抗菌药物神经毒性的机制及危险因素研究进展
熊立广?李昕?向德标?袁芳?童焕
摘要:抗菌药物作为治疗及预防细菌感染的特效药,在临床上被广泛使用,同时也存在一系列的副作用。其中,神经毒性是抗菌药物临床使用过程中最常见的一种严重且易混淆的毒副作用。本文就抗菌药物神经毒性的临床症状、作用机制、危险因素以及防治措施进行总结,为抗菌药物临床合理用药及有效防治抗菌药物的神经毒性提供科学依据。
关键词:抗菌药物;神经毒性;机制;危险因素;防治措施
中图分类号:R978.1文献标志码:A
Research progress on the mechanism and risk factors of antibiotics neurotoxicity
Xiong Li-guang1,2, Li Xin2,3, Xiang De-biao2,3, Yuan Fang2,3, and Tong Huan2,3
(1 Hunan University of Chinese Medicine, Changsha 410015; 2 The Third Hospital of Changsha, Changsha 410015;
3 Antibiotic Clinical Application Research Institute of Changsha, Changsha 410015)
Abstract Antibacterial agents are widely used clinically for the treatment and prevention of bacterial infections while a range of adverse effects exist. Neurotoxicity is one of the most common, serious, and confusing side effects in the clinical use of antibiotics. In this article, the clinical symptoms, mechanism of action, risk factors, and preventive measures of neurotoxicity of antibiotics are summarized to provide a scientific basis for the rational application of antibacterial drugs in clinical practice and the effective prevention and treatment of the neurotoxicity of antibiotics.
Key words Antibacterial agents; Neurotoxicity; Mechanism; Risk factors; Prevention
隨着全球微生物感染疾病的肆虐及传播,抗菌药物的使用日益广泛,其毒性也逐渐受到人们的重视。抗菌药物的毒性主要表现为肾毒性、神经毒性、肝毒性等。其中神经毒性呈浓度依赖性,且随着药物剂量的调整其神经毒性是可逆的,停药后可恢复。但往往在治疗中会与不同的神经病征或患者本身罹患疾病混淆,同时存在着老龄、肾功能不全以及既往病史等危险因素,导致抗菌药物难以合理使用,造成进一步神经损害[1]。抗菌药物神经毒性产生机制比较广泛,主要与GABA(γ-氨基丁酸)、NMDA(N-methyl-D-aspartic acid, N-甲基-D-天冬氨酸)受体、乙酰胆碱等神经递质的损伤相关,同时也与氧化应激以及线粒体功能障碍关系密切。目前针对抗菌药物神经毒性的防治措施除停药外,也采用药物进行预防或控制神经毒性症状的发生,如增强GABAA活性的苯二氮卓类药物,具有神经保护作用的谷胱甘肽、神经节苷脂、雷帕霉素和红景天苷等,同时越来越多的药物临床使用建议采用TDM(治疗药物血药浓度监测)来制定个体化给药方案,以此优化抗菌药物的合理应用。
1 抗菌药物神经毒性的临床表现
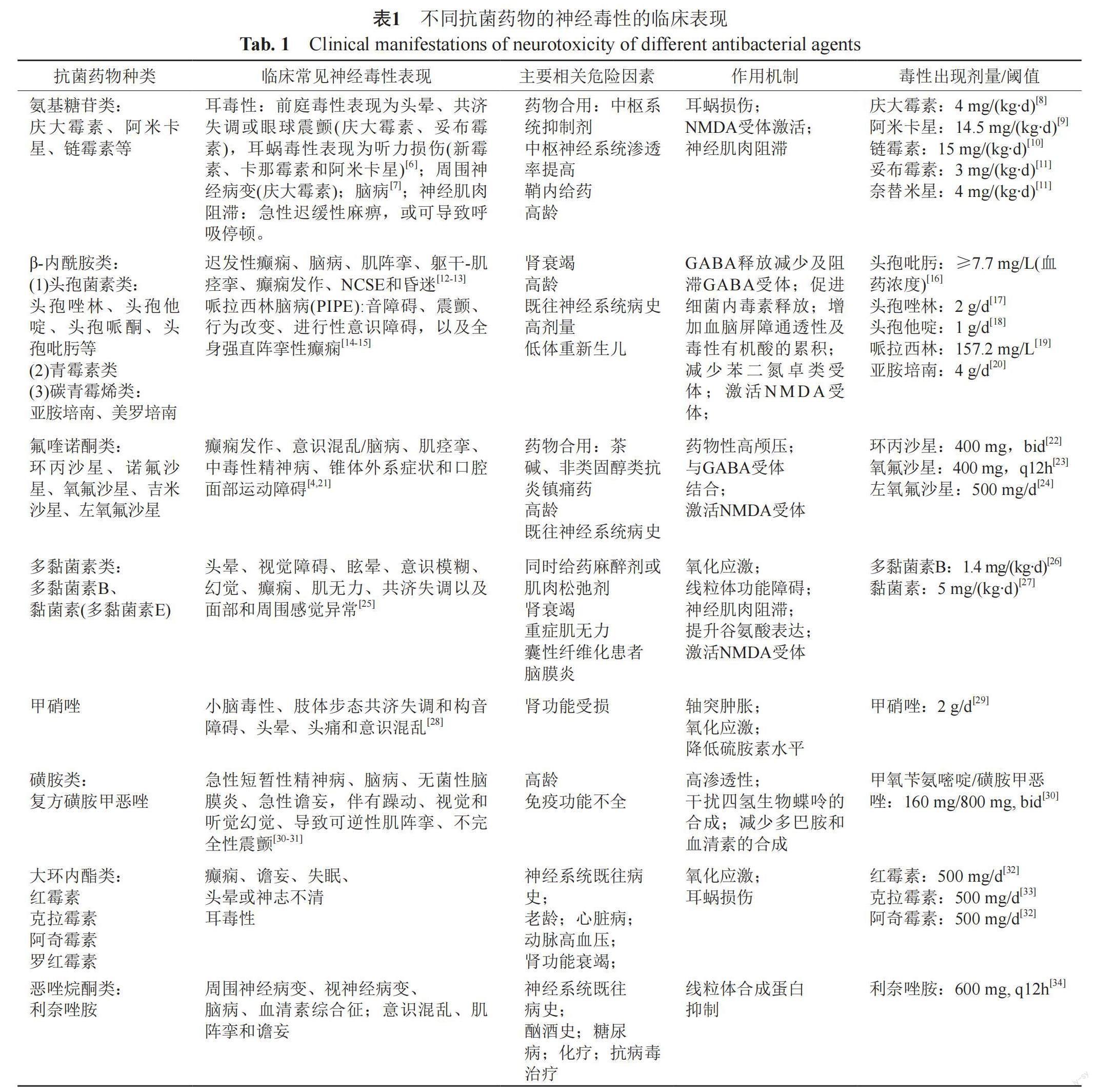
临床上评估神经毒性主要通过相关生化指标、脑电图表现、心理和行为测试和神经学检查等途径。抗菌药物临床症状表现多样,且不同抗菌药物的神经毒性临床表现有所不同,分为中枢神经毒性以及周围神经毒性。中枢神经毒性主要表现为以精神状态改变、记忆丧失、激动、认知能力丧失、失眠和幻觉为特征的脑病[2]、耳毒性、视觉和听觉幻觉、癫痫发作、非惊厥性癫痫持续状态(nonconvulsive epileptic, NCSE)和昏迷[3];周围神经毒性主要表现为口腔面部运动障碍、肌阵挛与肌无力[4-5],详见表1。
2 抗菌药物神经毒性发生机制
抗菌药物产生神经毒性的机制主要有以下几种:①与γ-氨基丁酸(GABA)结合减少,或直接拮抗GABAA受体阻断GABA结合位点,导致抑制性神经递质浓度降低和皮质传入兴奋,从而导致神经传递过度兴奋诱发神经毒性[21,35];②激活NMDA受体,造成神经兴奋、ROS生成增加和Ca2+胞内浓度升高,诱导细胞凋亡[36-38];③轴突变性[39-40];④抑制乙酰胆碱突触前释放,阻滞或减弱乙酰胆碱与受体的结合[41-42];⑤钙离子消耗引起的去极化[43];⑥氧化应激以及线粒体功能障碍[25]。
2.1 氨基糖苷类
氨基糖苷类药物诱发神经毒性以耳毒性最为常见,同时也有研究报道氨基糖苷类的周围神经系统损伤、脑病以及神经肌肉阻滞等相关神经毒性。
(1)耳毒性 氨基糖苷类药物造成的耳毒性包括耳蜗损伤和前庭器官损伤,耳毒性整体发生率范围在2%~25%[44-45]。氨基糖苷类产生耳毒性的机制为氨基糖苷类药物进入内耳后透过赖斯纳氏膜、血管纹以及基底膜等结构进入耳蜗,主要在静纤毛的机械力电转导作用下(次要途径为内吞作用和基底外侧TRPA1通道)进入毛细胞[46-50],并与毛细胞中tRNA结合,导致线粒体的RNA翻译受损以及蛋白质合成抑制,减少ATP的产生[51-52],从而促进活性氧的生成,破坏线粒体的完整性,促进细胞色素C的释放,激活细胞凋亡级联反应[36,53]。同时研究表明氨基糖苷类药物能够激活耳蜗内NMDA受体,而过度激活NMDA受体会增加一氧化氮(NO)的形成,从而促进活性氧的产生,同时可能增加Ca2+通过NMDA受体进入细胞,诱导急性肿胀破坏突触后结构,随后导致钙离子级联反应,进一步导致神经元细胞凋亡和损伤[36,54]。
