五味子中1个新的联苯环辛烯类木脂素
邸同莲,刘小清,吴 楠,陈孝源,范君婷,谭宁华
· 化学成分 •
五味子中1个新的联苯环辛烯类木脂素
邸同莲1,刘小清2,吴 楠1,陈孝源1,范君婷3*,谭宁华1*
1. 中国药科大学中药学院,江苏 南京 211198 2. 扬子江药业集团北京海燕药业有限公司,北京 102206 3. 南京医科大学药学院,江苏 南京 211116
研究中药五味子的木脂素类化学成分。采用硅胶柱色谱、中压反相柱色谱、半制备和制备液相对五味子中富含木脂素类成分的石油醚萃取部位进行分离、纯化,通过波谱数据鉴定化合物的结构。从五味子中分离得到了26个化合物,分别鉴定为绿叶五味子素B(1)、sphaerandrin A(2)、sieverlignan E(3)、arisanschinin G(4)、widdaranal F(5)、戈米辛A(6)、戈米辛B(7)、戈米辛D(8)、戈米辛G(9)、(−)-戈米辛K1(10)、(+)-戈米辛K2(11)、(−)-戈米辛L1(12)、(+)-戈米辛M1(13)、戈米辛N(14)、当归酰戈米辛H(15)、当归酰戈米辛O(16)、当归酰戈米辛Q(17)、苯甲酰戈米辛Q(18)、五味子醇甲(19)、五味子甲素(20)、五味子乙素(21)、五味子丙素(22)、五味子酯丙(23)、neglschisandrin E(24)、7(18)-dehydroschisandro A(25)、安五脂素(26),其中24个为联苯环辛烯类木脂素(1~4、6~25)。化合物1为新化合物,化合物2~5为首次从该植物中分离得到。
五味子;绿叶五味子素B;联苯环辛烯类木脂素;sphaerandrin A;sieverlignan E;arisanschinin G;widdaranal F;五味子醇甲
五味子为木兰科植物五味子(Turcz.) Baill.的干燥成熟果实,始载于《神农本草经》,列为上品,习称“北五味子”,道地产区为黑龙江、吉林、辽宁等地。五味子具有收敛固涩、益气生津、补肾宁心等功效,可用于治疗久咳虚喘、久泻不止、津伤口渴等症[1]。五味子中富含木脂素、萜类、挥发油、多糖、酚酸、黄酮、植物甾醇等多类成分,其中木脂素类是其特征活性成分[2]。五味子作为苏黄止咳胶囊复方中的臣药,研究发现其木脂素类成分具有舒张气管平滑肌[3]、抗炎[4-6]、抗纤维化[7-8]、镇咳平喘[9-11]的功效,可能是该中成药发挥药效的活性成分。前期课题组利用LC-MS技术对苏黄止咳胶囊的化学成分进行分析和药材溯源,鉴定出300多个化合物[12-13],其中包含29个木脂素并主要来源于五味子,且研究结果表明五味子醇甲是影响该药质量的主要成分[14]。因此本实验研究苏黄止咳胶囊臣药五味子的化学成分,有助于进一步阐明苏黄止咳胶囊的药效物质基础及其质量标准研究。
本实验采用硅胶柱色谱、中压反相柱色谱、半制备和制备液相对五味子水提物富含木脂素的石油醚萃取部位进行分离纯化,共得到25个木脂素和1个倍半萜,包括24个联苯环辛烯类木脂素(1~4、6~25),分别鉴定为绿叶五味子素B(schiviridin B,1)、sphaerandrin A(2)、sieverlignan E(3)、arisanschinin G(4)、widdaranal F(5)、戈米辛A(gomisin A,6)、戈米辛B(gomisin B,7)、戈米辛D(gomisin D,8)、戈米辛G(gomisin G,9)、(−)-戈米辛K1[(−)-gomisin K1,10]、(+)-戈米辛K2[(+)-gomisin K2,11]、(−)-戈米辛L1[(−)-gomisin L1,12]、(+)-戈米辛M1[(+)-gomisin M1,13]、戈米辛N(gomisin N,14)、当归酰戈米辛H(angeloylgomisin H,15)、当归酰戈米辛O(angeloylgomisin O,16)、当归酰戈米辛Q(angeloylgomisin Q,17)、苯甲酰戈米辛Q(benzoylgomisin Q,18)、五味子醇甲(schisandrin,19)、五味子甲素(schisandrin A,20)、五味子乙素(γ-schisandrin,21)、五味子丙素(schisandrin C,22)、五味子酯丙(schisantherin C,23)、neglschisandrin E(24)、7(18)-dehydroschisandro A(25)、安五脂素(anwulignan,26)。其中化合物1为新化合物,化合物2~5为首次从该药材中分离得到。
1 药材、仪器与材料
五味子(10 kg)由扬子江药业集团北京海燕药业有限公司提供,样品(ID-210831)保存于中国药科大学中药学院谭宁华教授团队实验室,经中国药科大学中药学院秦民坚教授鉴定为植物五味子(Turcz.) Baill.的干燥成熟果实。
Shimadzu UV-2600i型紫外光谱仪(日本Shimadzu有限公司);Bruker AV-400型核磁共振仪(德国Bruker公司);Rudolph Autopl IV型旋光仪(美国Rudolph有限公司);Bruker Tensor-27型红外光谱仪(德国Bruker公司);Japan Jasco J810型圆二光谱仪(日本分光JASCO公司);Angilent 1260-6230 TOF液质联用仪(美国Angilent公司);Waters XevoTQD质谱仪(美国Waters公司);LC 3000 Ⅰ型制备型HPLC(北京创新通恒科技有限公司)。半制备型液相色谱柱、制备型液相色谱柱以及分析型液相色谱柱分别为YMC-Pack ODS-A C18柱系列(250 mm×10 mm,5 μm,日本YMC公司)、YMC-Pack ODS-A C18柱系列(250 mm×20 mm,5 μm,日本YMC公司)、Waters ACQUITY UPLCHSS T3色谱柱(100 mm×2.1 mm,1.8 μm,美国Waters公司)。柱色谱硅胶(100~200、200~300目)以及GF254薄层板(青岛裕明源硅胶试剂厂);反相材料Lichroprep RP-18(40~63 µm,美国Merck公司);显色剂为5%硫酸乙醇溶液;实验所用试剂均为分析纯(无锡市亚盛集团化学试剂有限公司)和色谱纯(美国TEDIA公司和Merck公司);LC-MS级甲酸(德国Sigma-Aldrich公司)。
2 提取与分离
干燥的五味子10 kg,粉碎,用10倍量的水煎煮3次,每次1 h,水提液减压浓缩得浸膏。浸膏以水溶解,用石油醚、醋酸乙酯、正丁醇依次萃取3~4次,萃取液减压浓缩得各萃取部位浸膏。各萃取部位浸膏经TLC、LC-MS检测,确定所需木脂素类成分主要集中在石油醚萃取部位。然后石油醚部位(80.4 g)首先通过硅胶柱色谱(200~300目硅胶),以石油醚-醋酸乙酯(10∶0~0∶10)梯度洗脱,减压浓缩,TLC、LC-MS检测合并,得到6个流分(Fr. 1~6)。
Fr. 2(5.936 g)经硅胶柱色谱,以石油醚-醋酸乙酯5个梯度(80∶1、40∶1、20∶1、10∶1、0∶1)进行洗脱,利用TLC和LC-MS检测,合并获得7个亚流分(Fr. 2-1~2-7)。Fr. 2-3(473 mg)经反复硅胶柱色谱及制备型HPLC(75%乙腈)纯化得到化合物16(34 mg,R=51.6 min)、22(18 mg,R=38.8 min)、26(33 mg,R=23.1 min)。Fr. 2-5(3.7 g)经反复硅胶柱色谱及制备型HPLC(72%乙腈)纯化得到化合物14(958 mg,R=33.0 min)、21(304 mg,R=35.3 min)。Fr. 3(22.555 g)经硅胶柱色谱,石油醚-醋酸乙酯(50∶1、40∶1、20∶1、10∶1、5∶1、3∶1、0∶1)梯度洗脱,得到9个亚流分(Fr. 3-1~3-9)。Fr. 3-5(5.385 g)经反复硅胶柱色谱及制备型HPLC(60%乙腈)纯化得到化合物1(8 mg,R=33.6 min)、5(56 mg,R=41.6 min)、10(16 mg,R=27.6 min)、11(10 mg,R=24.9 min)、12(19 mg,R=54.0 min)、13(35 mg,R=55.8 min)、20(206 mg,R=29.6 min)、24(22 mg,R=21.0 min)、25(16 mg,R=22.5 min)。Fr. 3-7(8.056 g)经反复硅胶柱色谱及制备型HPLC(60%乙腈)纯化得到化合物9(24 mg,R=36.6 min)。Fr. 4(22.91 g)经硅胶柱色谱,石油醚-醋酸乙酯(20∶1、10∶1、5∶1、3∶1、0∶1)梯度洗脱,得到5个亚流分Fr. 4-1~4-5。Fr. 4-2(2.474 g)经制备型HPLC(60%乙腈)纯化得到化合物4(6 mg,R=38.2 min)、6(252 mg,R=19.1 min)、7(51 mg,R=34.5 min)、17(217 mg,R=26.5 min)、23(21 mg,R=31.4 min)。Fr. 4-3(15.896 g)经RP-18中压反相柱色谱,甲醇-水(30%~100%)梯度洗脱,利用TLC和LC-MS检测合并,得到4个亚流分Fr. 4-3-1~4-3-4,其中Fr. 4-3-2(10.202 g)经检测为化合物19(10.202 g)。Fr. 4-3-3(1.802 g)经制备型HPLC(60%乙腈)纯化得到化合物2(15 mg,R=19.7 min)、3(21 mg,R=23.8 min)、8(71 mg,R=15.8 min)、15(608 mg,R=25.6 min)、18(57 mg,R=27.1 min)。
3 结构鉴定
