Molecular simulation study of the stabilization process of NEPE propellant
Ling-ze Kong ,Ke-hi Dong ,* ,Ai-min Jing ,Chun-lu Yng ,Yn-hui Tng ,Yun-dong Xio
a Department of Aircraft Engineering, Naval Aviation University, Yantai, 264001, China
b School of Physics and Optoelectronic Engineering, Ludong University, Yantai, 264001, China
Keywords:NEPE propellant Stabilizer Stabilization process Molecular simulation DFT VTST
ABSTRACT In this reported study,the density functional theory(DFT)was used at the(U)B3LYP/6-311G(d,p)level to investigate the stabilization process of the nitrate ester plasticized polyether propellant (NEPE).Molecular simulations were conducted of the reaction that generates NO2,the autocatalytic and aging reaction triggered by the NO2,and the nitrogen dioxide absorption reaction of the stabilizers during the propellent stabilization process.These simulations were derived using the transition-state theory (TST)and variational transition-state theory (VTST).The simulation results suggested that the stabilization of the NEPE propellant consisted of three stages.First,heat and NO2 were generated during the denitrification reaction of nitroglycerine (NG) and 1,2,4-butanetriol trinitrate(BTTN) in the NEPE propellant.Second,nitroso products were generated by the reactions of N-Methyl-4-nitroaniline (MNA) and 2-nitrodiphenylamine (2NDPA) with NO2.Third,the stabilizers were exhausted and the autocatalytic reaction of NG and BTTN and the aging reaction of polyethylene glycol (PEG) were triggered by the heat and NO2 generated in the first stage.By comparing the energy barriers of the various reactions,it was found that the NO2 generated from the denitrification reaction significantly reduced the reaction energy barrier to 105.56-126.32 kJ/mol,also increased the reaction rate constant,and decreased the thermal stability and energetic properties of the NEPE propellant.In addition,the NO2 also weakened the mechanical properties of the NEPE propellant by attacking the -CH2 groups and the O atoms in the PEG molecular chain.The energy barriers of the reactions of MNA and 2NDPA with NO2 (94.61-133.61 kJ/mol)were lower than those of the autocatalytic and decomposition reactions of NG,BTTN,and the aging reactions of PEG (160.30-279.46 kJ/mol).This indicated that,by eliminating NO2,the stabilizer in the NEPE propellant can effectively prevent NO2 from reacting with the NG,BTTN,and PEG in the NEPE propellant.Consequently,this would help maintain the energy and mechanical properties of the NEPE propellant,thereby improving its thermal stability.
1.Introduction
Nitrate ester plasticized polyether propellant (NEPE) has good energy characteristics and excellent mechanical properties due to the addition of plasticizer components such as nitroglycerine(NG)and 1,2,4-butanetriol trinitrate(BTTN).However,a large number of experimental studies have shown that the addition of these agents to the NEPE propellant will gradually decompose,and produce NO2,alkoxyl radicals,and other substances.These reaction products can then further accelerate the decomposition of NG,BTTN,which will degrade the energetic performance of the propellant.In addition,this process will also lead to the aging of the PEG binder matrix,and degrade the mechanical properties of the propellant [1-8].The combined effects of the two features will dramatically shorten the service life of the propellant.Consequently,to inhibit the effects of the NO2on the NEPE propellant,N-Methyl-4-nitroaniline(MNA),2-nitrodiphenylamine (2NDPA),and other stabilizers are usually added to the propellant mixture.Understanding the stabilization process of the NEPE propellant is the key to improving its thermal stability,energy,and physical properties,thus extending its service life.
To date,most domestic and foreign researchers have focused on the aging of the different components of the NEPE propellant.For instance,Vogelsanger et al.[9-15] studied the thermal decomposition process of nitrate,calculated the activation energies for decomposition of nitrate ester groups,and found the energy of the RO-NO2ester bond to be small.Following decomposition,the resulting NO2can immediately initiate a series of exothermic side reactions with neighboring nitrate and PEG molecules,which accelerates the decomposition of nitrate and PEG and makes various decomposition products appear [9,11,12,16-19].Zayed et al.[20-22] showed that stabilizers can slow the decomposition and autocatalytic process of the plasticizer by absorbing NO2and other plasticizer thermal decomposition products by bonding with them thus precluding further reaction of NO2with other propellant components.Subsequently,Lindblom et al.[23-25]found that the stabilizers cannot prevent the nitrate ester decomposition but can only slow down the rate of the catalytic reaction caused by the decomposition products.However,these reported observations were mostly speculations based on experimental results and did not explain how NO2affects propellant aging or the process of absorption of NO2by stabilizers during the NEPE propellant stabilization process.In recent years,with the rapid development of computational chemistry,molecular simulation technology has become a powerful tool for investigating chemical reaction processes,reaction mechanisms,and predicting material properties.Pei et al.[26-31]analyzed the thermal decomposition mechanism of PEG and different kinds of nitrate esters based on density functional theory(DFT)and molecular dynamics method and identified the possible decomposition path of its monomers.Yan et al.[32-35] used quantum chemical (QC) simulation to analyze the decomposition of plasticizers such as NG,BTTN and the stability of different stabilizers such as EC,MENA,NMA,and 2NDPA in the NEPE propellant and reported a good correlation between their simulations and experimental results.However,according to the present studies,the experiment is still the main method to study the stabilization process of the NEPE propellant and the stability mechanism of stabilizers.Also,relevant theoretical and kinetic studies of these systems have rarely been reported.It is still not clear how nitrogen dioxide catalyzes the decomposition of the plasticizers and binders during the propellant stabilization process,which accelerates the degradation of thermal stability and mechanical properties of the propellant,and how the stabilizers improve the propellant storage performance by inhibiting nitrogen dioxide.
To explore the stabilization process of the NEPE propellant and to reveal the chemical reactions and stability mechanism of the stabilizers,the density functional theory(DFT)method at the level of 6-311G(d,p) was applied to study PEG,NG,BTTN,MNA,and 2NDPA.Using the DFT method,the mechanism of the generation of NO2,and the autocatalytic and aging reactions initiated by NO2were initially simulated and analyzed.Then,the reaction energy barrier and rate constants of various reactions between MNA,2NDPA and NO2were calculated,and finally,the energy barrier and rate constants of each reaction were analyzed to elucidate the stability mechanisms of MNA and 2NDPA and the effect of NO2on the stabilization process of the NEPE propellant.
2.Methods of simulation and calculation
2.1.Quantum chemistry simulation methods
The molecular structures required for the simulation were constructed in GaussView,and different molecular structures were optimized at the B3LYP/6-311G(d,p) level,so that the molecular models with the lowest energy were obtained after frequency analysis to confirm that there was no imaginary frequency (IF).Subsequently,the transition state(TS)involved in the reaction was calculated at the same level,and the intrinsic reaction coordinate(IRC) of the transition state was simulated to ensure the authenticity of the transition state in the reaction.The quantum chemical simulation calculations are all performed using the Gaussian program.The structural formulae of NG,BTTN,PEG(n=2),MNA,and 2NDPA are shown in Fig.1.From the calculations of the dissociation energy required for the reaction of PEG molecules with different degrees of polymerization by Pei et al.,it was clear that the degree of polymerization was less influential on the aging reaction of PEG molecules,and PEG(n=2)better represented the aging state of PEG molecules.Therefore,to optimize computational time,a PEG molecular model with a degree of polymerization of 2 was used as the object of this study [36].