(2)神经肌肉阻滞 氨基糖苷类药物的神经肌肉阻滞机制多样,如新霉素能阻滞Ca2+通道从而抑制突触前乙酰胆碱的释放;氨基糖苷类药物如庆大霉素和新霉素还能通过阻滞Ca2+进入胆碱能受体来抑制毛细胞中的胆碱能K+电流传导,诱导神经肌肉阻滞[55-56]。也有研究表明氨基糖苷类药物能促进对乙酰胆碱受体(AChR,acetycholine report)的免疫应答,提高血清中AChR抗体水平,阻断ACh与受体的结合,加速肌肉神经接点上AChR的丢失,破坏突触前和突触后膜结构从而诱导神经肌肉阻滞[57]。
2.2 β-内酰胺类
(1)头孢菌素类 头孢素类药物中第一代头孢菌素(头孢唑林)、第二代头孢菌素(头孢呋辛)、第三代头孢菌素(头孢他啶)以及第四代头孢菌素(头孢吡肟)都有神经毒性的相关报道。根据药物警戒数据库对1987—2017年的病例统计分析,各类头孢菌素类药物的神经毒性发生率分别为:头孢吡肟(33.1%)、头孢曲松(29.7%)、头孢他啶(19.6%)、头孢噻肟(9%)和头孢唑林(2.9%)[58]。头孢菌素类药物造成神经毒性的主要机制有以下方面:①减少神经末梢释放γ-氨基丁酸(GABA),同时与GABA-A受体竞争性结合抑制GABA诱导的Cl-电流,导致神经元过度兴奋和突触后膜去极化,降低癫痫发作阈值[59-61]。值得注意的是,青霉素与GABA受体的结合是非竞争性的,意味着头孢菌素类更可能发生神经毒性[62]。②头孢他啶能诱导革兰阴性菌细胞释放内毒素,如LPS,而LPS能够造成小胶质细胞的激活,释放TNF-a和IL-1β,同时头孢他啶本身也能提升促炎细胞促进因子的转录,如TNF-α和IL-6等,这些细胞因子能够诱导神经元细胞的凋亡和损伤[63-64]。③在肾功能不全的患者中,头孢吡肟随着肌酐清除率的降低而排泄减少,而高血浆浓度的头孢吡肟能够介导血尿素增加、氨甲酰化、糖基化或其他化学蛋白修饰,导致血脑屏障通透性增加以及脑脊液毒性有机酸的积累[65]。④研究表明使用微透析技术将头孢噻利注入海马体后,实验大鼠的神经元细胞外谷氨酸显著升高,尽管该项研究结果表明头孢噻利诱导神经毒性的机制更倾向于GABA受体的阻滞,但多项研究也表明谷氨酸的过度释放能引起神经功能障碍和退化,提示谷氨酸介导的兴奋作用可能作为潜在的机制诱导神经毒性[66-68]。
(2)青霉素类 青霉素类药物诱导神经毒性的机制主要为:①研究表明青霉素在毫摩尔浓度下能阻滞GABA-A受体的电压依赖性离子通道(voltage-dependent channel),减少GABA与受体的结合,从而降低抑制信号传递,使神经元过度兴奋发生
癫痫[62,69]。②高剂量青霉素能够减少苯二氮卓受体,从而降低抑制和改变神经元兴奋性[70]。
(3)碳青霉烯类 碳青霉烯类药物神经毒性常表现为癫痫发作,其中亚胺培南的癫痫发作率在3%~33%之间,而多利培南和厄他培南则小于1%[71]。该类药物造成神经毒性的机制主要有:①与GABA-A受体结合并抑制,碳青霉烯类药物分子的侧链碱性越强,与GABA-A受体的亲和力越高,其致癫痫潜能就越大,美罗培南的C2侧链比亚胺培南和帕尼培南的碱性小得多,因此前者的神经毒性小于后者[72];②与NMDA受体结合造成兴奋性毒性[73];③与α-氨基-3-羟基-5-甲基异恶唑-4-丙酸受体的结合也被认为与癫痫发作有关[74]。
2.3 氟喹诺酮类
研究表明左氧氟沙星和环丙沙星为氟喹诺酮类药物中最常见引起神经毒性的药物[3-4,75-76]。氟喹诺酮类药物的神经系统渗透性与其致癫痫性并不完全相关,有报道称与环丙沙星相比,氧氟沙星的神经系统渗透浓度较高,能达到血清中浓度的50%,但后者相比前者,其臨床神经毒性的报道较少[77-78]。氟喹诺酮类药物造成神经毒性的机制主要有:①氟喹诺酮类药物的母核上6位的氟原子具有疏水性,脂溶性较好,易渗透进血脑屏障进入脑组织,药物浓度过高会增加细胞渗透压,使神经元细胞水肿导致药物性高颅压[79];②氟喹诺酮类药物上的7-哌嗪环与GABA结构相似,能与GABA-A受体结合阻滞GABA传递抑制性神经电流,导致神经兴奋[79-80];③激活NMDA受体使神经兴奋[37]。
2.4 多黏菌素类
多黏菌素类药物主要包括多黏菌素B和黏菌素(多黏菌素E)。早期研究报道多黏菌素在肌肉注射和静脉注射给药后神经毒性的发生率分别约为7.3%和27%[81]。近些年可能由于对给药剂量及联合用药的优化,近期的研究统计表明多黏菌素的神经毒性发生率在0~7%[82]。多黏菌素类药物造成神经毒性的机制主要有:①氧化应激:黏菌素能显著提升细胞内活性氧(ROS)水平,降低谷胱甘肽(GSH)水平和抗氧化酶超氧化物歧化酶和过氧化氢酶(CAT)活性,导致脂质、蛋白质和DNA受损,并最终导致神经元细胞死亡而表现为神经毒性[25,83];②线粒体功能障碍:研究表明多黏菌素能够诱导线粒体中Bax/Bcl-2蛋白比值上升,增加Ca2+诱导的线粒体通透性转变降低膜电位和降低琥珀酸脱氢酶,使线粒体中超微结构发生病理变化,如嵴破裂以及广泛肿胀,从而促进细胞色素C的释放并激活Caspase蛋白相关的凋亡级联
通路[40,83];③神经肌肉阻滞:多黏菌素能非竞争性地与突触前受体结合,阻断乙酰胆碱释放到突出间隙中,同时研究表明多黏菌素能提高机体内乙酰胆碱酯酶水平,加速乙酰胆碱的降解,从而延长去极化,持续消耗Ca2+,导致神经肌肉阻滞[84-85];④神经兴奋:研究表明静脉注射黏菌素后能显著提升小鼠大脑皮层中谷氨酸以及NMDA受体的表达,促进Na+和Ca2+内流到神经元导致兴奋性毒性[38]。
2.5 甲硝唑