化合物1:黄色固体(甲醇),易溶于三氯甲烷。[α]20 D+82.1°(0.067,MeOH),HR-ESI-MS(/519.197 31 [M+Na]+;计算值519.198 94),可确定化合物1分子式为C28H32O8,不饱和度为13。紫外光谱显示其在225 nm处有最大吸收,表明化合物结构中存在较强的共轭系统。红外光谱在3398、1641、1456、1396、1033 cm−1处有吸收,提示分子中含有苯环、羰基、双键。化合物1的1H-NMR和13C-NMR谱(表1)与schiviridin A[15]较为相似,提示其为联苯环辛烯类木脂素。
化合物1的1H-NMR谱(表1)显示2个芳环质子信号H6.73 (1H, s), 6.48 (1H, s);1个连酯基质子信号H6.42 (1H, s);1个亚甲二氧基质子信号H5.99 (1H, d,= 1.4 Hz), 5.97 (1H, d,= 1.4 Hz);2个末端烯氢质子信号H4.83 (1H, s), 4.60 (1H, s);4个甲氧基质子信号H3.88 (3H, s), 3.84 (3H, s), 3.78 (3H, s), 3.61 (3H, s);1个次甲基质子信号H2.58 (1H, overlapped);1个亚甲基质子信号H2.54 (1H, overlapped), 2.24 (1H, dd,= 12.2, 9.6 Hz);1个甲基质子信号H1.21 (3H, d,= 6.8 Hz);还具有偶合系统的质子信号H5.95 (1H, m), 1.87 (3H, dq,= 7.2, 1.6 Hz), 1.51 (3H, q,= 1.6 Hz),结合13C-NMR谱(表1)的酯基碳(C166.3) 和烯碳(C139.6, 127.5),以及1H-1H COSY谱中H-3/H-4相关信号,可以推导为当归酰基信号。13C-NMR结合HSQC谱显示化合物1中有28个碳信号,包括4个甲氧基、3个甲基、1个亚甲二氧基、2个亚甲基、5个次甲基、12个季碳、1个酯基。
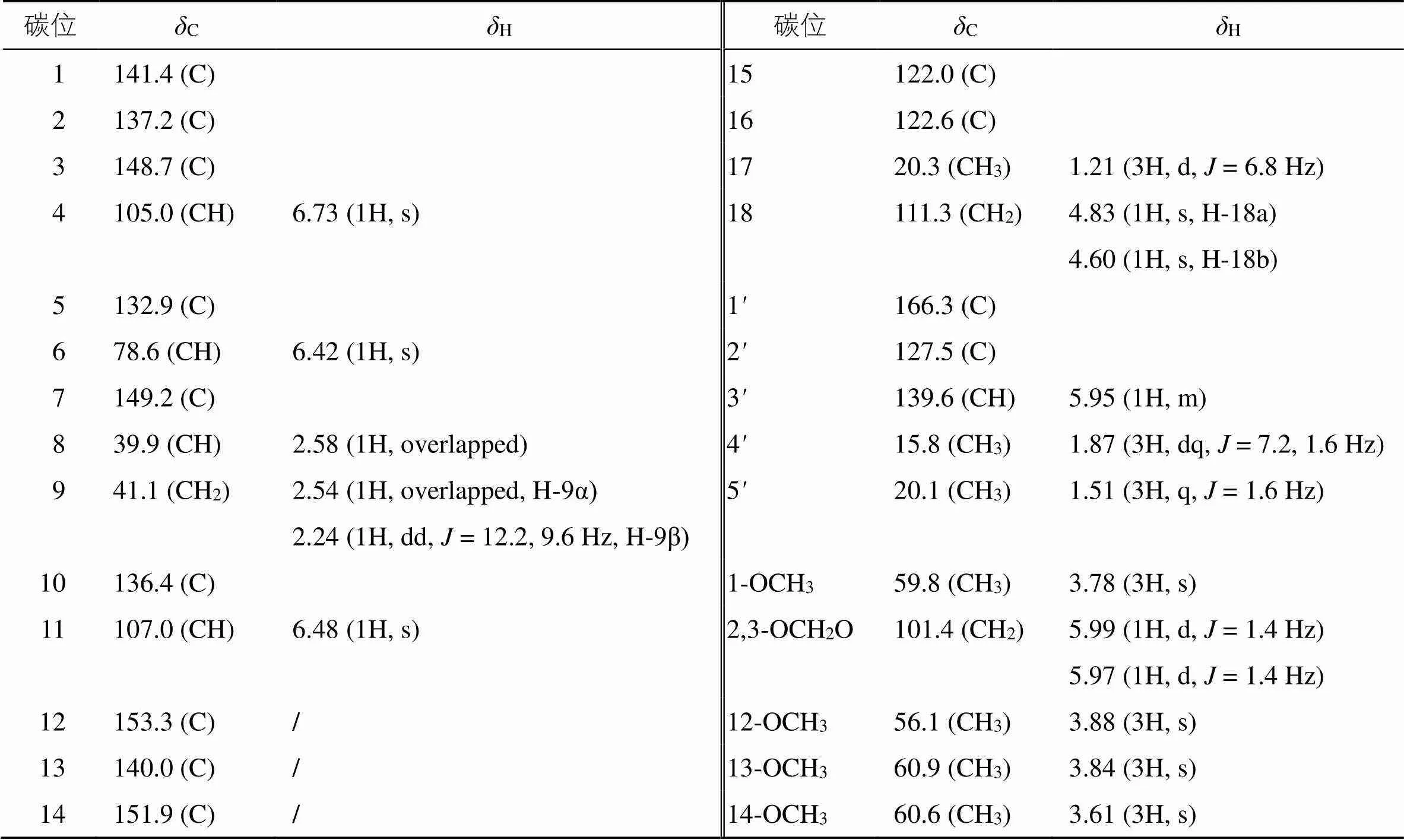
表1 化合物1的1H-NMR和13C-NMR数据(400/100 MHz, CDCl3)
1H-1H COSY谱中(图1),H-8与H-9、H-17相关,表明C-8、C-9、C-17相连。HMBC谱中(图1)显示,H-6 (H6.42) 与C-4、C-5、C-7、C-8、C-16、C-18、C-1相关;H-9 (H2.24, 2.54) 与C-11、C-8、C-10、C-15、C-7存在相关信号;芳环H-4 (H6.73) 与C-2、C-3、C-16相关,芳环氢H-11 (H6.48) 与C-12、C-13、C-15相关;这些相关信号提示含有联苯环辛烯类木脂素的基本骨架。其中,H-6与C-1存在相关信号,表明C-6位是当归酰基取代;H-6还与C-18相关,显示末端烯键连接在C-7位;亚甲二氧基H5.99、5.97分别与C-2、C-3相关,表明亚甲二氧基与C-2、C-3连接;4个甲氧基H3.61、3.78、3.84、3.88分别与C-14、C-1、C-13、C-12相关,确定了甲氧基的连接位置。综上所述,化合物1的平面结构得以确定。
化合物1的立体结构利用CD光谱和ROESY谱来确定。文献报道型联苯环辛烯类木脂素的CD光谱在215~225 nm为正Cotton效应,在235~255 nm为负Cotton效应,而型的CD光谱则相反[15]。化合物1的实验ECD光谱显示在222 nm有正Cotton效应,在243 nm有负Cotton效应,表明1是型联苯环辛烯类木脂素。同时,ROESY谱中(图1)H-4/H-6、H-6/H-18相关,显示H-4、H-6和H-18处于同侧;H-11/H-8、H-8/H-9β相关,也显示H-8、H-9β和H-11处于同侧。最后,通过计算ECD和实验ECD比较(图2),确定其绝对构型为6、8。与已知化合物schiviridin A[15]的结构进行比对,二者苯环上的4个甲氧基和亚甲二氧基取代位置存在差异,且schiviridin A的绝对构型是6、8。因此,化合物1的结构鉴定为(a)-(5,7)- 5,6,7,8-四氢-1,11,12,13-四甲氧基-7-甲基-6-亚甲苯并[3,4]环辛基[1,2:4,5]苯并[1,2-f][1,3]二氧-5-基(2)-2-甲基-丁烯酸酯。经SciFinder检索为未见文献报道的新化合物,命名为绿叶五味子素B(schiviridin B)。
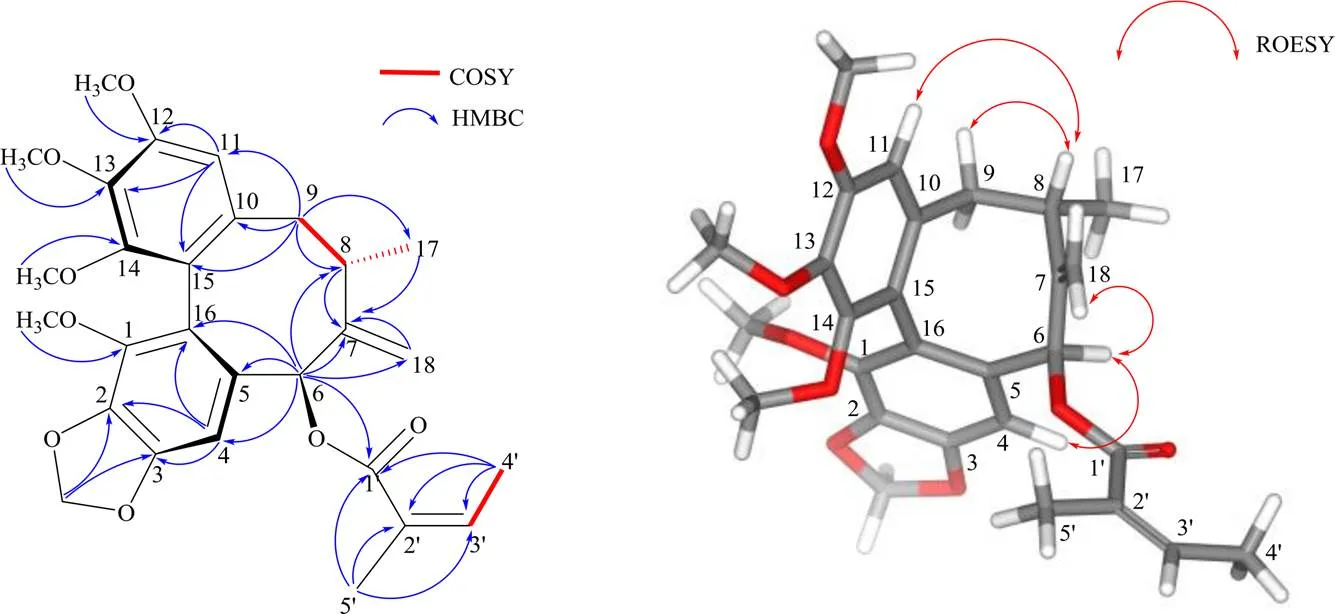
图1 化合物1的结构及其主要1H-1H COSY、HMBC和ROESY相关信号
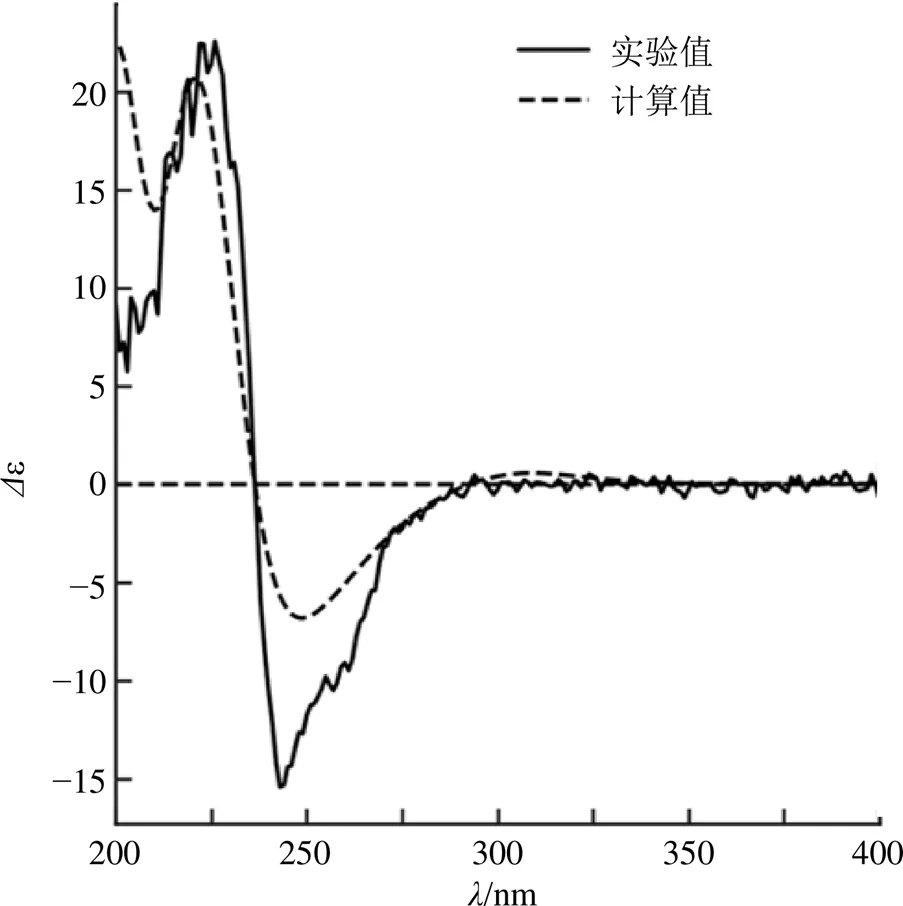
图2 化合物1的计算ECD和实验ECD图谱
化合物2:白色晶体(甲醇);[α]20 D−42.3°(0.052,MeOH);C27H32O9;ESI-MS/: 523.25 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.86 (1H, s, H-4), 5.51 (1H, s, H-6), 2.00 (1H, m, H-8), 2.26 (1H, dd,= 14.1, 9.6 Hz, H-9β), 2.18 (1H, dd,= 14.1, 1.3 Hz, H-9α), 6.48 (1H, s, H-11), 1.14 (3H, d,= 7.1 Hz, H-17), 1.36 (3H, s, H-18), 5.95 (1H, m, H-3), 1.84 (3H, dq,= 7.3, 1.5 Hz, H-4), 1.42 (3H, d,= 1.5 Hz, H-5), 3.55 (3H, s, 1-OCH3), 3.93 (3H, s, 2-OCH3), 3.93 (3H, s, 3-OCH3), 5.97 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 5.89 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 5.15 (1H, s, 14-OH);13C-NMR (100 MHz, CDCl3): 150.9 (C-1), 142.0 (C-2), 152.6 (C-3), 111.5 (C-4), 132.5 (C-5), 84.8 (C-6), 72.3 (C-7), 42.6 (C-8), 36.6 (C-9), 135.7 (C-10), 102.2 (C-11), 148.6 (C-12), 133.6 (C-13), 136.7 (C-14), 118.5 (C-15), 120.0 (C-16), 20.0 (C-17), 28.4 (C-18), 166.2 (C-1), 126.9 (C-2), 140.1 (C-3), 15.9 (C-4), 18.8 (C-5), 61.5 (1-OCH3), 61.3 (2-OCH3), 56.1 (3-OCH3), 101.4 (12, 13-OCH2O)。以上数据与文献报道对比基本一致[16],鉴定化合物2为sphaerandrin A。
化合物3:白色晶体(甲醇);[α]20 D+126.6°(0.064,MeOH);C28H36O8;ESI-MS/: 501.25 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.55 (1H, s, H-4), 2.71 (1H, d,= 13.7 Hz, H-6α), 2.33 (1H, d,= 13.7 Hz, H-6β), 1.89 (1H, m, H-8), 2.69 (1H, dd,= 14.2, 1.8 Hz, H-9α), 2.41 (1H, dd,= 14.2, 7.6 Hz, H-9β), 6.68 (1H, s, H-11), 0.85 (3H, d,= 7.3 Hz, H-17), 1.24 (3H, s, H-18), 6.82 (1H, m, H-3), 1.71 (3H, overlapped, H-4), 1.69 (3H, overlapped, H-5), 3.50 (3H, s, 1-OCH3), 3.83 (3H, s, 2-OCH3), 3.87 (3H, s, 3-OCH3), 3.82 (3H, s, 13-OCH3), 3.90 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 152.0 (C-1), 140.5 (C-2), 152.6 (C-3), 110.3 (C-4), 133.2 (C-5), 40.8 (C-6), 72.1 (C-7), 42.1 (C-8), 34.4 (C-9), 133.9 (C-10), 112.9 (C-11), 142.8 (C-12), 140.0 (C-13), 151.8 (C-14), 123.3 (C-15), 123.1 (C-16), 16.0 (C-17), 30.0 (C-18), 165.9 (C-1), 128.1 (C-2), 138.3 (C-3), 12.2 (C-4), 14.5 (C-5), 61.0 (1-OCH3), 61.1 (2-OCH3), 56.2 (3-OCH3), 60.8 (13-OCH3), 56.2 (14-OCH3)。以上数据与文献报道对比基本一致[17],鉴定化合物3为sieverlignan E。
招聘需求人数规模度指标用来表征岗位的人数需求紧缺度,即岗位在同行业中的人数需求紧缺情况。计算方法是先对招聘需求实际人数进行修正,修正值为招聘需求人数和发布频率的比值。招聘需求人数规模度指标为岗位招聘需求人数修正值和岗位所在细分领域内招聘需求人数修正值总和的比值。发布频率指标用于表征岗位招聘需求在时间上的紧缺程度,意思是平均每家公司每天发布的岗位需求情况,计算方法是招聘发布公司数量和发布天数的乘积比招聘发布条数。