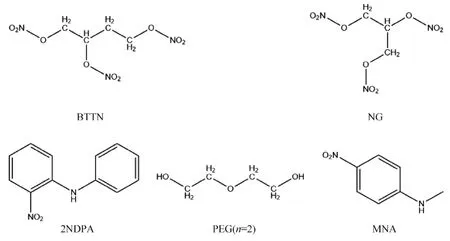
Fig.1.Structural formulae of NG,BTTN,PEG(n=2),MNA and 2NDPA.
2.2.Calculation of chemical reaction rate constants
The reaction rate constantkCVTwas calculated using the variational transition state theory of the CVT method.The calculation method ofkCVTis represented by Eq.(1)
where σ is the degeneracy of the reaction path and its value is the ratio of the number of rotational symmetries of the reactants to the transition state;kBis Boltzmann's constant,J/K;Tis the temperature;his Planck's constant,6.626×10-34J s;QGT(T,s)andQR(T)are the partition functions of the transition state and the reactants at the reaction coordinate(s) at the temperatureT,respectively;VEMPis the value of potential energy along the minimum energy path.
3.Results and discussion
3.1.Generation mechanism of NO2
The results of thermal decomposition experiments conducted by Bohn et al.showed that the bond energy of the nitrate ester RO-NO2bond is low,and it can be directly cleaved to form NO2during long-term storage [37].The denitrification reactions of NG with BTTN to generate NO2were obtained by molecular simulation.
The bond dissociation energy (BDE) of different N-O bonds in NG and BTTN molecules was calculated to determine the preferential position of the denitrification reaction.The dissociation positions and bond dissociation energies of NG and BTTN are shown in Fig.2 and Table 1 respectively.

Table 1 Bond dissociation energy of denitrification reaction.
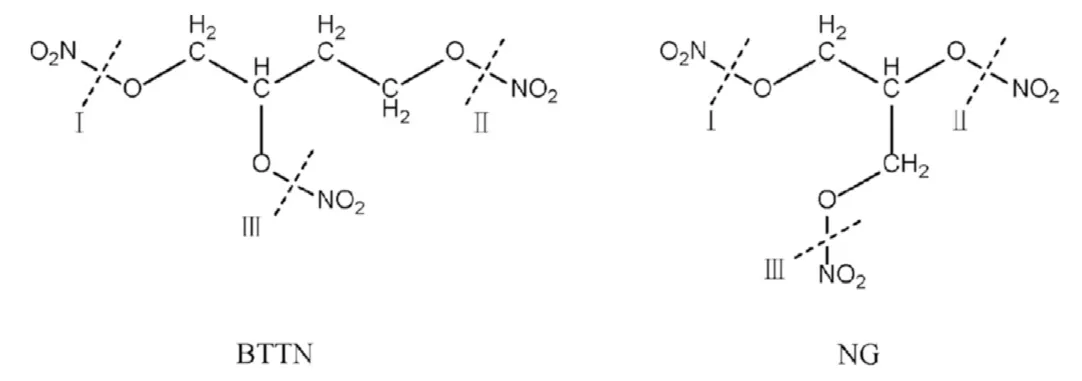
Fig.2.The dissociation position of denitrification reaction of NG and BTTN.
It can be seen from Table 1 that the dissociation energy required for cleaving I in BTTN molecule and I/III in NG molecule was lower than that required for cleaving other N-O bonds,indicating that I(BTTN) and I/III (NG) bonds are the easiest to cleave in the decomposition process of NG and BTTN respectively,thus triggering the denitrification reaction.The equations for denitrification reactions of NG and BTTN are shown in Eq.(R1) and Eq.(R2)respectively.
where TS1and TS2are the transition states of the denitrification reactions of NG and BTTN respectively(see Fig.3).
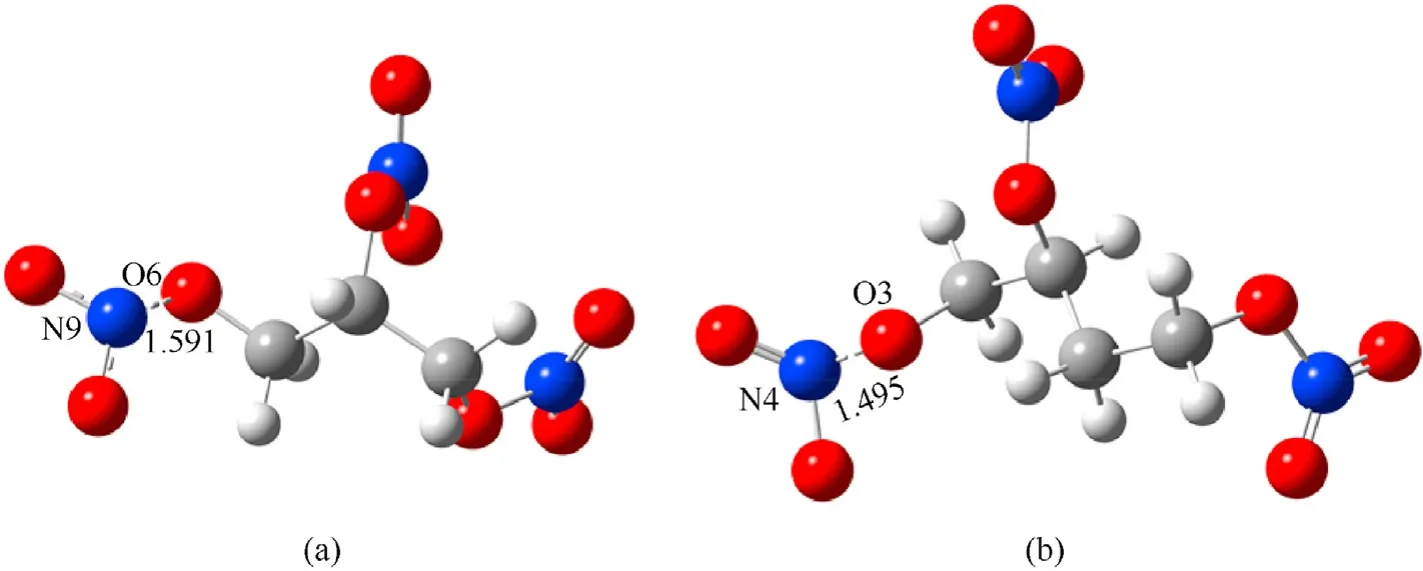
Fig.3.Transition state of denitration reaction of NG and BTTN: (a) TS1;(b)TS2.
To clearly explain the decomposition mechanisms of NG and BTTN,the energy barrier for the denitrification reactions of NG and BTTN was calculated using the transition state theory,as shown in Table 2.Fig.4 shows the IRC curves connecting the transition states between reactants and products.