甲硝唑长期使用易导致神经毒性,造成小脑病变,但停药后3~7 d症状会得到缓解[86]。有研究表明甲硝唑造成神经毒性的机制为甲硝唑诱导血管源性水肿继发的轴突肿胀引起的;也有研究表明甲硝唑能导致脂质过氧化物MDA(丙二醛)的累积,同时诱导体内CAT、SOD水平下降,导致氧化应激失衡,同时该研究还提及甲硝唑能够降低机体内硫胺素(维生素B1)水平,造成磷酸戊糖代谢障碍,影响磷脂类的合成,使周围和中枢神经组织出现脱髓鞘和轴索变性样改变,导致神经元细胞损伤[86-87]。
2.6 磺胺类药物
研究表明,当甲氧嘧啶的剂量从<12 mg/(kg·d)调高至>18 mg/(kg·d)时,精神病的发生率由0升至23.5%[88]。磺胺类药物引起神经毒性的机制可能是由于磺胺类药物具有的神经系统高渗透性,同时甲氧苄啶能抑制二氢叶酸还原酶(DHFR),磺胺甲恶唑为二氢蝶酸合酶的竞争性抑制剂,二者结合能干扰四氢生物蝶呤的合成,从而减少多巴胺和血清素的合成,影响神经系统的信号传导,导致神经系统的毒副作用[89-90]。
2.7 大环内酯类药物
大环内酯类药物主要包括红霉素及其衍生物(阿奇霉素和克拉霉素),该类药物的神经毒副作用主要为癫痫、谵妄、头晕、神志不清以及耳毒性[33,91]。该药物造成中枢神经系统受损的机制尚不明确,但一篇研究报道了球藻暴露在罗红霉素时,能造成MDA累积以及SOD和CAT水平的降低,提示大环内酯类药物可能通过氧化应激的方式对中枢神经系统造成损伤[92]。而耳毒性在报道中认为静脉注射大剂量大环内酯类药物时产生,这可能是由血管纹水肿导致外毛细胞功能障碍引起的[93]。
2.8 恶唑烷酮类药物
利奈唑胺作为恶唑烷酮类药物的代表药物,其神经毒副作用主要体现为周围神经病变、视神经病变、脑病、血清素综合征;意识混乱、肌阵挛和谵妄[94]。该药的毒副作用机制目前尚未定论,不过许多研究鉴于利奈唑胺的抗菌机制为通过与50S核糖体亚基结合来抑制细菌蛋白质,普遍支持其神经毒性的机制为抑制线粒体蛋白合成,从而造成后续的骨髓抑制、神经病变和乳酸中毒[95]。
3 抗菌药物神经毒性的危险因素
3.1 年龄
年龄是抗菌药物造成神经毒性的主要危险因素之一。老年患者是细菌感染(肺炎、流感和败血症)的高发人群,同时老年患者存在不同程度的肝肾功能减退, 机体肌肉含量下降,脂肪含量提高,其药物动力学(PK)特性发生变化,药物代谢减缓以及药物分布发生变化使药物易于累积,造成血药浓度增高。此外许多老年患者往往罹患各种较严重的基础疾病,存在不同程度的脑萎缩或脑动脉硬化、中枢神经系统耐受性差、血浆蛋白含量降低等因素。上述原因导致这一人群的神经系统不良反应风险增高[96-98]。
3.2 肾衰竭
在抗菌药物的诱发的神经毒性中,肾衰竭也是重要的危险因素之一。肾衰竭能从药代学以及药效学两个方面增加抗菌药物神经毒性的发生风险。
(1)药代学方面:①肾衰竭中的尿毒症毒素能降低胃肠道、肝以及肾中的细胞色素P含量,降低药物代谢酶的活性,从而导致抗菌药物代谢缓慢造成累积[99];②终末期肾病患者肝组织中P-糖蛋白(P-gp, P-glycoprotein)的药物外排功能和有机阴离子多肽转运体(OATP, organic anion transporting polypeptide)介导的药物摄取转运蛋白功能都有不同程度的缺陷,从而减少药物的非肾性消除导致药物累积[100-101];③肾衰竭导致的低白蛋白血症(与肾脏疾病中蛋白尿和硫胺素缺乏相关)减少抗菌药物的蛋白结合,导致血液中抗菌药物的游离浓度升高,增加中枢神经系统毒性风险[102];④肾衰竭患者往往肌肉和皮下脂肪含量减少,这两者都会改变亲脂性抗菌药物的药代动力学特征容积[103];总之,肾功能衰竭能降低抗菌药物的代谢消除、蛋白结合以及分布,从而导致其神经毒性风险增加。
(2)药效学方面:肾衰竭导致的电解质紊乱和神经元膜上蛋白质的化学修饰可能会降低癫痫发作的阈值,同时神经递质受体或其信号通路的化学修饰可能影响广泛的神经元功能[103]。除此之外,研究表明肾衰竭小鼠中脑脊液中的蛋白质浓度增加,同时尿毒症会介导涉及rOat3和rOatp2受体的脑-血液运输受到抑制,导致内源性代谢物和药物在大脑中积累,这表明肾衰竭能造成血脑屏障的通透性改变或中枢神经系统功能障碍[104-105]。
值得一提的是,碳青霉烯类药物在肾衰竭诱导的神经毒性机制存在一定的特殊性。一项研究表明,亚胺培南通过非肾消除产生的代谢物能够导致动物癫痫发作,且这种代谢物在肾衰竭病症中具有较长的半衰期[106]。另外一项研究则将亚胺培南的神经毒性归因于其在脑脊液的缓慢消除,该研究认为尿毒症毒素能竞争性抑制脑脊液的主动外排转运机制,因此在肾衰竭的情况下,毒性有机酸的积累或pH值的改变可能会导致亚胺培南(以及青霉素和头孢菌素)从脑脊液向血液的主动转运受阻,从而产生与脑组织药物累积量相关的神经毒性[66,107]。
3.3 既往神经系统病史及其他病症
已有多项研究表明既往神经系统病史是抗菌药物神经毒性的重要危险因素之一。神经系统疾病如中风、脑血脉硬化、帕金森症等都有潜在的加重抗菌药物神经毒性作用;对于有癫痫史患者,使用抗菌药物可能会降低癫痫发作阈值[108]。早期以及最近的研究表明,对于重症肌无力患者,使用抗菌药物会加重病症[1,41,109]。一项评估以多黏菌素B为主治療革兰阴性菌脑膜炎的有效性以及安全性的研究表明,有28%的患者在治疗过程发生了神经毒性,原因可能在于细菌性脑膜炎能够增加血脑屏障的通透性,使抗菌药物易于渗透进入脑组织造成神经毒性[110-111]。除此之外,还有一些特殊病症也会诱发或加重抗菌药物的神经毒性,有研究表明原发性甲状腺毒症是环丙沙星致癫痫的潜在危险因素[112];脓毒症与囊性纤维化症也被认为具有潜在增加抗菌药物神经毒性的作用,原因可能为这些病症诱导的部分或全身炎症导致血脑屏障通透性增加,从而增加抗菌药物血脑屏障渗透率[35,113-114]。
3.4 联合用药