化合物4:白色晶体(甲醇);[α]20 D−24.1°(0.058,MeOH);C22H28O6;ESI-MS/: 411.36 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.37 (1H, s, H-4), 2.24 (1H, dd,= 13.3, 9.4 Hz, H-6β), 2.04 (1H, d,= 13.3 Hz, H-6α), 1.81 (1H, m, H-7), 1.89 (1H, m, H-8), 2.56 (1H, dd,= 13.5, 7.4 Hz, H-9β), 2.44 (1H, d,= 13.5, 1.9 Hz, H-9α), 6.66 (1H, s, H-11), 0.73 (3H, d,= 7.1 Hz, H-17), 1.00 (3H, d,= 7.1 Hz, H-18), 5.74 (1H, s, 1-OH), 3.87 (3H, s, 2-OCH3), 3.90 (3H, s, 3-OCH3), 5.68 (1H, s, 12-OH), 3.93 (3H, s, 13-OCH3), 3.60 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 146.7 (C-1), 133.5 (C-2), 151.9 (C-3), 104.0 (C-4), 140.0 (C-5), 35.6 (C-6), 41.0 (C-7), 33.9 (C-8), 38.9 (C-9), 135.5 (C-10), 113.8 (C-11), 147.9 (C-12), 137.7 (C-13), 150.3 (C-14), 121.6 (C-15), 115.9 (C-16), 12.6 (C-17), 22.0 (C-18), 61.2 (2-OCH3), 55.9 (3-OCH3), 61.2 (13-OCH3), 60.6 (14-OCH3)。以上数据与文献报道对比基本一致[18],鉴定化合物4为arisanschinin G。
化合物5:白色针晶(甲醇);[α]20 D−82.6°(0.086,MeOH);C15H22O2;ESI-MS/: 235.25 [M+H]+。1H-NMR (400 MHz, CDCl3): 2.24 (2H, m, H-1), 7.10 (1H, m, H-2), 2.34 (1H, m, H-4a), 1.84 (1H, m, H-4b), 1.54 (2H, m, H-5), 1.78 (1H, m, H-8a), 1.19 (1H, m, H-8b), 2.08 (1H, m, H-9a), 1.41 (1H, m, H-9b), 2.16 (2H, m, H-10), 4.86 (1H, s, H-12a), 4.36 (1H, s, H-12b), 0.88 (3H, s, H-13), 0.85 (3H, s, H-14);13C-NMR (100 MHz, CDCl3): 30.1 (C-1), 142.2 (C-2), 128.9 (C-3), 21.8 (C-4), 23.7 (C-5), 45.0 (C-6), 37.3 (C-7), 37.0 (C-8), 25.6 (C-9), 32.1 (C-10), 148.5 (C-11), 110.9 (C-12), 23.2 (C-13), 25.0 (C-14), 173.0 (C-15)。以上数据与文献报道对比基本一致[19],鉴定化合物5为widdaranal F。
化合物6:白色晶体(甲醇);[α]20 D+59.5°(0.074,MeOH);C23H28O7;ESI-MS/: 439.26 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.61 (1H, s, H-4), 2.67 (1H, d,= 13.5 Hz, H-6α), 2.35 (1H, d,= 13.5 Hz, H-6β), 2.00 (1H, s, 7-OH), 1.85 (1H, m, H-8), 2.57 (1H, dd,= 14.1, 1.6 Hz, H-9α), 2.32 (1H, dd,= 14.1, 7.4 Hz, H-9β), 6.47 (1H, s, H-11), 0.81 (3H, d,= 7.3 Hz, H-17), 1.25 (3H, s, H-18), 3.51 (3H, s, 1-OCH3), 3.90 (3H, s, 2-OCH3), 3.91 (3H, s, 3-OCH3), 5.96 (2H, s, 12, 13-OCH2O), 3.83 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 152.2 (C-1), 140.8 (C-2), 152.4 (C-3), 110.4 (C-4), 132.1 (C-5), 40.6 (C-6), 71.7 (C-7), 42.1 (C-8), 33.8 (C-9), 132.6 (C-10), 106.1 (C-11), 148.0 (C-12), 135.0 (C-13), 141.3 (C-14), 121.9 (C-15), 124.2 (C-16), 15.9 (C-17), 30.2 (C-18), 60.7 (1-OCH3), 61.1 (2-OCH3), 56.1 (3-OCH3), 101.0 (12, 13-OCH2O), 59.8 (14-OCH3)。以上数据与文献报道对比基本一致[20],鉴定化合物6为gomisin A。
化合物7:白色晶体(甲醇);[α]20 D−20.9°(0.067,MeOH);C28H34O9;ESI-MS/: 537.35 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.76 (1H, s, H-4), 5.61 (1H, s, H-6), 1.93 (1H, m, H-8), 2.29 (1H, dd,= 14.0, 9.7 Hz, H-9β), 2.13 (1H, d,= 14.0 Hz, H-9α), 6.45 (1H, s, H-11), 1.12 (3H, d,= 7.1 Hz, H-17), 1.32 (3H, s, H-18), 5.99 (1H, m, H-3), 1.84 (3H, dq,= 7.3, 1.6 Hz, H-4), 1.38 (3H, q,= 1.6 Hz, H-5), 3.72 (3H, s, 1-OCH3), 3.88 (3H, s, 2-OCH3), 3.89 (3H, s, 3-OCH3), 5.89 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 5.86 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 3.55 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 152.2 (C-1), 141.8 (C-2), 152.0 (C-3), 109.9 (C-4), 130.7 (C-5), 84.5 (C-6), 72.3 (C-7), 42.5 (C-8), 36.5 (C-9), 135.3 (C-10), 102.8 (C-11), 148.8 (C-12), 134.3 (C-13), 140.6 (C-14), 121.3 (C-15), 122.3 (C-16), 19.1 (C-17), 28.2 (C-18), 166.0 (C-1), 127.2 (C-2), 140.1 (C-3), 15.8 (C-4), 19.9 (C-5), 60.8 (1-OCH3), 61.0 (2-OCH3), 55.9 (3-OCH3), 100.7 (12, 13-OCH2O), 59.2 (14-OCH3)。以上数据与文献报道对比基本一致[21],鉴定化合物7为gomisin B。
化合物8:白色晶体(甲醇);[α]20 D−47.4°(0.076,MeOH);C28H34O10;ESI-MS/: 553.36 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.79 (1H, s, H-4), 5.72 (1H, s, H-6), 1.76 (1H, m, H-8), 2.37 (1H, dd,= 14.1, 8.3 Hz, H-9β), 2.00 (1H, d,= 14.1 Hz, H-9α), 6.47 (1H, s, H-11), 1.05 (3H, d,= 7.0 Hz, H-17), 1.22 (3H, s, H-18), 1.65 (1H, m, H-21), 3.71 (2H, m, H-22), 1.28 (3H, s, H-23), 1.14 (3H, d,= 7.2 Hz, H-24), 3.56 (3H, s, 1-OCH3), 3.84 (3H, s, 2-OCH3), 3.91 (3H, s, 3-OCH3), 6.00 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 5.91 (1H, d,= 1.4 Hz, 12, 13-OCH2O);13C-NMR (100 MHz, CDCl3): 152.0 (C-1), 142.5 (C-2), 152.2 (C-3), 110.9 (C-4), 130.3 (C-5), 87.6 (C-6), 72.2 (C-7), 44.0 (C-8), 36.1 (C-9), 136.5 (C-10), 102.7 (C-11), 148.6 (C-12), 137.3 (C-13), 138.4 (C-14), 121.4 (C-15), 122.6 (C-16), 19.0 (C-17), 27.6 (C-18), 177.7 (C-19), 75.0 (C-20), 37.9 (C-21), 73.0 (C-22), 24.8 (C-23), 11.4 (C-24), 60.8 (1-OCH3), 60.9 (2-OCH3), 56.1 (3-OCH3), 101.2 (12, 13-OCH2O)。以上数据与文献报道对比基本一致[22],鉴定化合物8为gomisin D。