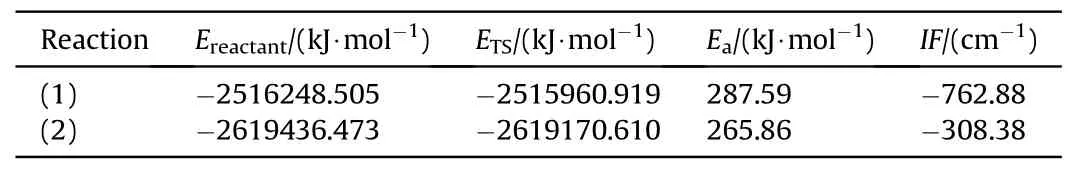
Table 2 Energy barrier of denitration reaction of NG and BTTN.

Fig.4.IRC curves of denitration reaction of NG and BTTN: (a) Reaction (1);(b) Reaction (2).
It can be seen from Fig.3 and Fig.4 that the reaction mechanisms for the denitrification reaction between NG and BTTN were similar.At the initial stage of the reaction,the bond length of the N-O bond with lower bond energy in the NG and BTTN gradually elongates from 1.43 Å and 1.43 Å to 1.59 and 1.50 Å in TS1and TS2respectively.At this point,the N-O bonds in NG and BTTN have been broken and NO2has been released.It can be seen from the IRC curves that these reactions are exothermic.Therefore,as the reactions continue,other N-O bonds with highly active denitrification products from NG and BTTN will undergo further homogeneous cracking reactions and release more NO2as the temperature increases.Zhao et al.investigated the thermal decomposition of different components of the NEPE propellant using thermolysis/RSFT-IR.They found that as the temperature increased,the intensity of the absorption peak of O-NO2began to decline slowly,indicating that NG began to decompose,which was consistent with the results of simulation results [38,39].
3.2.Autocatalytic and the aging reaction initiated by NO2
JB levy et al.employed thermal decomposition and highperformance liquid chromatography to show that NO2can accelerate the decomposition of NG and BTTN thereby releasing copious quantities of heat,and the generated NO2will then react with PEG to deteriorate the physical properties of the NEPE propellant[4,5,40].The types of reactions initiated by NO2in the stabilization process of the NEPE propellant were obtained by molecular simulation,including the autocatalytic decomposition of NG and BTTN,and the dehydrogenation and decomposition of PEG.
3.2.1.Autocatalytic decomposition of NG and BTTN
The reactions for the autocatalytic decomposition of NG and BTTN were obtained by molecular simulation,as shown in Eq.(R3)and Eq.(R4).The transition state and energy barrier for the autocatalytic decomposition reactions of NG and BTTN are shown in Fig.5 and Table 3 respectively.Fig.6 shows the IRC curves that connect the transition states between reactants and products.
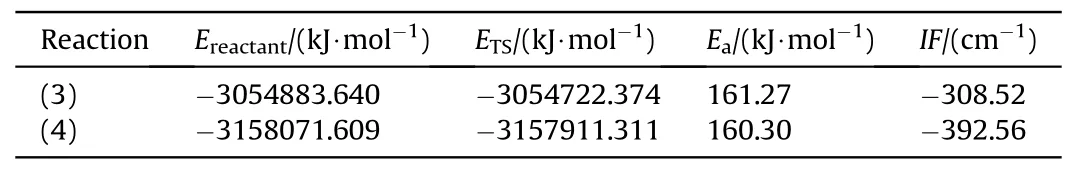
Table 3 Energy barrier of autocatalytic reaction NG and BTTN.
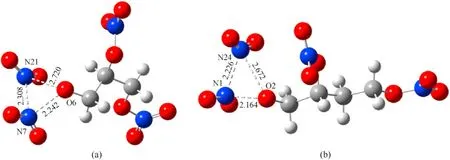
Fig.5.Transition state of autocatalytic reaction of NG and BTTN: (a) TS3;(b)TS4.
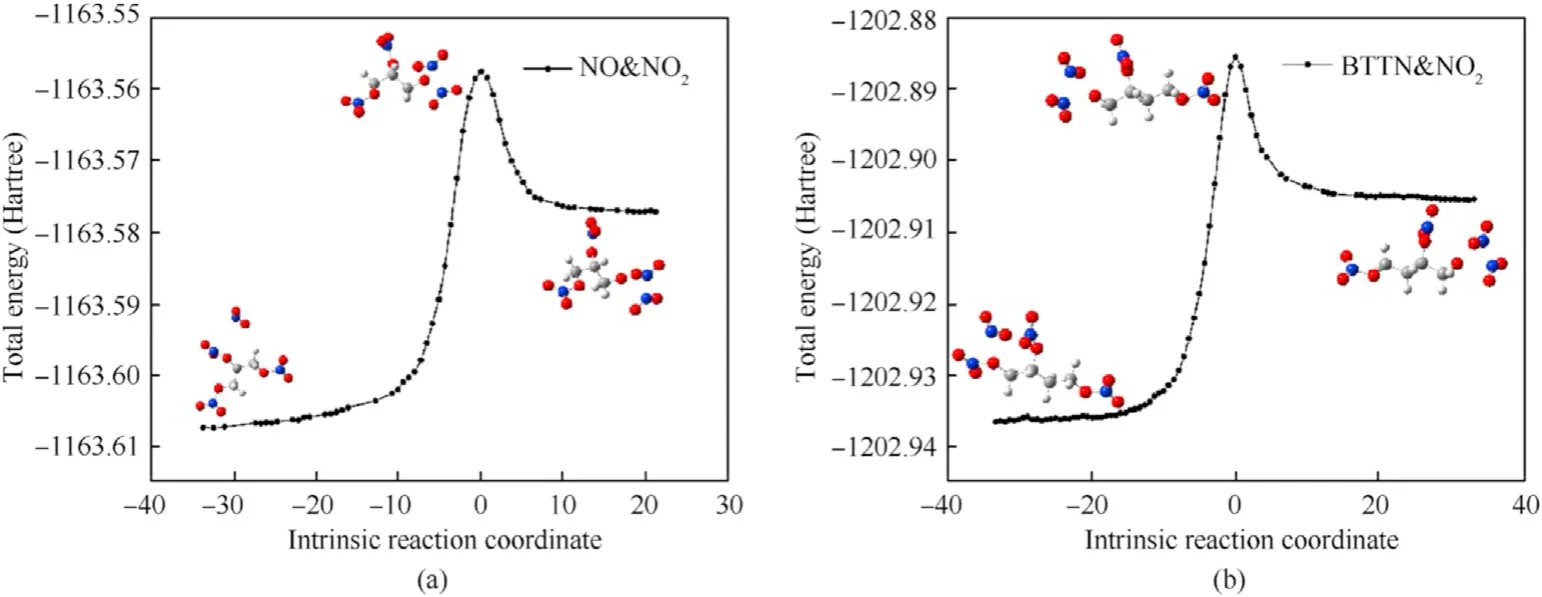
Fig.6.IRC curves of autocatalytic reaction of NG and BTTN: (a) Reaction (3);(b) Reaction (4).
where TS3and TS4are the transition states of the autocatalytic reactions of NG and BTTN respectively.