在使用抗菌药物治疗的时候,往往因为患者并发症而联合用药,但不合理的联合用药可能会增加抗菌药物发生神经毒性的风险。其诱发的机制可能为影响药物代谢或共同作用增加其神经毒性。如氟喹诺酮类可抑制肝细胞色素(CYP450)酶活性,从而显著降低茶碱肝清除率,使血中茶碱浓度明显增高, 引起茶碱神经中毒症状[115];氟喹诺酮类与非类固醇类消炎镇痛药联合用药时还能协同增强氟喹诺酮类的抑制GABA作用从而诱发神经毒性[116-117]。同时还有研究表明多黏菌素与镇静剂、麻醉剂、皮质类固醇和肌肉松弛剂等药物共同给药时能提高多黏菌素发生神经毒性的风险[35]。
4 防治措施
4.1 严格把握适应症以及给药方案
对肾功能不全或肾衰竭、既往神经系统病史如癫痫、帕金森症和精神病史等以及高龄患者应谨慎用药,同时应当根据患者的机体代谢情况制定合理的给药方案, 调整给药剂量和用药时间[118],例如在一项研究对比庆大霉素一次性给药与分3次给药造成耳毒性的研究中,结果表明一次性给药方案相比分3次给药不仅在治愈率上更具优势(分别为87.5%和69.2%),在耳毒性风险方面也更为安全(一次性给药方案0例,分3次给药3例)[119];并且在出现神经毒性时应当及时停药,同时根据用药种类应避免药物相互作用。
4.2 药物控制神经毒性症状
有研究表明,在应对抗菌药物尤其是碳青霉烯类药物诱导的癫痫发作的治疗中,苯二氮卓类被推荐为一线治疗药物,因其能通过增加氯离子通道开放的速率来增强GABAA的活性,从而导致神經元超极化,有效地控制由GABA拮抗引起的癫痫[120]。另外还能选取一些具有神经保护作用药物如谷胱甘肽、神经节苷脂、雷帕霉素和红景天苷等缓解抗菌药物诱导的神经毒性[25,121]。
4.3 血药浓度检测(TDM)
对于治疗指数窄,毒性作用强,个体差异大的抗菌药物,应进行血药浓度检测,同时观测患者在治疗过程是否有癫痫发作、意识混乱、脑病以及痉挛等不良反应,结合患者病症、血药浓度以及生化指标制定更合理的给药方案,为把控药物的合理应用、建立个体化治疗提供科学依据,如一项针对利奈唑胺个体精细化给药的研究中,该文献对TDM测定的利奈唑胺群体PK模型进行蒙特卡洛模拟,结果表明使用标准的给药方案利奈唑胺(600 mg, q12h),对于MIC高于4 mg/L的菌株难以有效杀灭,对于这些菌株需要更高的药物暴露量才能达到理想的临床效果,但剂量增加可能导致超过30%的患者有潜在的神经毒副作用发生率,需考虑个体化给药保证有效安全的药物暴露[122]。
5 总结
神经毒性是抗菌药物的一种常见且严重的毒副作用,其危险因素主要有肾衰竭、既往神经系统史、脓毒血症、囊性纤维化、老龄患者以及药物联用。因此在治疗前应充分了解患者既往病史及患者机体状况,同时做好预防措施,如药物浓度监测、脑电图监测,结合患者生化指标提供合理的给药方案,从而有效将患者血药浓度控制在安全窗口内。神经毒性发生机制主要为呈浓度依赖性抑制或激活GABA、NMDA以及乙酰胆碱等神经递质的传递,同时也有药物能够通过氧化应激诱导细胞凋亡导致神经毒性。对此,使用苯二氮卓类药物以及神经保护剂在维持抗菌效果的同时减少抗菌药物对神经系统的损伤也具有一定的前景。值得注意的是研究表明在神经系统感染、全身感染(脓毒症)以及肾功能不全的患者中,血脑屏障受到炎症因子的破坏导致渗透率增加,或者抑制脑-血转运体都能增加神经毒性风险。因此进一步研究完善抗菌药物的脑内转运机制以及保护血脑屏障损伤也不失为减少抗菌药物神经毒性不良反应的一个突破点。
参 考 文 献
Rezaei N J, Bazzazi A M, Alavi S A N. Neurotoxicity of the antibiotics: A comprehensive study[J]. Neurol India, 2018, 66(6): 1732-1740.
Gschwind M, Simonetta F, Vulliemoz S. Reversible encephalopathy with photoparoxysmal response during imipenem/cilastatin treatment[J]. J Neurol Sci, 2016, 360: 23-24.
Grill M F, Maganti R K. Neurotoxic effects associated with antibiotic use: Management considerations[J]. Br J Clin Pharmacol, 2011, 72(3): 381-393.
Morales D, Pacurariu A, Slattery J, et al. Association between peripheral neuropathy and exposure to oral fluoroquinolone or amoxicillin-clavulanate therapy[J]. JAMA Neurol, 2019, 76(7): 827-833.
Goolsby T A, Jakeman B, Gaynes R P. Clinical relevance of metronidazole and peripheral neuropathy: A systematic review of the literature[J]. Int J Antimicrob Agents, 2018, 51(3): 319-325.
Leis J A, Rutka J A, Gold W L, et al. Aminoglycoside-induced ototoxicity[J]. CMAJ, 2015, 187(1): E52.
Bischoff A, Meier C, Roth F. Gentamicin neurotoxicity (polyneuropathy-encephalopathy)[J]. Schweiz Med Wochenschr, 1977, 107(1): 3-8.
Tange R A, Dreschler W A, Prins J M, et al. Ototoxicity and nephrotoxicity of gentamicin vs netilmicin in patients with serious infections: A randomized clinical trial[J]. Clin Otolaryngol Allied Sci, 1995, 20(2): 118-123.