化合物9:白色晶体(甲醇);[α]20 D−126.5°(0.068,MeOH);C30H32O9;ESI-MS/: 559.25 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.77 (1H, s, H-4), 5.88 (1H, s, H-6), 2.13 (1H, m, H-8), 2.48 (1H, dd,= 14.0, 10.1 Hz, H-9β), 2.28 (1H, d,= 14.0 Hz, H-9α), 6.69 (1H, s, H-11), 1.20 (3H, d,= 7.0 Hz, H-17), 1.33 (3H, s, H-18), 7.36 (5H, overlapped, H-2~6), 3.39 (3H, s, 1-OCH3), 6.00 (2H, d,= 1.6 Hz, 2, 3-OCH2O), 3.98 (3H, s, 12-OCH3), 3.81 (3H, s, 13-OCH3), 3.14 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 141.8 (C-1), 137.2 (C-2), 148.2 (C-3), 107.4 (C-4), 128.9 (C-5), 84.5 (C-6), 72.6 (C-7), 42.3 (C-8), 36.9 (C-9), 136.6 (C-10), 106.3 (C-11), 153.4 (C-12), 140.0 (C-13), 150.8 (C-14), 122.4 (C-15), 121.9 (C-16), 19.1 (C-17), 28.4 (C-18), 164.9 (-CO), 129.6 (C-1), 129.3 (C-2, 6), 128.2 (C-3, 5), 133.3 (C-4), 60.0 (1-OCH3), 101.5 (2, 3-OCH2O), 56.2 (12-OCH3), 60.2 (13-OCH3), 59.8 (14-OCH3)。以上数据与文献报道对比基本一致[21],鉴定化合物9为gomisin G。
化合物10:白色晶体(甲醇);[α]20 D−112.5°(0.056,MeOH);C23H30O6;ESI-MS/: 403.30 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.54 (1H, s, H-4), 2.54 (2H, m, H-6), 1.84 (1H, overlapped, H-7), 1.84 (1H, overlapped, H-8), 2.22 (1H, dd,= 13.1, 9.4 Hz, H-9β), 2.01 (1H, dd,= 13.1, 1.3 Hz, H-9α), 6.62 (1H, s, H-11), 0.96 (3H, d,= 7.1 Hz, H-17), 0.72 (3H, d,= 7.1 Hz, H-18), 3.54 (3H, s, 1-OCH3), 3.88 (3H, s, 2-OCH3), 3.88 (3H, s, 3-OCH3), 5.86 (1H, s, 12-OH), 3.90 (3H, s, 13-OCH3), 3.55 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 151.6 (C-1), 140.1 (C-2), 151.6 (C-3), 110.6 (C-4), 134.2 (C-5), 39.3 (C-6), 33.8 (C-7), 40.9 (C-8), 35.3 (C-9), 140.1 (C-10), 110.1 (C-11), 148.8 (C-12), 137.4 (C-13), 150.3 (C-14), 121.5 (C-15), 123.3 (C-16), 21.8 (C-17), 12.7 (C-18), 60.6 (1-OCH3), 61.1 (2-OCH3), 56.0 (3-OCH3), 61.0 (13-OCH3), 60.2 (14-OCH3)。以上数据与文献报道对比基本一致[20],鉴定化合物10为(−)-gomisin K1。
化合物11:白色晶体(甲醇);[α]20 D+3.8°(0.053,MeOH);C23H30O6;ESI-MS/: 403.31 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.54 (1H, s, H-4), 2.16 (2H, m, H-6), 1.84 (1H, overlapped, H-7), 1.84 (1H, overlapped, H-8), 2.51 (2H, m, H-9), 6.62 (1H, s, H-11), 0.73 (3H, d,= 7.1 Hz, H-17), 0.99 (3H, d,= 7.1 Hz, H-18), 3.55 (3H, s, 1-OCH3), 3.89 (3H, s, 2-OCH3), 3.92 (3H, s, 3-OCH3), 5.70 (1H, s, 12-OH), 3.88 (3H, s, 13-OCH3), 3.55 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 151.5 (C-1), 139.9 (C-2), 153.0 (C-3), 107.4 (C-4), 139.5 (C-5), 35.7 (C-6), 41.0 (C-7), 33.9 (C-8), 38.9 (C-9), 134.8 (C-10), 113.1 (C-11), 147.6 (C-12), 137.7 (C-13), 150.4 (C-14), 122.7 (C-15), 122.3 (C-16), 12.6 (C-17), 22.0 (C-18), 60.7 (1-OCH3), 61.1 (2-OCH3), 56.0 (3-OCH3), 61.1 (13-OCH3), 60.2 (14-OCH3)。以上数据与文献报道对比基本一致[23],鉴定化合物11为(+)-gomisin K2。
化合物12:白色晶体(甲醇);[α]20 D−5.9°(0.051,MeOH);C22H26O6;ESI-MS/: 387.26 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.51 (1H, s, H-4), 2.51 (2H, m, H-6), 1.84 (1H, overlapped, H-7), 1.84 (1H, overlapped, H-8), 2.16 (2H, m, H-9), 6.37 (1H, s, H-11), 1.00 (3H, d,= 7.2 Hz, H-17), 0.73 (3H, d,= 7.2 Hz, H-18), 3.87 (3H, s, 1-OCH3), 5.95 (2H, d,= 1.6 Hz, 2, 3-OCH2O), 3.90 (3H, s, 12-OCH3), 3.90 (3H, s, 13-OCH3), 5.72 (1H, s, 14-OH);13C-NMR (100 MHz, CDCl3): 141.3 (C-1), 135.1 (C-2), 148.0 (C-3), 106.6 (C-4), 133.2 (C-5), 39.1 (C-6), 34.0 (C-7), 40.9 (C-8), 35.5 (C-9), 140.1 (C-10), 104.0 (C-11), 151.8 (C-12), 133.2 (C-13), 146.8 (C-14), 115.8 (C-15), 121.4 (C-16), 22.1 (C-17), 12.4 (C-18), 59.9 (1-OCH3), 101.0 (2, 3-OCH2O), 55.8 (12-OCH3), 61.2 (13-OCH3)。以上数据与文献报道对比基本一致[24],鉴定化合物12为(−)-gomisin L1。
化合物13:白色晶体(甲醇);[α]20 D+3.2°(0.062,MeOH);C22H26O6;ESI-MS/: 387.26 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.51 (1H, s, H-4), 2.14 (2H, m, H-6), 1.84 (1H, overlapped, H-7), 1.84 (1H, overlapped, H-8), 2.55 (2H, m, H-9), 6.38 (1H, s, H-11), 0.75 (3H, d,= 7.2 Hz, H-17), 0.97 (3H, d,= 7.2 Hz, H-18), 3.87 (3H, s, 1-OCH3), 5.95 (1H, d,= 1.5 Hz, 2, 3-OCH2O), 5.93 (1H, d,= 1.5 Hz, 2, 3-OCH2O), 3.91 (3H, s, 12-OCH3), 3.89 (3H, s, 13-OCH3), 5.72 (1H, s, 14-OH);13C-NMR (100 MHz, CDCl3): 141.1 (C-1), 134.6 (C-2), 149.0 (C-3), 103.6 (C-4), 138.5 (C-5), 35.8 (C-6), 40.9 (C-7), 33.6 (C-8), 39.3 (C-9), 134.8 (C-10), 107.3 (C-11), 150.5 (C-12), 133.6 (C-13), 147.0 (C-14), 116.7 (C-15), 120.3 (C-16), 13.0 (C-17), 21.6 (C-18), 59.9 (1-OCH3), 100.9 (2, 3-OCH2O), 55.8 (12-OCH3), 61.1 (13-OCH3)。以上数据与文献报道对比基本一致[25],鉴定化合物13为(+)-gomisin M1。