As can be seen in Fig.5,with the increase in the amount of NO2released by NG and BTTN during denitrification,the bond lengths of N7-O6 in the NG and N1-O2 in the BTTN gradually elongate from 1.43 Å and 1.43 Å to 2.24 and 2.16 Å in TS3and TS4respectively.At this point,the N-O bonds in NG and BTTN have been cleaved.As the reaction continues,the NO2produced by the fracture of the N-O bonds gradually approaches the reactant to form N-N bonds and produces N2O4.Because N2O4is relatively unstable,it rapidly decomposes into a large amount of NO2.Fig.6 shows that these reactions are endothermic.By comparing the energy barrier of the denitrification reaction and autocatalytic reaction in Table 2 and Table 3,it can be seen that the presence of NO2greatly reduces the energy barrier of the denitrification reaction of NG and BTTN,which accelerates the rate of the denitrification reaction.Moriaty et al.studied the mixture of nitrocellulose and NO2vapor.They found that the addition of NO2would accelerate the decomposition by autocatalysis,resulting in heat and more NO2.The simulation results are consistent with the experimental results[41].Therefore,with the increase in the storage time of the NEPE propellant,the rate of decomposition of the NG and BTTN continues to increase,releasing a large amount of heat and NO2.At the same time,the large quantity of generated NO2also increases the pressure in the NEPE propellant matrix producing microcracks and holes.
3.2.2.Dehydrogenation reaction of PEG
To determine when the PEG dehydrogenation reaction first occurs,the bond dissociation energy (BDE) of various C-H bonds in the PEG molecules was calculated,as shown in Table 4.Fig.7 shows the dissociation positions of different C-H bonds in the PEG.

Table 4 Dissociation energy of dehydrogenation reaction of PEG(n=2).
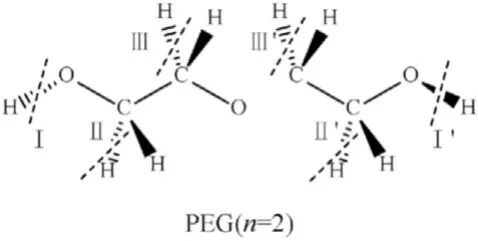
Fig.7.Dissociation position of dehydrogenation reaction of PEG(n=2).
As shown in Table 4,the dissociation energy required for the fracture at the III/III'position in the PEG is smaller than the energy of other positions,indicating that the III/III' position is the most vulnerable for reaction with NO2,leading to a dehydrogenation reaction.The chemical equations for the dehydrogenation reactions between PEG and NO2are shown in Eq.(R5) and Eq.(R6).
where TS5and TS6are the transition states for the reaction of NO2with PEG respectively(see Fig.8).
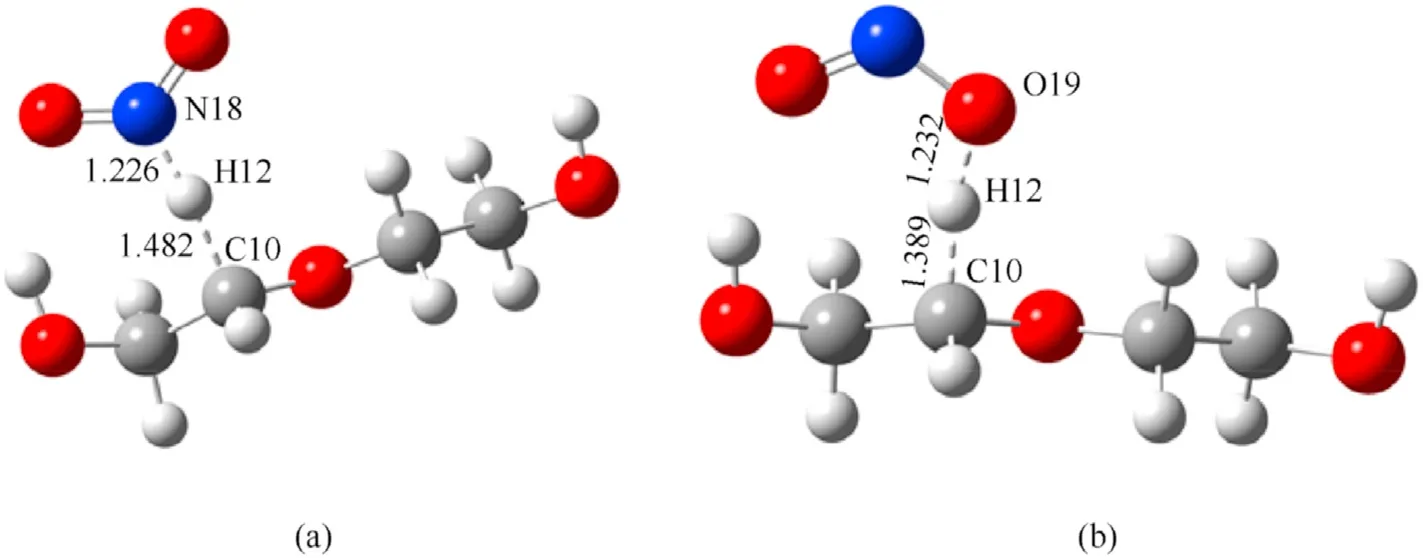
Fig.8.Transition state of dehydrogenation reaction of PEG(n=2): (a) TS5;(b)TS6.
The energy barrier for the dehydrogenation reactions between PEG and NO2was obtained from molecular simulation,as shown in Table 5.Fig.9 is the IRC curves that connect the transition states between the reactants and products.
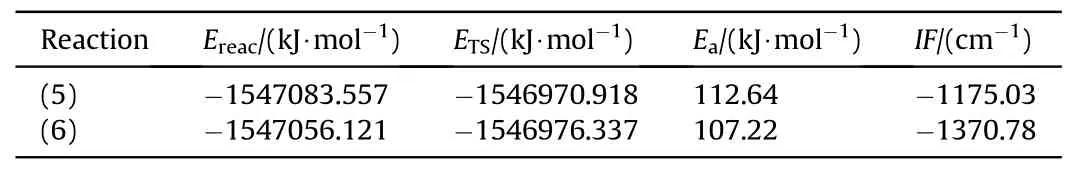
Table 5 Energy barrier of dehydrogenation reaction of PEG(n=2).
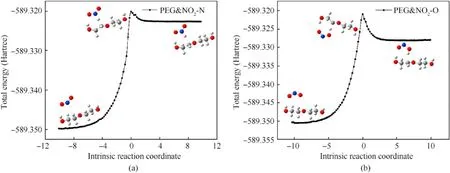
Fig.9.IRC curves of dehydrogenation reaction of PEG(n=2): (a) Reaction (5);(b) Reaction (6).