Tulkens P M. Pharmacokinetic and toxicological evaluation of a once-daily regimen versus conventional schedules of netilmicin and amikacin[J]. J Antimicrob Chemother, 1991, 27(Suppl C): 49-61.
Klis S, Stienstra Y, Phillips R O, et al. Long term streptomycin toxicity in the treatment of Buruli Ulcer: follow-up of participants in the BURULICO drug trial[J]. PLoS Negl Trop Dis, 2014, 8(3): e2739.
Lerner A M, Reyes M P, Cone L A, et al. Randomised, controlled trial of the comparative efficacy, auditory toxicity, and nephrotoxicity of tobramycin and netilmicin[J]. Lancet, 1983, 1(8334): 1123-1126.
Bhattacharyya S, Berkowitz A L. Cephalosporin neurotoxicity: An overlooked cause of toxic-metabolic encephalopathy[J]. J Neurol Sci, 2019, 398: 194-195.
Triplett J D, Lawn N D, Chan J, et al. Cephalosporin-related neurotoxicity: Metabolic encephalopathy or non-convulsive status epilepticus?[J]. J Clin Neurosci, 2019, 67: 163-166.
Lin C S, Cheng C J, Chou C H, et al. Piperacillin/tazobactam-induced seizure rapidly reversed by high flux hemodialysis in a patient on peritoneal dialysis[J]. Am J Med Sci, 2007, 333(3): 181-184.
Huang W T, Hsu Y J, Chu P L, et al. Neurotoxicity associated with standard doses of piperacillin in an elderly patient with renal failure[J]. Infection, 2009, 37(4): 374-376.
Boschung-Pasquier L, Atkinson A, Kastner L K, et al. Cefepime neurotoxicity: Thresholds and risk factors. A retrospective cohort study[J]. Clin Microbiol Infect, 2020, 26(3): 333-339.
Grill M F, Maganti R. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring[J]. Ann Pharmacother, 2008, 42(12): 1843-1850.
Chow K M, Szeto C C, Hui A C, et al. Retrospective review of neurotoxicity induced by cefepime and ceftazidime[J]. Pharmacotherapy, 2003, 23(3): 369-373.
Quinton M C, Bodeau S, Kontar L, et al. Neurotoxic concentration of piperacillin during continuous infusion in critically III patients[J]. Antimicrob Agents Chemother, 2017, 61(9): e00654-17.
Olthof E, Tostmann A, Peters W H, et al. Hydrazine-induced liver toxicity is enhanced by glutathione depletion but is not mediated by oxidative stress in HepG2 cells[J]. Int J Antimicrob Agents, 2009, 34(4): 385-386.
Xiao C, Han Y, Liu Y, et al. Relationship between fluoroquinolone structure and neurotoxicity revealed by zebrafish neurobehavior[J]. Chem Res Toxicol, 2018, 31(4): 238-250.
Bhalerao S, Talsky A, Hansen K, et al. Ciprofloxacin-induced manic episode[J]. Psychosomatics, 2006, 47(6): 539-540.