化合物14:白色晶体(甲醇);[α]20 D−10.3°(0.078,MeOH);C23H28O6;ESI-MS/: 401.25 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.55 (1H, s, H-4), 2.58 (1H, dd,= 13.6, 7.2 Hz, H-6β), 2.51 (1H, dd,= 13.6, 2.4 Hz, H-6α), 1.84 (1H, overlapped, H-7), 1.84 (1H, overlapped, H-8), 2.23 (1H, dd,= 13.3, 9.4 Hz, H-9β), 2.02 (1H, dd,= 13.3, 1.5 Hz, H-9α), 6.48 (1H, s, H-11), 0.99 (3H, d,= 7.2 Hz, H-17), 0.73 (3H, d,= 7.2 Hz, H-18), 3.54 (3H, s, 1-OCH3), 3.88 (3H, s, 2-OCH3), 3.89 (3H, s, 3-OCH3), 5.94 (2H, s, 12, 13-OCH2O), 3.82 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 151.7 (C-1), 140.1 (C-2), 151.7 (C-3), 110.7 (C-4), 134.2 (C-5), 39.2 (C-6), 33.9 (C-7), 40.8 (C-8), 35.6 (C-9), 137.9 (C-10), 103.0 (C-11), 148.8 (C-12), 134.6 (C-13), 141.2 (C-14), 121.4 (C-15), 123.4 (C-16), 21.7 (C-17), 12.9 (C-18), 60.6 (1-OCH3), 61.1 (2-OCH3), 56.0 (3-OCH3), 100.8 (12, 13-OCH2O), 59.7 (14-OCH3)。以上数据与文献报道对比基本一致[26],鉴定化合物14为gomisin N。
化合物15:白色晶体(甲醇);[α]20 D+2.2°(0.091,MeOH);C28H36O8;ESI-MS/: 523.20 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.53 (1H, s, H-4), 2.71 (1H, d,= 13.7 Hz, H-6α), 2.30 (1H, d,= 13.7 Hz, H-6β), 1.84 (1H, m, H-8), 2.67 (1H, dd,= 14.6, 1.8 Hz, H-9α), 2.38 (1H, dd,= 14.6, 7.6 Hz, H-9β), 6.66 (1H, s, H-11), 0.81 (3H, d,= 7.3 Hz, H-17), 1.21 (3H, s, H-18), 5.85 (1H, m, H-3), 1.73 (3H, overlapped, H-4), 1.72 (3H, overlapped, H-5), 3.50 (3H, s, 1-OCH3), 3.80 (3H, s, 2-OCH3), 3.83 (3H, s, 3-OCH3), 3.87 (3H, s, 12-OCH3), 3.80 (3H, s, 13-OCH3);13C-NMR (100 MHz, CDCl3): 151.7 (C-1), 140.3 (C-2), 152.6 (C-3), 110.1 (C-4), 133.1 (C-5), 40.7 (C-6), 72.0 (C-7), 41.9 (C-8), 34.3 (C-9), 133.9 (C-10), 112.8 (C-11), 151.8 (C-12), 139.7 (C-13), 142.3 (C-14), 123.2 (C-15), 122.9 (C-16), 15.9 (C-17), 29.9 (C-18), 165.6 (C-1), 127.6 (C-2), 137.3 (C-3), 15.3 (C-4), 20.3 (C-5), 60.6 (1-OCH3), 60.9 (2-OCH3), 56.0 (3-OCH3), 56.0 (12-OCH3), 60.8 (13-OCH3)。以上数据与文献报道对比基本一致[20],鉴定化合物15为angeloylgomisin H。
化合物16:白色晶体(甲醇);[α]20 D+20.8°(0.051,MeOH);C28H34O8;ESI-MS/: 521.26 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.75 (1H, s, H-4), 5.75 (1H, d,= 8.5 Hz, H-6), 1.89 (1H, m, H-7), 1.89 (1H, m, H-8), 2.18 (2H, m, H-9), 6.40 (1H, s, H-11), 0.94 (3H, d,= 6.9 Hz, H-17), 0.83 (3H, d,= 6.9 Hz, H-18), 5.92 (1H, overlapped, H-3), 1.84 (3H, d,= 7.2 Hz, H-4), 1.57 (3H, s, H-5), 3.51 (3H, s, 1-OCH3), 3.88 (3H, s, 2-OCH3), 3.89 (3H, s, 3-OCH3), 5.92 (2H, m, 12, 13-OCH2O), 3.80 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 151.9 (C-1), 141.7 (C-2), 151.9 (C-3), 111.2 (C-4), 132.9 (C-5), 80.9 (C-6), 37.1 (C-7), 37.3 (C-8), 37.9 (C-9), 135.2 (C-10), 102.4 (C-11), 148.8 (C-12), 134.6 (C-13), 142.0 (C-14), 121.7 (C-15), 123.7 (C-16), 18.0 (C-17), 15.7 (C-18), 167.1 (C-1), 128.1 (C-2), 138.2 (C-3), 15.7 (C-4), 20.2 (C-5), 60.5 (1-OCH3), 61.0 (2-OCH3), 56.1 (3-OCH3), 100.1 (12, 13-OCH2O), 59.4 (14-OCH3)。以上数据与文献报道对比基本一致[27],鉴定化合物16为angeloylgomisin O。
化合物17:白色晶体(甲醇);[α]20 D−8.8°(0.068,MeOH);C29H38O9;ESI-MS/: 553.34 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.78 (1H, s, H-4), 5.73 (1H, s, H-6), 1.90 (1H, m, H-8), 2.33 (1H, dd,= 14.0, 9.8 Hz, H-9β), 2.18 (1H, d,= 14.0 Hz, H-9α), 6.53 (1H, s, H-11), 1.15 (3H, d,= 7.1 Hz, H-17), 1.31 (3H, s, H-18), 5.94 (1H, m, H-3), 1.80 (3H, dq,= 7.4, 1.7 Hz, H-4), 1.29 (3H, q,= 1.7 Hz, H-5), 3.50 (3H, s, 1-OCH3), 3.80 (3H, s, 2-OCH3), 3.88 (3H, s, 3-OCH3), 3.87 (3H, s, 12-OCH3), 3.86 (3H, s, 13-OCH3), 3.55 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 152.2 (C-1), 141.0 (C-2), 152.0 (C-3), 110.1 (C-4), 130.4 (C-5), 84.3 (C-6), 72.4 (C-7), 42.2 (C-8), 36.8 (C-9), 136.6 (C-10), 107.3 (C-11), 153.2 (C-12), 140.0 (C-13), 150.9 (C-14), 122.3 (C-15), 122.5 (C-16), 19.2 (C-17), 28.2 (C-18), 166.0 (C-1), 127.0 (C-2), 142.0 (C-3), 15.7 (C-4), 19.9 (C-5), 60.6 (1-OCH3), 60.9 (2-OCH3), 55.9 (3-OCH3), 56.2 (12-OCH3), 60.8 (13-OCH3), 60.3 (14-OCH3)。以上数据与文献报道对比基本一致[28-29],鉴定化合物17为angeloylgomisin Q。
