷场中心区探索时间与对照组小鼠相比显著增加,提示小鼠并未出现焦虑症状。以往有研究显示,纹状体注射5 μg以上6-OHDA,3周后小鼠黑质多巴胺能神经元丢失超过50%,并出现运动障碍以及焦虑等非运动症状[31-34]。与之相比,本实验中6-OHDA用药剂量低、作用时间较短,因此推测这可能是小鼠未出现焦虑症状的原因。
綜上,本研究通过单侧纹状体注射6-OHDA观察其对小鼠运动和焦虑症状以及黑质-纹状体系统功能的影响,结果表明4 μg的6-OHDA单侧纹状体注射在2周后可以造成黑质-纹状体通路的部分损失,小鼠出现运动障碍。本实验为6-OHDA制备小鼠PD模型提供了良好的注射位点,为PD的研究提供了有效的实验工具。
[参考文献]
[1]BALESTRINO R, SCHAPIRA A H V. Parkinson disease[J]. European Journal of Neurology, 2020,27(1):27-42.
[2]JANKOVIC J, TAN E K. Parkinsons disease: etiopathoge-nesis and treatment[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 2020,91(8):795-808.
[3]GRAYSON M. Parkinsons disease[J]. Nature, 2016,538(7626):S1.
[4]TIEU K. A guide to neurotoxic animal models of Parkinsons disease[J]. Cold Spring Harbor Perspectives in Medicine, 2011,1(1):a009316.
[5]CHIA S J, TAN E K, CHAO Y X. Historical perspective: models of Parkinsons disease[J]. International Journal of Molecular Sciences, 2020,21(7):2464.
[6]MUSTAPHA M, MAT TAIB C N. MPTP-induced mouse model of Parkinsons disease: a promising direction of therapeutic strategies[J]. Bosnian Journal of Basic Medical Sciences, 2021,21(4):422-433.
[7]SAUER H, OERTEL W H. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tra-cing and immunocytochemical study in the rat[J]. Neuroscience, 1994,59(2):401-415.
[8]PRZEDBORSKI S, LEVIVIER M, JIANG H, et al. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine[J]. Neuroscience, 1995,67(3):631-647.
[9]IRAVANPOUR F, DARGAHI L, REZAEI M, et al. Intranasal insulin improves mitochondrial function and attenuates motor deficits in a rat 6-OHDA model of Parkinsons disease[J]. CNS Neuroscience & Therapeutics, 2021,27(3):308-319.
[10]SIMOLA N, MORELLI M, CARTA A R. The 6-Hydroxydopamine model of Parkinsons disease[J]. Neurotoxicity Research, 2007,11(3):151-167.
[11]GUIMARES R P, LEANDRO RIBEIRO D, DOS SANTOS K B, et al. The 6-hydroxydopamine rat model of Parkinsons disease[J]. Journal of Visualized Experiments, 2021(176):1-17.
[12]BOUCHATTA O, ABY F, SIFEDDINE W, et al. Pain hypersensitivity in a pharmacological mouse model of attention-deficit/hyperactivity disorder[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022,119(30):e2114094119.
[13]MAGNO L A V, TENZA-FERRER H, COLLODETTI M, et al. Optogenetic stimulation of the M2 cortex reverts motor dysfunction in a mouse model of Parkinsons disease[J]. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 2019,39(17):3234-3248.
[14]ZHANG H, ZHANG C K, QU Z W, et al. STN-ANT plasti-city is crucial for the motor control in Parkinsons disease mo-del[J]. Signal Transduction and Targeted Therapy, 2021,6(1):215.
[15]ARMSTRONG M J, OKUN M S. Diagnosis and treatment of Parkinson disease: a review[J]. JAMA, 2020,323(6):548-560.
[16]VIJIARATNAM N, SIMUNI T, BANDMANN O, et al. Progress towards therapies for disease modification in Parkinsons disease[J]. The Lancet Neurology, 2021,20(7):559-572.