Traeger S M, Bonfiglio M F, Wilson J A, et al. Seizures associated with ofloxacin therapy[J]. Clin Infect Dis, 1995, 21(6): 1504-1506.
Nishikubo M, Kanamori M, Nishioka H. Levofloxacin-associated neurotoxicity in a patient with a high concentration of levofloxacin in the blood and cerebrospinal fluid[J]. Antibiotics (Basel), 2019, 8(2): 78.
Dai C, Xiao X, Li J, et al. Molecular mechanisms of neurotoxicity induced by polymyxins and chemoprevention[J]. ACS Chem Neurosci, 2019, 10(1): 120-131.
Zhou Y, Li Y, Xie X, et al. Higher incidence of neurotoxicity and skin hyperpigmentation in renal transplant patients treated with polymyxin B[J]. Br J Clin Pharmacol, 2022, 88(11): 4742-4750 .
Kelesidis T, Falagas M E. The safety of polymyxin antibiotics[J]. Expert Opin Drug Saf, 2015, 14(11): 1687-1701.
Quickfall D, Daneman N, Dmytriw A A, et al. Metronidazole-induced neurotoxicity[J]. CMAJ, 2021, 193(42): E1630.
Kim E, Na D G, Kim E Y, et al. MR imaging of metronidazole-induced encephalopathy: Lesion distribution and diffusion-weighted imaging findings[J]. AJNR Am J Neuroradiol, 2007, 28(9): 1652-1658.
Saidinejad M, Ewald M B, Shannon M W. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration[J]. Pediatrics, 2005, 115(6): e739-741.
Gray D A, Foo D. Reversible myoclonus, asterixis, and tremor associated with high dose trimethoprim-sulfamethoxazole: A case report[J]. J Spinal Cord Med, 2016, 39(1): 115-117.
Ikeda A K, Prince A A, Chen J X, et al. Macrolide-associated sensorineural hearing loss: A systematic review[J]. Laryngoscope, 2018, 128(1): 228-236.
Bandettini di Poggio M, Anfosso S, Audenino D, et al. Clarithromycin-induced neurotoxicity in adults[J]. J Clin Neurosci, 2011, 18(3): 313-318.
Clark D B, Andrus M R, Byrd D C. Drug interactions between linezolid and selective serotonin reuptake inhibitors: case report involving sertraline and review of the literature[J]. Pharmacotherapy, 2006, 26(2): 269-276.
Wanleenuwat P, Suntharampillai N, Iwanowski P. Antibiotic-induced epileptic seizures: Mechanisms of action and clinical considerations[J]. Seizure, 2020, 81: 167-174.
Fu X, Wan P, Li P, et al. Mechanism and prevention of ototoxicity induced by aminoglycosides[J]. Front Cell Neurosci, 2021, 15: 692762.
Leung K. 4-Acetoxy-7-chloro-3-(3-(-4-[11C]methoxybenzyl)phenyl)-2(1H)-quinolone[M]. Molecular Imaging and Contrast Agent Database (MICAD). Bethesda (MD), 2009.
Wang J, Yi M, Chen X, et al. Effects of colistin on amino acid neurotransmitters and blood-brain barrier in the mouse brain[J]. Neurotoxicol Teratol, 2016, 55: 32-37.
Nar Z, Edizer D T, Yiit Z, et al. Does calcium dobesilate have therapeutic effect on gentamicin-induced cochlear nerve ototoxicity? An experimental study[J]. Otol Neurotol, 2020, 41(10): e1185-e1192.
Dai C, Tang S, Biao X, et al. Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice[J]. Mol Biol Rep, 2019, 46(2): 1963-1972.
Gummi R R, Kukulka N A, Deroche C B, et al. Factors associated with acute exacerbations of myasthenia gravis[J]. Muscle Nerve, 2019, 60(6): 693-699.
Sheikh S, Alvi U, Soliven B, et al. Drugs that induce or cause deterioration of myasthenia gravis: An update[J]. J Clin Med, 2021, 10(7): 1537.
Kuznetsov A V, Margreiter R, Amberger A, et al. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death[J]. Biochim Biophys Acta, 2011, 1813(6): 1144-1152.
Rizzi M D, Hirose K. Aminoglycoside ototoxicity[J]. Curr Opin Otolaryngol Head Neck Surg, 2007, 15(5): 352-357.
Van Hecke R, Van Rompaey V, Wuyts F L, et al. Systemic aminoglycosides-induced vestibulotoxicity in humans[J]. Ear Hear, 2017, 38(6): 653-662.
Steyger P S, Peters S L, Rehling J, et al. Uptake of gentamicin by bullfrog saccular hair cells in vitro[J]. J Assoc Res Otolaryngol, 2003, 4(4): 565-578.
Marcotti W, van Netten S M, Kros C J. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels[J]. J Physiol, 2005, 567(Pt 2): 505-521.
Waguespack J R, Ricci A J. Aminoglycoside ototoxicity: Permeant drugs cause permanent hair cell loss[J]. J Physiol, 2005, 567(Pt2): 359-360.
Warchol M E. Cellular mechanisms of aminoglycoside ototoxicity[J]. Curr Opin Otolaryngol Head Neck Surg, 2010, 18(5): 454-458.
Steyger P S. Mechanisms of aminoglycoside- and cisplatin-induced ototoxicity[J]. Am J Audiol, 2021, 30(3S): 887-900.
Hobbie S N, Akshay S, Kalapala S K, et al. Genetic analysis of interactions with eukaryotic rRNA identify the mitoribosome as target in aminoglycoside ototoxicity[J]. Proc Natl Acad Sci U S A, 2008, 105(52): 20888-20893.
Guan M X. Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity[J]. Mitochondrion, 2011, 11(2): 237-245.
Kros C J, Steyger P S. Aminoglycoside- and cisplatin-induced ototoxicity: Mechanisms and otoprotective strategies[J]. Cold Spring Harb Perspect Med, 2019, 9(11): a033548.
Zareifopoulos N, Panayiotakopoulos G. Neuropsychiatric effects of antimicrobial agents[J]. Clin Drug Investig, 2017, 37(5): 423-437.
Shi L J, Liu L A, Cheng X H, et al. Decrease in acetylcholine-induced current by neomycin in PC12 cells[J]. Arch Biochem Biophys, 2002, 403(1): 35-40.
Krenn M, Grisold A, Wohlfarth P, et al. Pathomechanisms and clinical implications of myasthenic syndromes exacerbated and induced by medical treatments[J]. Front Mol Neurosci, 2020, 13: 156.