Based on the calculated results for the transition state,thelength of the C-H bond in the PEG gradually elongates from 1.10 to 1.48/1.39 Å in TS5/TS6due to its attraction to the N/O atom at the beginning of the reaction.As the reaction moves through the transition state,the C-H bond is gradually broken,and the distance between N or O atoms to the H atom gradually shortens to form N-H or O-H bonds.During the reaction between NO2and PEG,the energy barrier of the O atom to initiate the dehydrogenation reaction is slightly lower than that required for the N atom to react.To explore the reason for the difference between these energy barriers,the penetration diagrams of the van der Waals surface electrostatic potential between TS5and TS6molecules were plotted as shown in Fig.10.It can be seen from Fig.10 that there are two reasons for these variations.On one hand,due to the difference in the electronegativity of N and O,the electronegativity of the O atom in NO2is relatively large,so it tends to be attracted by the less electronegative H atom of the PEG molecule during the reaction.This triggers the dehydrogenation reaction and generates HNO2.On the other hand,compared to the N atom,the O atom has a relatively smaller steric hindrance that facilitates its approach to the H atom,which leads to the dehydrogenation reaction.These were also confirmed by the nuclear magnetic resonance (NMR) and Fourier transform infrared (FTIR) results obtained by Luo et al.Luo et al.found that substances such as nitrogen dioxide and alkoxy radicals produced by the thermal decomposition of nitrate esters can acquire protons from the main chain of polyurethane and produce acids and alcohols [42].With the attack of the NO2on the -CH2group in the PEG molecule,the C-H bonds in the main chain of the PEG molecule continue to shrink,and the flexibility of the molecular chain gradually increases,resulting in an increase in the maximum elongation in the early stage of aging of NEPE propellant[43,44].Comparing Table 3 and Table 5,it can be seen that the energy barrier for the dehydrogenation reaction of PEG is lower than that of the autocatalytic reaction of NG and BTTN.This indicates that the NO2released by the decomposition of the plasticizers NG and BTTN will initially dehydrogenate the PEG producing an acidic environment that is conducive to the decomposition of NG and BTTN.Consequently,this will accelerate the decomposition and autocatalytic reactions of NG and BTTN.Note:Ereactantis the free energies of the reactant;ETSis the free energies of the transition state;Eais the energy barrier;IFis the imaginary frequency.The data were all obtained at the (U)B3LYP/6-311++G(d,p) level.
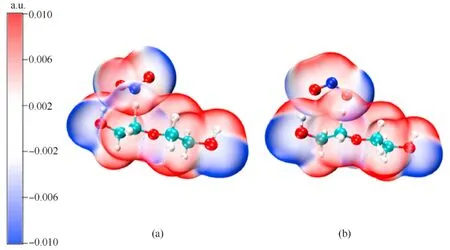
Fig.10.Penetration diagram of van der Waals surface electrostatic potential between (a) TS5 and (b) TS6 molecules.
3.2.3.Decomposition reaction of PEG
The chemical reaction equation corresponding to the decomposition reaction of PEG is shown in Eq.(R7).
where TS7is the transition state of the PEG decomposition reaction(see Fig.11).
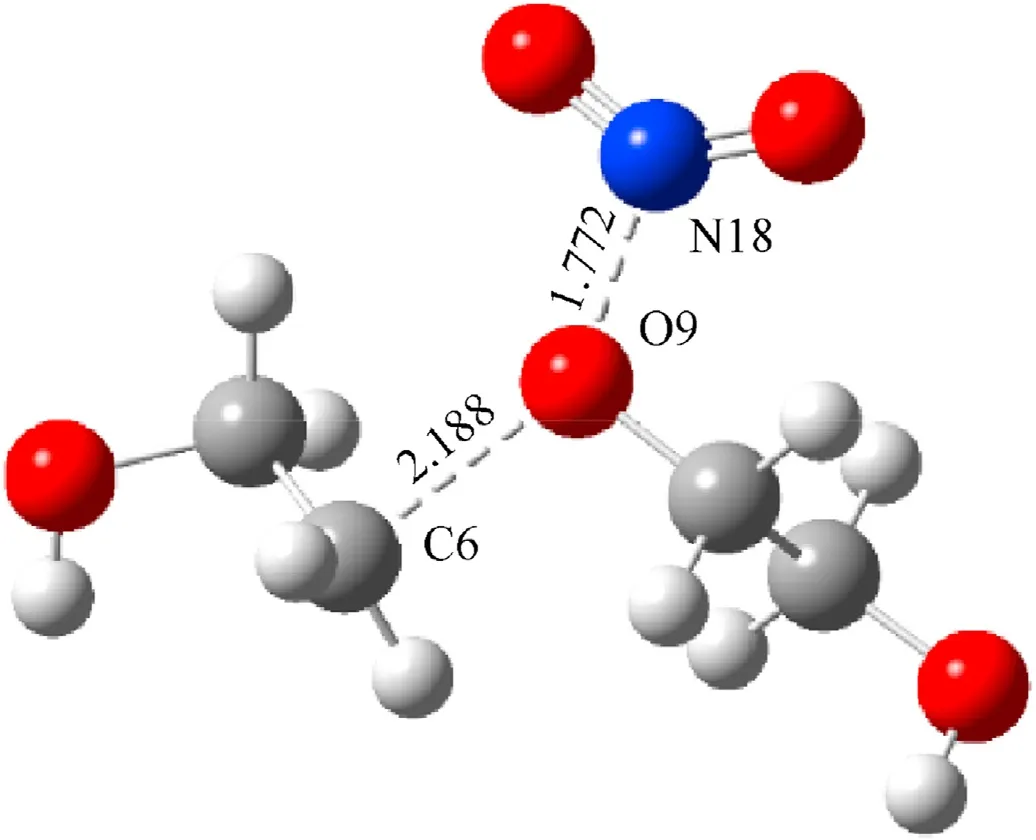
Fig.11.Transition state of decomposition reaction of PEG(n=2).
The transition state energy barrier of the PEG decomposition reaction was calculated as shown in Table 6.Fig.12 is the IRC curve connecting the transition state between reactants and products.

Table 6 Energy barrier of decomposition reaction of PEG(n=2).
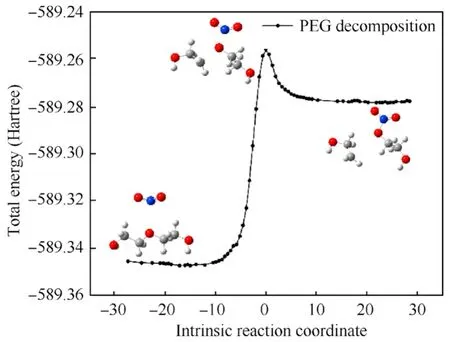
Fig.12.IRC curve of the decomposition reaction of PEG(n=2).
According to calculated results for the transition state,the positively charged N atom in the reaction between NO2and PEG can attack the methylene in the PEG molecule but it can also attack the negatively charged O atom due to the relatively small steric hindrance of the O atom in the PEG.At the initial stage of the reaction,the C-O bond in the PEG molecule is attracted by the NO2,and the bond length of C-O gradually increases from 1.42 to 2.19 Å in TS7.As the reaction continues,the distance between the N atom and O atom decreases from 1.77 Å in TS7to 1.42 Å,forming an N-O bond.At this point,the PEG molecular chain chemically deteriorated.There have been literature reports such as that by Zhao et al.showing that as the rate of nitrate ester decomposition increases,the absorption band for the C-O-C bond in the propellant decreased significantly[38].Comparing Table 5 and Table 6,it can be seen that compared to the dehydrogenation reaction,the energy barrier for the PEG decomposition reaction is higher and the reaction time is longer,which is also one of the reasons for the significant reduction of the maximum elongation in the later stage of aging of the NEPE propellant [43,44].
3.3.Stability mechanism of stabilizers
By analyzing their chromatographic experimental results,Lussier et al.speculated that the propellent stabilizer primarily inhibited the autocatalytic decomposition of the nitrate ester by reacting with the nitrogen oxide from the decomposed nitrate ester[45,46].From the molecular simulation of the reactions between NO2with 2NDPA and MNA,it was found that 2NDPA and MNA played a stabilizing role through the dehydrogenation reaction.The chemical equations that correspond to the dehydrogenation reaction of MNA and 2NDPA are exhibited by Eq.(R8)-Eq.(R11).
where TS8-TS11are the transition states of the reactions of NO2with 2NDPA and MNA respectively(see Fig.13).