[17]TAGUCHI T, IKUNO M, YAMAKADO H, et al. Animal model for prodromal Parkinsons disease[J]. International Journal of Molecular Sciences, 2020,21(6):1961.
[18]DAUER W, PRZEDBORSKI S. Parkinsons disease: mechanisms and models[J]. Neuron, 2003,39(6):889-909.
[19]KIN K, YASUHARA T, KAMEDA M, et al. Animal models for Parkinsons disease research: trends in the 2000s[J]. International Journal of Molecular Sciences, 2019,20(21):5402.
[20]ASANUMA M, HIRATA H, CADET J L. Attenuation of 6-hydroxydopamine-induced dopaminergic nigrostriatal lesions in superoxide dismutase transgenic mice[J]. Neuroscience, 1998,85(3):907-917.
[21]SCHWARTING R K W, HUSTON J P. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae[J]. Progress in Neurobiology, 1996,49(3):215-266.
[22]KONNOVA E A, SWANBERG M. Animal models of Parkinsons disease[M]//STOKER T B, GREENLAND J C. Parkinsons disease: Pathogenesis and clinical aspects. Brisbane (AU): Codon Publications Copyright. 2018.
[23]THIRUGNANAM T, SANTHAKUMAR K. Chemically induced models of Parkinsons disease[J]. Comparative Biochemistry and Physiology Toxicology & Pharmacology, 2022,252:109213.
[24]TRONCI E, FRANCARDO V. Animal models of L-DOPA-induced dyskinesia: the 6-OHDA-lesioned rat and mouse[J]. Journal of Neural Transmission (Vienna, Austria:1996), 2018,125(8):1137-1144.
[25]TRIPANICHKUL W, JAROENSUPPAPERCH E O. Ame-liorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice[J]. European Review for Medical and Pharmacological Sciences, 2013,17(10):1360-1368.
[26]VARCIN M, BENTEA E, MERTENS B, et al. Acute versus long-term effects of 6-hydroxydopamine on oxidative stress and dopamine depletion in the striatum of mice[J]. Journal of Neuroscience Methods, 2011,202(2):128-136.
[27]KABUTO H, NISHIZAWA M, TADA, et al. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hyd-roxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum[J]. Neurochemical Research, 2005,30(3):325-332.
[28]COLETTE S, DAUBNER. Tyrosine hydroxylase and regulation of dopamine synthesis[J]. Archives of Biochemistry and Biophysics, 2011,508(1):1-12.
[29]NAGATSU T, NAKASHIMA A, WATANABE H, et al. Neuromelanin in Parkinsons disease: tyrosine hydroxylase and tyrosinase[J]. International Journal of Molecular Sciences, 2022,23(8):4176.
[30]NAGATSU T, NAKASHIMA A, ICHINOSE H, et al. Human tyrosine hydroxylase in Parkinsons disease and in related disorders[J]. Journal of Neural Transmission, 2019,126(4):397-409.
[31]ANTUNES M S, CATTELAN SOUZA L, LADD F V L, et al. Hesperidin ameliorates anxiety-depressive-like behavior in 6-OHDA model of Parkinsons disease by regulating striatal cytokine and neurotrophic factors levels and dopaminergic innervation loss in the striatum of mice[J]. Molecular Neuro-biology, 2020,57(7):3027-3041.
[32]MENDES-PINHEIRO B, SOARES-CUNHA C, MAROTE A, et al. Unilateral intrastriatal 6-hydroxydopamine lesion in mice: a closer look into non-motor phenotype and glial response[J]. International Journal of Molecular Sciences, 2021,22(21):11530.
[33]LIU X J, YU H, CHEN B X, et al. CB2 agonist GW842166x protected against 6-OHDA-induced anxiogenic- and depressive-related behaviors in mice[J]. Biomedicines, 2022,10(8):1776.
[34]MASINI D, PLEWNIA C, BERTHO M, et al. A guide to the generation of a 6-hydroxydopamine mouse model of Parkin-sons disease for the study of non-motor symptoms[J]. Biomedicines, 2021,9(6):598.
(本文編辑马伟平)