Liu C, Hu F. Investigation on the mechanism of exacerbation of myasthenia gravis by aminoglycoside antibiotics in mouse model[J]. J Huazhong Univ Sci Technolog Med Sci, 2005, 25(3): 294-296.
Lacroix C, Kheloufi F, Montastruc F, et al. Serious central nervous system side effects of cephalosporins: A national analysis of serious reports registered in the French Pharmacovigilance Database[J]. J Neurol Sci, 2019, 398: 196-201.
Tamune H, Hamamoto Y, Aso N, et al. Cefepime-induced encephalopathy: Neural mass modeling of triphasic wave-like generalized periodic discharges with a high negative component (Tri-HNC)[J]. Psychiatry Clin Neurosci, 2019, 73(1): 34-42.
Cock H R. Drug-induced status epilepticus[J]. Epilepsy Behav, 2015, 49: 76-82.
Sugimoto M, Uchida I, Mashimo T, et al. Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins[J]. Neuropharmacology, 2003, 45(3): 304-314.
Sugimoto M, Fukami S, Kayakiri H, et al. The beta-lactam antibiotics, penicillin-G and cefoselis have different mechanisms and sites of action at GABA(A) receptors[J]. Br J Pharmacol, 2002, 135(2): 427-432.
Alkharfy K M, Kellum J A, Frye R F, et al. Effect of ceftazidime on systemic cytokine concentrations in rats[J]. Antimicrob Agents Chemother, 2000, 44(11): 3217-3219.
Block M L, Zecca L, Hong J S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms[J]. Nat Rev Neurosci, 2007, 8(1): 57-69.
Preston R A, Mamikonyan G, Mastim M, et al. Single-center investigation of the pharmacokinetics of WCK 4282 (Cefepime-Tazobactam Combination) in renal impairment[J]. Antimicrob Agents Chemother, 2019,63(10): e00873-19.
Ohtaki K, Matsubara K, Fujimaru S, et al. Cefoselis, a beta-lactam antibiotic, easily penetrates the blood-brain barrier and causes seizure independently by glutamate release[J]. J Neural Transm (Vienna), 2004, 111(12): 1523-1535.
Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration[J]. Pflugers Arch, 2010, 460(2): 525-542.
Mahmoud S, Gharagozloo M, Simard C, et al. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release[J]. Cells, 2019, 8(2): 184.
Rossokhin A V, Sharonova I N, Bukanova J V, et al. Block of GABA(A) receptor ion channel by penicillin: electrophysiological and modeling insights toward the mechanism[J]. Mol Cell Neurosci, 2014, 63: 72-82.
Shiraishi H, Ito M, Go T, et al. High doses of penicillin decreases [3H]flunitrazepam binding sites in rat neuron primary culture[J]. Brain Dev, 1993, 15(5): 356-361.
Ayd?n A, Bar?? Aykan M, Sa?lam K, et al. Seizure induced by ertapenem in an elderly patient with dementia[J]. Consult Pharm, 2017, 32(10): 561-562.
Sunagawa M, Matsumura H, Sumita Y, et al. Structural features resulting in convulsive activity of carbapenem compounds: effect of C-2 side chain[J]. J Antibiot (Tokyo), 1995, 48(5): 408-416.
Zivanovic D, Lovic O S, Susic V. Effects of manipulation of N-methyl-D-aspartate receptors on imipenem/cilastatin-induced seizures in rats[J]. Indian J Med Res, 2004, 119(2): 79-85.
Koppel B S, Hauser W A, Politis C, et al. Seizures in the critically ill: The role of imipenem[J]. Epilepsia, 2001, 42(12): 1590-1593.
Ali A K. Peripheral neuropathy and Guillain-Barré syndrome risks associated with exposure to systemic fluoroquinolones: A pharmacovigilance analysis[J]. Ann Epidemiol, 2014, 24(4): 279-285.
Bhattacharyya S, Darby R R, Raibagkar P, et al. Antibiotic-associated encephalopathy[J]. Neurology, 2016, 86(10): 963-971.
Schwartz M T, Calvert J F. Potential neurologic toxicity related to ciprofloxacin[J]. DICP, 1990, 24(2): 138-140.
Kaur K, Fayad R, Saxena A, et al. Fluoroquinolone-related neuropsychiatric and mitochondrial toxicity. A collaborative investigation by scientists and members of a social network[J]. J Community Support Oncol, 2016, 14(2): 54-65.
De Sarro A, De Sarro G. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects[J]. Curr Med Chem, 2001, 8(4): 371-384.
高偉波, 朱继红. 氟喹诺酮类药物中枢神经系统不良反应及其防治[J]. 疑难病杂志, 2008, 7(8): 507-509.
Koch-Weser J, Sidel V W, Federman E B, et al. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy[J]. Ann Intern Med, 1970, 72(6): 857-868.
Molina J, Cordero E, Pachón J. New information about the polymyxin/colistin class of antibiotics[J]. Expert Opin Pharmacother, 2009, 10(17): 2811-2828.
Dai C, Tang S, Velkov T, et al. Colistin-induced apoptosis of neuroblastoma-2a cells involves the generation of reactive oxygen species, mitochondrial dysfunction, and autophagy[J]. Mol Neurobiol, 2016, 53(7): 4685-700.
Ajiboye T O. Colistin sulphate induced neurotoxicity: Studies on cholinergic, monoaminergic, purinergic and oxidative stress biomarkers[J]. Biomed Pharmacother, 2018, 103: 1701-1707.
Yachan N, Yanqi C, You W, et al. Case report: Respiratory paralysis associated with polymyxin B therapy[J]. Front Pharmacol, 2022, 13: 963140.
Lefkowitz A, Shadowitz S. Reversible cerebellar neurotoxicity induced by metronidazole[J]. CMAJ, 2018, 190(32): E961.
Hassan M H, Awadalla E A, Ali R A, et al. Thiamine deficiency and oxidative stress induced by prolonged metronidazole therapy can explain its side effects of neurotoxicity and infertility in experimental animals: Effect of grapefruit co-therapy[J]. Hum Exp Toxicol, 2020, 39(6): 834-847.
Lee K Y, Huang C H, Tang H J, et al. Acute psychosis related to use of trimethoprim/sulfamethoxazole in the treatment of HIV-infected patients with Pneumocystis jirovecii pneumonia: A multicentre, retrospective study[J]. J Antimicrob Chemother, 2012, 67(11): 2749-2754.
Patey O, Lacheheb A, Dellion S, et al. A rare case of cotrimoxazole-induced eosinophilic aseptic meningitis in an HIV-infected patient[J]. Scand J Infect Dis, 1998, 30(5): 530-531.