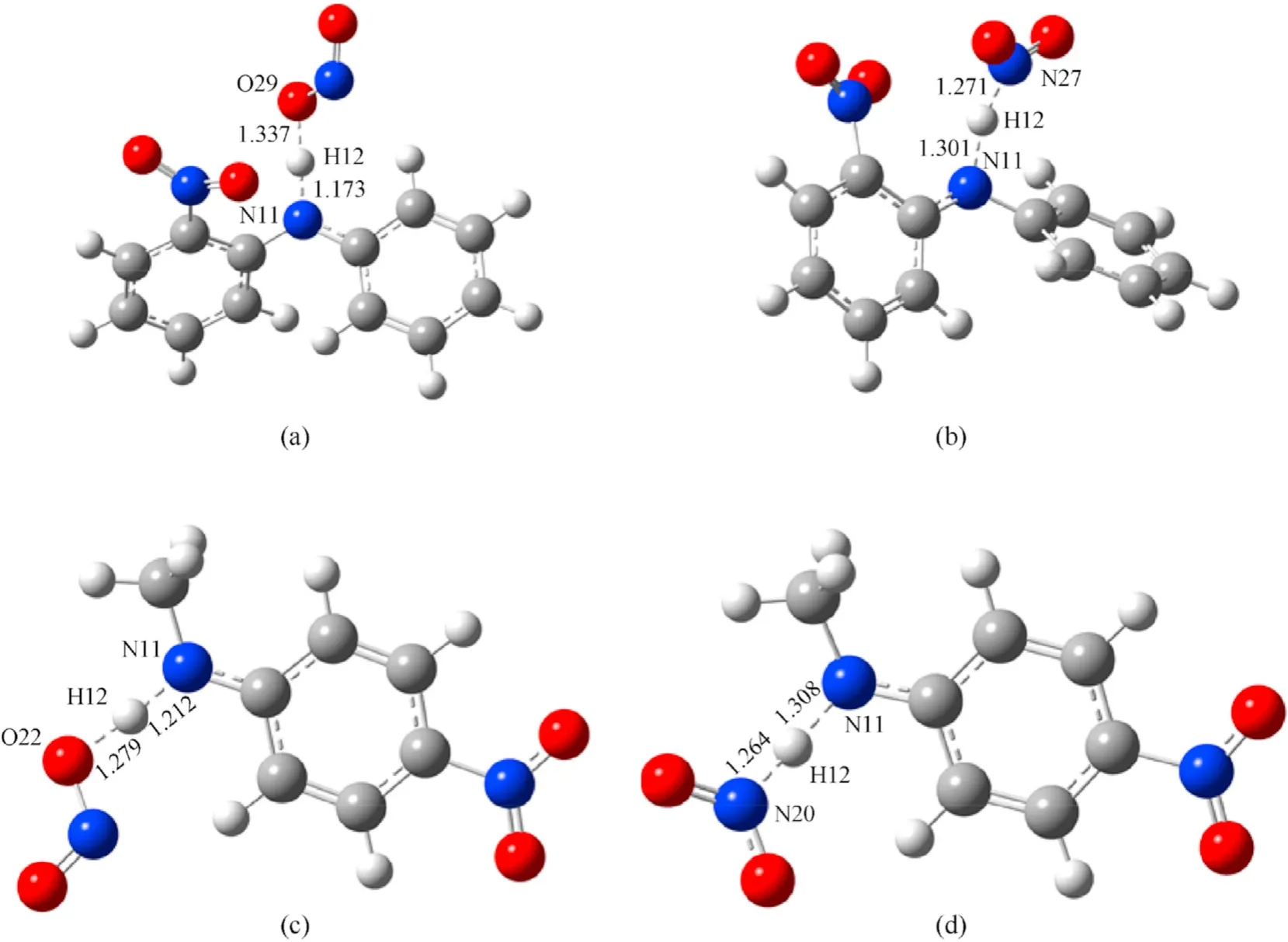
Fig.13.Transition state of dehydrogenation reaction of 2NDPA and MNA: (a) TS8;(b)TS9;(c) TS10;(d) TS11.
The energy barrier of the dehydrogenation reaction of 2NDPA and MNA was obtained from a molecular simulation,as shown in Table 7.Fig.14 is the IRC curves for the transition state between reactants and products.
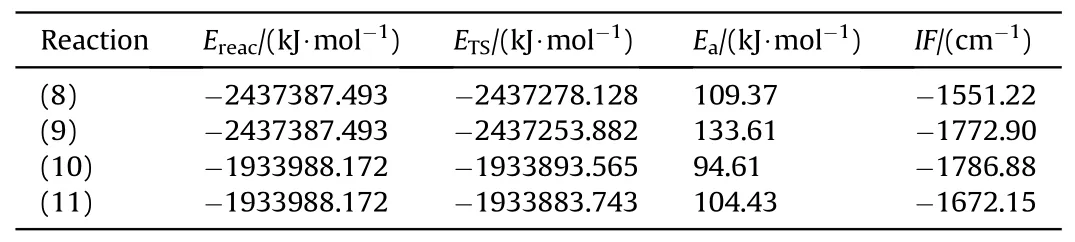
Table 7 Energy barrier of dehydrogenation reaction of 2NDPA and MNA.