化合物18:白色晶体(甲醇);[α]20 D−115.9°(0.063,MeOH);C31H36O9;ESI-MS/: 575.14 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.83 (1H, s, H-4), 5.97 (1H, s, H-6), 2.14 (1H, m, H-8), 2.48 (1H, dd,= 14.1, 10.0 Hz, H-9β), 2.28 (1H, d,= 14.1 Hz, H-9α), 6.68 (1H, s, H-11), 1.20 (3H, d,= 7.1 Hz, H-17), 1.34 (3H, s, H-18), 7.35 (5H, overlapped, H-2~6), 3.16 (3H, s, 1-OCH3), 3.57 (3H, s, 2-OCH3), 3.97 (3H, s, 3-OCH3), 3.89 (3H, s, 12-OCH3), 3.88 (3H, s, 13-OCH3), 3.38 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 152.3 (C-1), 142.1 (C-2), 152.0 (C-3), 110.3 (C-4), 130.1 (C-5), 84.5 (C-6), 72.6 (C-7), 42.4 (C-8), 36.9 (C-9), 136.5 (C-10), 107.2 (C-11), 153.2 (C-12), 140.1 (C-13), 150.7 (C-14), 122.5 (C-15), 122.6 (C-16), 19.1 (C-17), 28.2 (C-18), 165.0 (-CO), 129.3 (C-1), 129.6 (C-2, 6), 128.2 (C-3, 5), 133.3 (C-4), 60.2 (1-OCH3), 60.9 (2-OCH3), 56.0 (3-OCH3), 56.2 (12-OCH3), 60.8 (13-OCH3), 59.9 (14-OCH3)。以上数据与文献报道对比基本一致[28],鉴定化合物18为benzoylgomisin Q。
化合物19:白色晶体(甲醇);[α]20 D+87.1°(0.124,MeOH);C24H32O7;ESI-MS/: 455.36 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.59 (1H, s, H-4), 2.63 (1H, d,= 13.4 Hz, H-6β), 2.34 (1H, d,= 13.4 Hz, H-6α), 1.97 (1H, s, 7-OH), 1.85 (1H, m, H-8), 2.57 (1H, dd,= 14.2, 1.8 Hz, H-9α), 2.35 (1H, dd,= 14.2, 7.7 Hz, H-9β), 6.51 (1H, s, H-11), 0.79 (3H, d,= 7.3 Hz, H-17), 1.23 (3H, s, H-18), 3.51 (3H, s, 1-OCH3), 3.85 (3H, s, 2-OCH3), 3.88 (3H, s, 3-OCH3), 3.86 (3H, s, 12-OCH3), 3.86 (3H, s, 13-OCH3), 3.56 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 151.8 (C-1), 140.7 (C-2), 152.4 (C-3), 110.4 (C-4), 131.9 (C-5), 40.8 (C-6), 71.8 (C-7), 41.8 (C-8), 34.2 (C-9), 133.9 (C-10), 110.0 (C-11), 152.0 (C-12), 140.1 (C-13), 151.6 (C-14), 124.2 (C-15), 122.7 (C-16), 15.9 (C-17), 29.9 (C-18), 60.7 (1-OCH3), 61.0 (2-OCH3), 56.0 (3-OCH3), 55.9 (12-OCH3), 61.0 (13-OCH3), 60.6 (14-OCH3)。以上数据与文献报道对比一致[20],鉴定化合物19为schisandrin。
化合物20:白色针晶(甲醇);[α]20 D+9.3°(0.086,MeOH);C24H32O6;ESI-MS/: 417.36 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.55 (1H, s, H-4), 2.59 (1H, dd,= 13.5 , 7.3 Hz, H-6β), 2.06 (1H, d,= 13.5 Hz, H-6α), 1.85 (1H, overlapped, H-7), 1.85 (1H, overlapped, H-8), 2.50 (1H, d,= 13.2 Hz, H-9α), 2.28 (1H, dd,= 13.2 , 9.5 Hz, H-9β), 6.54 (1H, s, H-11), 0.74 (3H, d,= 7.1 Hz, H-17), 1.00 (3H, d,= 7.1 Hz, H-18), 3.59 (3H, s, 1-OCH3), 3.87 (3H, s, 2-OCH3), 3.89 (3H, s, 3-OCH3), 3.88 (3H, s, 12-OCH3), 3.88 (3H, s, 13-OCH3), 3.59 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 151.4 (C-1), 139.9 (C-2), 152.7 (C-3), 107.0 (C-4), 139.0 (C-5), 35.4 (C-6), 40.6 (C-7), 33.6 (C-8), 39.0 (C-9), 133.8 (C-10), 110.3 (C-11), 151.5 (C-12), 139.6 (C-13), 151.3 (C-14), 123.2 (C-15), 122.2 (C-16), 12.5 (C-17), 21.7 (C-18), 60.4 (1-OCH3), 60.8 (2-OCH3), 55.7 (3-OCH3), 55.7 (12-OCH3), 60.8 (13-OCH3), 60.4 (14-OCH3)。以上数据与文献报道对比基本一致[20],鉴定化合物20为schisandrin A。
化合物21:白色晶体(甲醇);[α]20 D0°(0.084,MeOH);C23H28O6;ESI-MS/: 401.30 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.54 (1H, s, H-4), 2.31 (1H, dd,= 13.2, 9.6 Hz, H-6β), 2.04 (1H, dd,= 13.2, 1.5 Hz, H-6α), 1.83 (1H, overlapped, H-7), 1.83 (1H, overlapped, H-8), 2.55 (1H, dd,= 13.5, 7.3 Hz, H-9β), 2.43 (1H, dd,= 13.5, 1.8 Hz, H-9α), 6.48 (1H, s, H-11), 0.73 (3H, dd,= 7.1 Hz, H-17), 0.99 (3H, d,= 7.1 Hz, H-18), 3.54 (3H, s, 1-OCH3), 3.88 (3H, s, 2-OCH3), 3.89 (3H, s, 3-OCH3), 5.95 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 5.94 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 3.83 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 151.5 (C-1), 139.8 (C-2), 152.9 (C-3), 107.5 (C-4), 139.5 (C-5), 35.5 (C-6), 40.8 (C-7), 33.9 (C-8), 38.9 (C-9), 132.6 (C-10), 106.0 (C-11), 147.7 (C-12), 134.9 (C-13), 141.3 (C-14), 122.3 (C-15), 122.5 (C-16), 12.5 (C-17), 22.1 (C-18), 60.7 (1-OCH3), 61.1 (2-OCH3), 55.9 (3-OCH3), 100.8 (12, 13-OCH2O), 59.7 (14-OCH3)。以上数据与文献报道对比基本一致[24],鉴定化合物21为γ-schisandrin。