Haruki H, Pedersen M G, Gorska K I, et al. Tetrahydrobiopterin biosynthesis as an off-target of sulfa drugs[J]. Science, 2013, 340(6135): 987-991.
Principi N, Esposito S. Comparative tolerability of erythromycin and newer macrolide antibacterials in paediatric patients[J]. Drug Saf, 1999, 20(1): 25-41.
Li J, Min Z, Li W, et al. Interactive effects of roxithromycin and freshwater microalgae, Chlorella pyrenoidosa: Toxicity and removal mechanism[J]. Ecotoxicol Environ Saf, 2020, 191: 110156.
Yorgason J G, Luxford W, Kalinec F. In vitro and in vivo models of drug ototoxicity: Studying the mechanisms of a clinical problem[J]. Expert Opin Drug Metab Toxicol, 2011, 7(12): 1521-1534.
Narita M, Tsuji B T, Yu V L. Linezolid-associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome[J]. Pharmacotherapy, 2007, 27(8):1189-1197.
Flanagan S, McKee E E, Das D, et al. Nonclinical and pharmacokinetic assessments to evaluate the potential of tedizolid and linezolid to affect mitochondrial function[J]. Antimicrob Agents Chemother, 2015, 59(1): 178-185.
Cunha B A. Antibiotic side effects[J]. Med Clin North Am, 2001, 85(1): 149-185.
Kumar A, Roberts D, Wood K E, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock[J]. Crit Care Med, 2006, 34(6): 1589-1596.
Jafarinasabian P, Inglis J E, Reilly W, et al. Aging human body: Changes in bone, muscle and body fat with consequent changes in nutrient intake[J]. J Endocrinol, 2017, 234(1): R37-R51.
李輝, 芮建中. 慢性肾衰竭对非肾消除药物体内代谢与转运的影响[J]. 中国临床药理学杂志, 2017, 33(21): 2179-2181.
Dro?dzik M, Oswald S, Dro?dzik A. Impact of kidney dysfunction on hepatic and intestinal drug transporters[J]. Biomed Pharmacother, 2021, 143: 112125.
Torres A M, Dnyanmote A V, Granados J C, et al. Renal and non-renal response of ABC and SLC transporters in chronic kidney disease[J]. Expert Opin Drug Metab Toxicol, 2021, 17(5): 515-542.
Hamed S A. Neurologic conditions and disorders of uremic syndrome of chronic kidney disease: Presentations, causes, and treatment strategies[J]. Exp Rev Clin Pharmacol, 2019, 12(1): 61-90.
Mattappalil A, Mergenhagen K A. Neurotoxicity with antimicrobials in the elderly: A review[J]. Clin Ther, 2014, 36(11): 1489-1511.e4.
Freeman R B, Sheff M F, Maher J F, et al. The blood-cerebrospinal fluid barrier in uremia[J]. Ann Intern Med, 1962, 56: 233-240.
Deguchi T, Isozaki K, Yousuke K, et al. Involvement of organic anion transporters in the efflux of uremic toxins across the blood-brain barrier[J]. J Neurochem, 2006, 96(4): 1051-1059.
Kahan F M, Kropp H, Sundelof J G, et al. Thienamycin: Development of imipenen-cilastatin[J]. J Antimicrob Chemother, 1983, 12(Suppl D): 1-35.
Chow K M, Szeto C C, Hui A C, et al. Mechanisms of antibiotic neurotoxicity in renal failure[J]. Int J Antimicrob Agents, 2004, 23(3): 213-217.
Czapińska-Ciepiela E. The risk of epileptic seizures during antibiotic therapy[J]. Wiad Lek, 2017, 70(4): 820-826.
Hokkanen E. The aggravating effect of some antibiotics on the neuromuscular blockade in myasthenia gravis[J]. Acta Neurol Scand, 1964, 40(4): 346-352.
Falagas M E, Bliziotis I A, Tam V H. Intraventricular or intrathecal use of polymyxins in patients with Gram-negative meningitis: A systematic review of the available evidence[J]. Int J Antimicrob Agents, 2007, 29(1): 9-25.
Yau B, Hunt N H, Mitchell A J, et al. Blood-brain barrier pathology and CNS Outcomes in Streptococcus pneumoniae Meningitis[J]. Int J Mol Sci, 2018, 19(11): 3555.
Agbaht K, Bitik B, Piskinpasa S, et al. Ciprofloxacin-associated seizures in a patient with underlying thyrotoxicosis: case report and literature review[J]. Int J Clin Pharmacol Ther, 2009, 47(5): 303-310.
Rekis N, Ambrose M, Sakon C. Neurotoxicity in adult patients with cystic fibrosis using polymyxin B for acute pulmonary exacerbations[J]. Pediatr Pulmonol, 2020, 55(5): 1094-1096.
Jin L, Li J, Nation R L, et al. Impact of p-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice[J]. Antimicrob Agents Chemother, 2011, 55(2): 502-507.
周义文. 抗感染药物对神经系统的毒性作用[J]. 中国实用内科杂志, 2001, 21(11): 692-694.
Giardina W J. Assessment of temafloxacin neurotoxicity in rodents[J]. Am J Med, 1991, 91(6A): 42S-44S.
Motomura M, Kataoka Y, Takeo G, et al. Hippocampus and frontal cortex are the potential mediatory sites for convulsions induced by new quinolones and non-steroidal anti-inflammatory drugs[J]. Int J Clin Pharmacol Ther Toxicol, 1991, 29(6): 223-227.
Hoff B M, Maker J H, Dager W E, et al. Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: An update[J]. Ann Pharmacother, 2020, 54(1): 43-55.
Raz R, Adawi M, Romano S. Intravenous administration of gentamicin once daily versus thrice daily in adults[J]. Eur J Clin Microbiol Infect Dis, 1995, 14(2): 88-91.
Payne L E, Gagnon D J, Riker R R, et al. Cefepime-induced neurotoxicity: A systematic review[J]. Crit Care, 2017, 21(1): 276.
郭昌, 趙文韬, 胡丰良. 奥沙利铂神经毒性的机制及防治研究进展[J]. 现代中西医结合杂志, 2020, 29(9): 1022-1026.
Luque S, Hope W, Sorli L, et al. Dosage individualization of linezolid: precision dosing of linezolid to optimize efficacy and minimize toxicity[J]. Antimicrob Agents Chemother, 2021, 65(6): e02490-20.