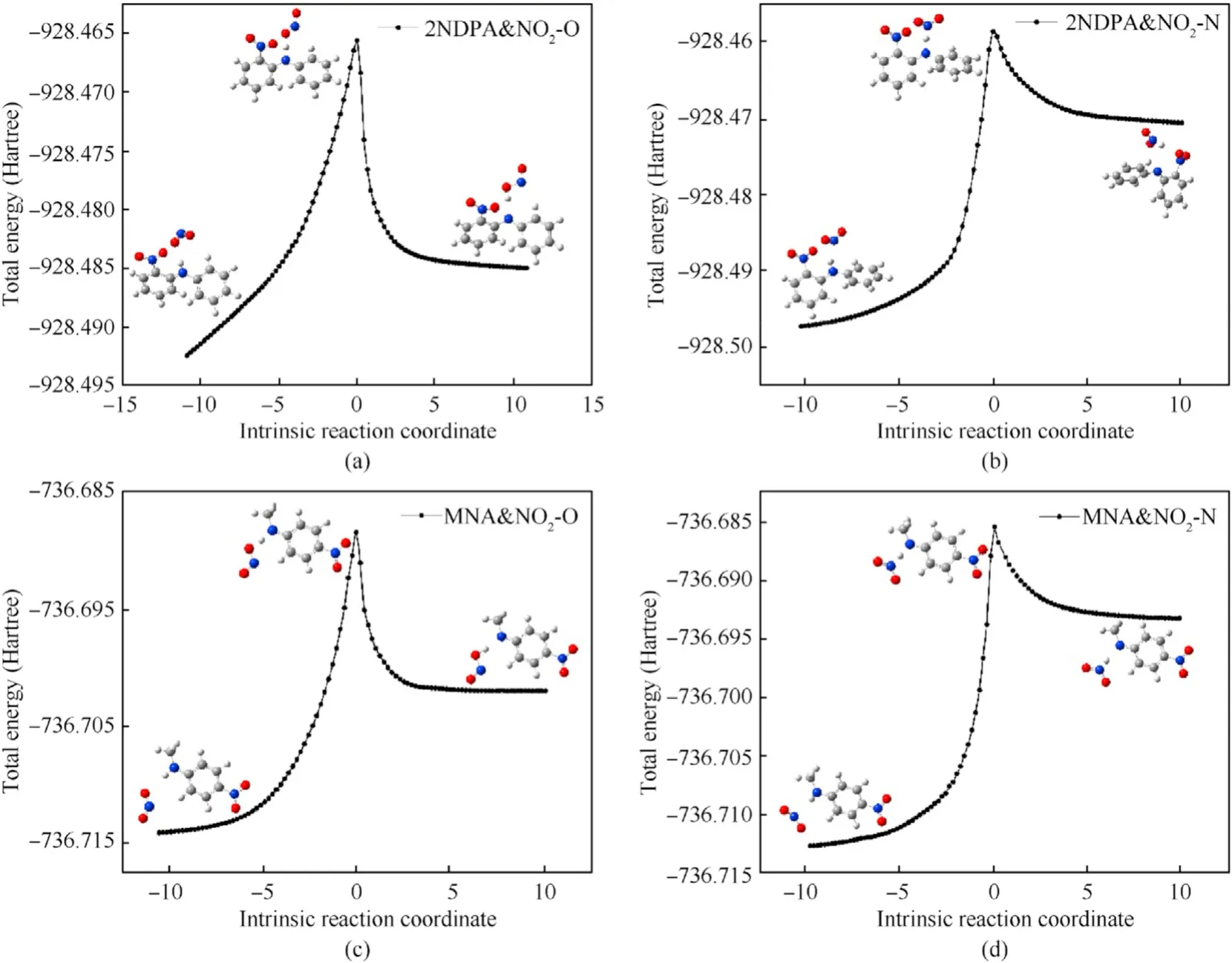
Fig.14.IRC curves of dehydrogenation reaction of 2NDPA and MNA: (a) Reaction (8);(b) Reaction (9);(c) Reaction (10);(d) Reaction (11).
Based on the simulation results of the transition state,the energy barriers of the reactions between NO2and MNA were lower than those of the reaction of NO2with 2NDPA,indicating that MNA is better than 2NDPA at inhibiting the attack from NO2in the aging of the NEPE propellant.Of the four reactions,the energy barrier for the HONO generation reaction was less than that required to generate ONHO.To explore the reasons for the differences in the energy barriers of the two stabilizers,the penetration diagrams of the van der Waals surface electrostatic potential of TS8-TS11were drawn as shown in Fig.15.It can be seen from Fig.15 that the presence of the phenyl and secondary amines,reduces the density of the electron cloud of the N atom.The electrostatic potential is positive near the α-H,which increases the reactivity of the α-H in aniline,facilitating its reaction with NO2which has a partial negative electrostatic potential.In addition,the electronegativity of the O atom in the NO2molecule is high,and its electrostatic potential and steric hindrance are low.Therefore,when the dehydrogenation reaction occurs,the O atom is more easily attracted by the H atom to produce HONO.
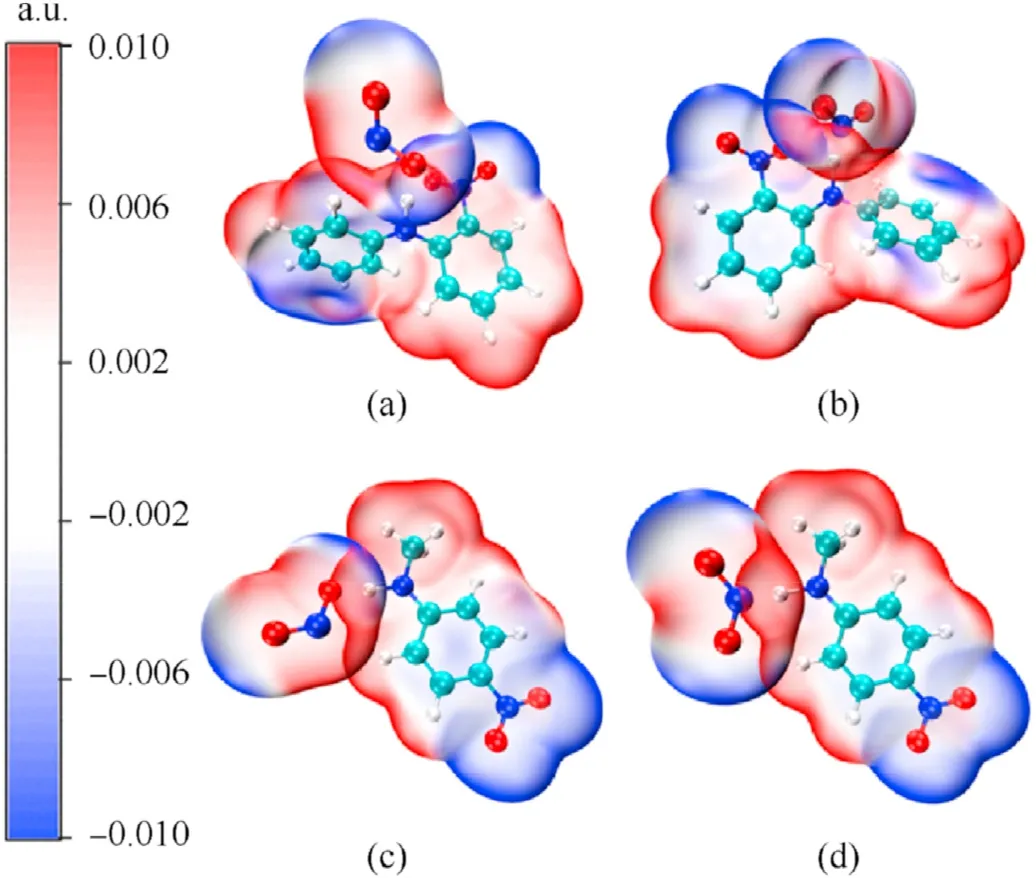
Fig.15.Penetration diagram of van der Waals surface electrostatic potential between TS8-TS11 molecules: (a) TS8;(b)TS9;(c) TS10;(d) TS11.
To further illustrate the mechanism of action of MNA and 2NDPA in the stabilization process of the NEPE propellant,the energy barriers of reaction (1) to reaction (11) were plotted as shown in Fig.16.It can be seen from Fig.16 that the value of energy barriers of the dehydrogenation reactions between NO2with MNA,2NDPA are in the range of 94.61-133.61 kJ/mol,which is less than the energy barrier required for denitrification and autocatalytic reactions of NG and BTTN.This fact indicates that both the stabilizers can effectively consume the decomposition products of NG and BTTN and inhibit NO2from initiating autocatalytic reactions.Moreover,the energy barriers of the dehydrogenation reactions between the two stabilizers and NO2were less than that required for the decomposition reactions of PEG,and only the energy barrier for the dehydrogenation reaction between MNA with NO2was less than that required for the PEG,indicating that both MNA and 2NDPA can effectively inhibit the decomposition of the PEG molecules in the aging process of PEG,but only MNA appeared to hinder the attack of NO2on the methylene group of the PEG molecular chain.