化合物22:白色固体(甲醇);[α]20 D−8.7°(0.072,MeOH);C22H24O6;ESI-MS/: 385.32 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.48 (1H, s, H-4), 2.54 (1H, dd,= 13.6, 7.2 Hz, H-6β), 2.44 (1H, dd,= 13.6, 1.9 Hz, H-6α), 1.82 (1H, m, H-7), 1.82 (1H, m, H-8), 2.24 (1H, dd,= 13.2 , 9.5 Hz, H-9β), 2.00 (1H, d,= 13.2 Hz, H-9α), 6.48 (1H, s, H-11), 0.96 (3H, d,= 7.2 Hz, H-17), 0.73 (3H, d,= 7.2 Hz, H-18), 3.82 (3H, s, 1-OCH3), 5.94 (2H, overlapped, 2, 3-OCH2O), 5.94 (2H, overlapped, 12, 13-OCH2O), 3.83 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 141.4 (C-1), 134.8 (C-2), 147.7 (C-3), 106.2 (C-4), 132.9 (C-5), 38.9 (C-6), 33.7 (C-7), 40.8 (C-8), 35.4 (C-9), 138.3 (C-10), 103.3 (C-11), 148.8 (C-12), 134.5 (C-13), 141.1 (C-14), 121.1 (C-15), 122.3 (C-16), 21.9 (C-17), 12.7 (C-18), 59.8 (1-OCH3), 100.8 (2, 3-OCH2O), 100.8 (12, 13-OCH2O), 59.8 (14-OCH3)。以上数据与文献报道对比基本一致[30],故鉴定化合物22为schisandrin C。
化合物23:白色晶体(甲醇);[α]20 D−150.0°(0.056,MeOH);C28H34O9;ESI-MS/: 537.28 [M+Na]+。1H-NMR (400 MHz, CDCl3): 6.76 (1H, s, H-4), 5.67 (1H, s, H-6), 1.95 (1H, m, H-8), 2.30 (1H, dd,= 14.0, 9.7 Hz, H-9β), 2.15 (1H, d,= 14.0 Hz, H-9α), 6.50 (1H, s, H-11), 1.13 (3H, d,= 7.0 Hz, H-17), 1.30 (3H, s, H-18), 6.01 (1H, m, H-3), 1.67 (3H, d,= 7.0 Hz, H-4), 1.56 (3H, s, H-5), 3.55 (3H, s, 1-OCH3), 3.86 (3H, s, 2-OCH3), 3.88 (3H, s, 3-OCH3), 5.92 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 5.86 (1H, d,= 1.4 Hz, 12, 13-OCH2O), 3.68 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 152.0 (C-1), 141.8 (C-2), 152.2 (C-3), 110.1 (C-4), 130.9 (C-5), 84.3 (C-6), 72.3 (C-7), 42.5 (C-8), 36.5 (C-9), 137.6 (C-10), 102.7 (C-11), 148.7 (C-12), 134.4 (C-13), 140.7 (C-14), 121.5 (C-15), 122.2 (C-16), 19.0 (C-17), 28.2 (C-18), 166.3 (C-1), 127.7 (C-2), 135.4 (C-3), 14.4 (C-4), 11.6 (C-5), 60.8 (1-OCH3), 61.0 (2-OCH3), 56.0 (3-OCH3), 100.6 (12, 13-OCH2O), 59.1 (14-OCH3)。以上数据与文献报道对比基本一致[31],鉴定化合物23为schisantherin C。
化合物24:白色晶体(甲醇);[α]20 D−13.5°(0.052,MeOH);C22H26O6;ESI-MS/: 387.26 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.63 (1H, s, H-4), 2.25 (1H, dd,= 13.2, 9.4 Hz, H-6β), 2.00 (1H, d,= 13.2 Hz, H-6α), 1.83 (1H, overlapped, H-7), 1.83 (1H, overlapped, H-8), 2.55 (1H, dd,= 13.6, 7.3 Hz, H-9β), 2.44 (1H, dd,= 13.6, 1.8 Hz, H-9α), 6.49 (1H, s, H-11), 0.73 (3H, d,= 7.1 Hz, H-17), 0.97 (3H, d,= 7.1 Hz, H-18), 3.50 (3H, s, 1-OCH3), 3.93 (3H, s, 2-OCH3), 5.70 (1H, s, 3-OH), 5.96 (2H, d,= 1.4 Hz, 12, 13-OCH2O), 3.78 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 150.4 (C-1), 137.5 (C-2), 148.8 (C-3), 110.3 (C-4), 140.4 (C-5), 35.2 (C-6), 41.0 (C-7), 33.9 (C-8), 39.1 (C-9), 132.8 (C-10), 106.3 (C-11), 147.8 (C-12), 135.2 (C-13), 141.4 (C-14), 121.6 (C-15), 122.6 (C-16), 12.5 (C-17), 22.0 (C-18), 60.3 (1-OCH3), 61.2 (2-OCH3), 100.9 (12, 13-OCH2O), 59.8 (14-OCH3)。以上数据与文献报道对比基本一致[32],鉴定化合物24为neglschisandrin E。
化合物25:黄色粉末(甲醇);[α]20 D+117.9°(0.056,MeOH);C24H30O6;ESI-MS/: 415.30 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.65 (1H, s, H-4), 3.00 (1H, d,= 12.3 Hz, H-6β), 2.93 (1H, d,= 12.3 Hz, H-6α), 2.70 (1H, m, H-8), 2.54 (2H, m, H-9), 6.55 (1H, s, H-11), 1.01 (3H, d,= 7.2 Hz, H-17), 4.86 (1H, d,= 2.0 Hz, H-18a), 4.72 (1H, d,= 2.0 Hz, H-18b), 3.60 (3H, s, 1-OCH3), 3.86 (3H, s, 2-OCH3), 3.90 (3H, s, 3-OCH3), 3.89 (3H, s, 12-OCH3), 3.89 (3H, s, 13-OCH3), 3.62 (3H, s, 14-OCH3);13C-NMR (100 MHz, CDCl3): 151.4 (C-1), 140.3 (C-2), 153.1 (C-3), 107.1 (C-4), 136.6 (C-5), 37.2 (C-6), 154.0 (C-7), 38.7 (C-8), 37.9 (C-9), 133.3 (C-10), 110.5 (C-11), 152.0 (C-12), 140.5 (C-13), 151.6 (C-14), 123.8 (C-15), 122.6 (C-16), 20.7 (C-17), 111.0 (C-18), 60.7 (1-OCH3), 61.1 (2-OCH3), 56.0 (3-OCH3), 56.1 (12-OCH3), 61.1 (13-OCH3), 60.7 (14-OCH3)。以上数据与文献报道对比基本一致[33],鉴定化合物25为7(18)-dehydroschisandro A。
化合物26:棕色油状物(甲醇);[α]20 D+11.5°(0.096,MeOH);C20H24O4;ESI-MS/: 329.20 [M+H]+。1H-NMR (400 MHz, CDCl3): 6.62 (1H, d,= 1.9 Hz, H-2), 6.83 (1H, d,= 7.9 Hz, H-5), 6.63 (1H, dd,= 7.9, 1.9 Hz, H-6), 2.74 (1H, dd,= 13.6, 6.0 Hz, H-7a), 2.24 (1H, dd,= 13.6, 9.1 Hz, H-7b), 1.73 (1H, overlapped, H-8), 0.84 (3H, d,= 6.8 Hz, H-9), 3.87 (3H, s, 3-OCH3), 5.45 (1H, s, 4-OH), 6.65 (1H, d,= 1.7 Hz, H-2), 6.73 (1H, d,= 7.9 Hz, H-5), 6.60 (1H, dd,= 7.9, 1.7 Hz, H-6), 2.70 (1H, dd,= 13.2, 6.4 Hz, H-7a), 2.29 (1H, dd,= 13.2, 9.1 Hz, H-7b), 1.73 (1H, overlapped, H-8), 0.82 (3H, d,= 6.8 Hz, H-9), 5.92 (2H, s, 3, 4-OCH2O);13C-NMR (100 MHz, CDCl3): 133.9 (C-1), 111.5 (C-2), 146.4 (C-3), 143.7 (C-4), 114.1 (C-5), 121.8 (C-6), 39.0 (C-7), 39.4 (C-8), 16.4 (C-9), 56.0 (3-OCH3), 135.8(C-1), 109.5 (C-2), 147.6 (C-3), 145.6 (C-4), 108.1 (C-5), 121.8 (C-6), 39.2 (C-7), 39.5 (C-8), 16.3 (C-9), 100.9 (3, 4-OCH2O)。以上数据与文献报道对比基本一致[34],鉴定化合物26为anwulignan。
4 讨论