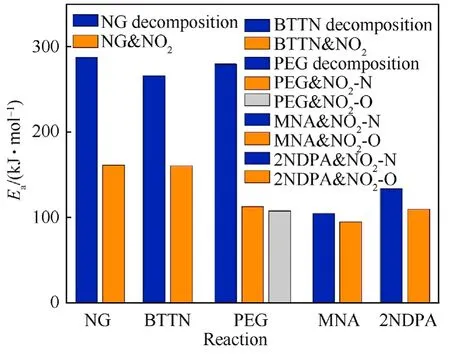
Fig.16.Comparison diagram of different reaction energy barriers of different reactions.
In addition to the energy barrier of the reactions,the reaction rate between NO2with 2NDPA and MNA was also a key factor that impacts the decomposition reactions of NG and BTTN and the aging reactions of PEG.To further quantify the difficulty of these different reactions and explore the mechanism of action of the stabilizers in the NEPE propellant,the reaction rate constants of the previous reactions in the range of 298 K and 298-338 K were calculated using the variational transition state theory (CVT).The rate constants for these reactions are shown in Fig.17 and Fig.18 respectively.As can be seen from Fig.17 and Fig.18,the reaction rate constants for the various reactions increased with temperature and were proportional to their energy barriers.For each of the NG and BTTN reactions,the rate constants for the denitrification reactions were more dependent on temperature than the rate constants of autocatalytic reactions.The reaction rate of the denitrification of BTTN was the fastest without the participation of NO2,and the rate of the autocatalytic reaction of NG was the fastest in the presence of NO2.For each of the PEG reactions,the rate constant of the dehydrogenation reaction was much higher than that of the decomposition reaction of the C-O bond,indicating that the former reaction was the main PEG reaction mode of PEG with NO2.For the reactions of MNA and 2NDPA,the rate constant of the reaction that produced HONO was greater than that of the reaction that yielded ONHO,indicating that the former reaction was more likely to occur.Comparing the reaction rate constants of the two stabilizers with the rate constants of the decomposition of NG and BTTN and the aging reaction of PEG,it was concluded that both stabilizers can effectively inhibit the degradation and chain-breaking of PEG and decrease the denitrification reaction and the autocatalytic reactions of NG and BTTN.However,only the MNA appeared capable of inhibiting the attack of NO2on the C-H bond of the PEG molecular chain.

Fig.17.Reaction rate constants of different reactions at 298 K.
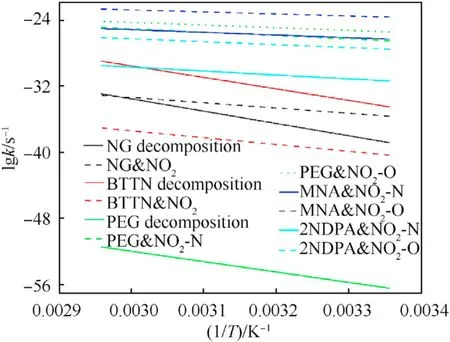
Fig.18.Reaction rate constants of different reactions at 298-338 K.
Based on the calculated results of the energy barriers and reaction rate constants,of the reaction(1)to reaction(11),the energy barriers of the reactions between MNA,2NDPA and NO2were relatively small,and the rate constants was large,indicating that both MNA and 2NDPA can effectively eliminate NO2and slow down the degradation of the energetic and physical properties of the NEPE propellant before NO2catalyze the decomposition of NG,BTTN,and PEG.
4.Conclusions
(1) The simulated results for the bond dissociation energy of the denitrification reactions of NG and BTTN showed that the bond energy of N-O in the NG and BTTN was small,making it susceptible to denitrification,and simultaneously producing NO2and much heat.With the participation of NO2and the heat generated by the denitrification reaction,other active N-O bonds in denitrification products continue to break,releasing more NO2and heat,which accelerates the decomposition of NG and BTTN in the NEPE propellant.
(2) The simulation results of the autocatalytic and aging reactions of the NEPE propellant initiated by NO2showed that the participation of NO2significantly reduced the energy barrier for the denitrification reactions of NG and BTTN,accelerated the reaction rate of the denitrification reaction,released more NO2and heat,and finally led to a decline in thermal stability and energy performance of the NEPE propellant.The participation of NO2also led to the dehydrogenation and decomposition reactions of PEG.The dehydrogenation reaction has a small energy barrier and a fast reaction rate,making it the major reaction in the aging of PEG.In the early stage of the PEG aging reaction,the methylene groups in PEG undergo dehydrogenation by the attack of NO2,resulting in the increased flexibility of the PEG polymer.As the reaction continues,NO2begins to attack the C-O bonds in PEG,initiating the decomposition reaction of the PEG,which eventually leads to a deterioration of the mechanical properties of the NEPE propellant.
(3) The simulation results for MNA and 2NDPA showed that both stabilizers effectively consumed the NO2produced by the decomposition of NG and BTTN,thereby decreasing the catalytic reaction rate of NO2with NG and BTTN and the decomposition reaction of PEG.However,only the MNA was found to prevent the NO2from attacking the methylene groups in the PEG molecular chain.
To summarize,the stabilization of the NEPE propellant can be mainly divided into three stages.In the first stage,NO2and heat are released by the reactions between NG and BTTN;In the second stage,MNA and 2NDPA react with NO2to produce nitroso products,which inhibits the decomposition of the nitrates and slows down the rate of their autocatalytic reactions;In the third stage,the stabilizers were exhausted and as a result,the NG,BTTN,and PEG undergo autocatalytic decomposition and aging reactions that are promoted by the heat and NO2released by the decomposition of NG and BTTN.This described stabilization process shows that the stabilizers not only effectively maintain the thermal stability and energy properties of the NEPE propellant,but also stabilize the physical properties of the NEPE propellant adhesive matrix,thereby delaying the aging of the NEPE propellant.In addition,this study mainly focused on the simulations of the main reactions that occurred in the stabilization process of the NEPE propellant based on previous experimental results and proved the feasibility of studying the stabilization process and stabilization mechanism by using molecular simulation software.In the follow on research,it will be necessary to further carry out relevant experiments on the influence of the side reactions and secondary reactions on the aging performance of NEPE propellant during the stabilization process based on the methods of nuclear magnetic resonance,infrared spectroscopy and liquid chromatography,so as to better specify the reaction path,validate the simulation results and provide experimental and theoretical support for the design and selection of the new generation of stabilizers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for the support provided by the School of Physics and Optoelectronic Engineering of Ludong University,especially in regard to the utilization of the Gaussian software.
- Defence Technology的其它文章
- Structural design of the fluted shaped charge liner using multi-section optimization method
- An aerial ammunition ad hoc network collaborative localizationalgorithm based on relative ranging and velocity measurement in a highly-dynamic topographic structure
- Damage assessment of aircraft wing subjected to blast wave with finite element method and artificial neural network tool
- Experimental and numerical studies of titanium foil/steel explosively welded clad plate
- Effects of connection types and elevated temperature on the impact behaviour of restrained beam in portal steel frame
- Thermal and ignition properties of hexanitrostilbene (HNS)microspheres prepared by droplet microfluidics