近年来课题组对苏黄止咳胶囊改善气道重塑[35]、痰阻塞[36]、抑制炎症反应[37]、以及治疗CVA[38-39]和PIC[40]等方面的药理机制进行了阐释,其功效成分及机制正在研究中。本研究作为该项目的一部分,进一步丰富了其臣药五味子化学成分的多样性,为后续开展苏黄止咳胶囊的药效物质基础研究及其质量控制奠定了基础。
利益冲突 所有作者均声明不存在利益冲突
[1] 中国药典[S]. 一部. 2020: 68.
[2] Zhou Y, Men L H, Sun Y X,. Pharmacodynamic effects and molecular mechanisms of lignans fromTurcz. (Baill.), a current review [J]., 2021, 892: 173796-173807.
[3] 陈丽华, 许智莹, 林程程, 等. 五味子木脂素舒张大鼠离体气管平滑肌的功能研究 [J]. 西北农林科技大学学报: 自然科学版, 2021, 49(10): 24-29.
[4] 孙靖辉, 吕希, 王春梅, 等. 五味子木脂素对哮喘模型小鼠Th2相关炎症因子的影响 [J]. 北华大学学报: 自然科学版, 2020, 21(3): 325-328.
[5] Zhong S, Nie Y C, Gan Z Y,. Effects ofextracts on cough and pulmonary inflammation in a cough hypersensitivity guinea pig model induced by cigarette smoke exposure [J]., 2015, 165: 73-82.
[6] 林豪, 王阔, 曾钦成, 等. 基于网络药理学和分子对接技术探讨五味子治疗慢性咳嗽的机制研究 [J]. 北华大学学报: 自然科学版, 2022, 23(4): 466-470.
[7] 赵红舟, 龙杞, 刘肇恒, 等. 五味子乙素对博来霉素诱导小鼠肺纤维化的干预作用及机制研究 [J]. 环球中医药, 2021, 14(4): 556-561.
[8] 魏菲, 刘斌, 肖娜, 等. 五味子乙素减轻博莱霉素诱导的肺纤维化 [J]. 天津中医药大学学报, 2017, 36(3): 200-204.
[9] Zhong S, Bai L P, Liu X D,. Cough inhibition activity ofin guinea pigs [J]., 2021, 24(4): 348-357.
[10] Lv X, Xu Z Y, Xu G Y,. Investigation of the active components and mechanisms ofin the treatment of asthma based on a network pharmacology approach and experimental validation [J]., 2020, 11(4): 3032-3042.
[11] Kim H, Ahn Y T, Kim Y S,. Antiasthmatic effects of schizandrae fructus extract in mice with asthma [J]., 2014, 10(Suppl 1): S80-S85.
[12] 李舒丰, 刘芹燕, 刘文东, 等. 苏黄止咳胶囊及其挥发油中间体中挥发性成分的分析 [J]. 中成药, 2017, 39(11): 2329-2334.
[13] 刘芹燕, 巫兴东, 陈东, 等. 苏黄止咳胶囊中非挥发性成分的LC-MS分析 [J]. 中成药, 2019, 41(6): 1434-1445.
[14] Wu X D, Liu Q Y, Chen D,. Identification of quality control markers in Suhuang antitussive capsule based on HPLC-PDA fingerprint and anti-inflammatory screening [J]., 2020, 180: 113053-113061.
[15] Tian T, Liu Y, Yu H Y,. Dibenzocyclooctadiene lignans from the fruits of[J]., 2015, 51(6): 1046-1048.
[16] Ying Y M, Yu H F, Rao G W,. Dibenzocyclooctadiene lignans from the stems of[J]., 2022, 36(1): 287-294.
[17] Zhou X D, Chen C X, Zheng X K,. Dibenzocyclooctadiene lignans fromand their anti-inflammatory activities [J]., 2021, 75(4): 1014-1020.
[18] Cheng Y B, Chang M T, Lo Y W,. Oxygenated lignans from the fruits of[J]., 2009, 72(9): 1663-1668.
[19] Amujuri D, Siva B, Bharathi K,. Bio-active constituents from: Isolation, semi-synthesis and evaluation of their anticancer activities [J]., 2021, 45: 184-189.
[20] Choi S K, Lee Y G, Wang R B,. Dibenzocyclooctadiene lignans from the fruits ofand their cytotoxicity on human cancer cell lines [J]., 2020, 63(1): 1-8.
[21] Ikeya Y, Taguchi H, Yosioka I,. The constituents ofBaill. I. Isolation and structure determination of five new lignans, gomisin A, B, C, F and G, and the absolute structure of schizandrin [J]., 1979, 27(6): 1383-1394.
[22] 刘俊霞, 窦凤鸣, 王英平. 五味子藤茎中木脂素类化合物的杀虫活性成分研究 [J]. 天然产物研究与开发, 2017, 29(7): 1210-1217.
[23] Ikeya Y, Taguchi H, Yosioka I. The constituents ofBaill. VII. The structures of three new lignans, (−)-gomisin K1and (+)-gomisins K2and K3[J]., 1980, 28(8): 2422-2427.
[24] Ikeya Y, Taguchi H, Yosioka I. The Constituents ofBaill. X. The structures of GAMMA - schizandrin and four new lignans, (−)-gomisins L1 and L2, (±)-gomisin M1 and (+)-gomisin M2 [J]., 1982, 30(1): 132-139.
[25] Rui T, Lian-Niang L, Qicheng F. Studies on the chemical constituents of: Isolation and structure elucidation of five new lignans [J]., 1984, 50(5): 414-417.
[26] Piao L Z, Lee Y J, PhamPhu T T,. Dibenzocyclooctene lignan compounds isolated from the fruits ofBaill [J]., 2005, 11(4): 248-252.
[27] Ikeya Y, Ookawa N, Taguchi H,. The constituents ofBaill. XI. The structures of three new lignans, angeloylgomisin O, and angeloyl- and benzoylisogomisin O [J]., 1982, 30(9): 3202-3206.
[28] Ikeya Y, Miki E, Okada M,. Benzoylgomisin Q and benzoylgomisin P, two new lignans fromRehd. et Wils [J]., 1990, 38(5): 1408-1411.
[29] Ikeya Y, Taguchi H, Yosioka I. The constituents ofBaill. The cleavage of the methylenedioxy moiety with lead tetraacetate in benzene, and the structure of angeloylgomisin Q [J]., 1979, 27(10): 2536-2538.
[30] Yan Z F, Guo J, Tian F H,. Active compounds fromexhibiting tyrosinase activity and melanin content inhibition in B16 melanoma cells [J]., 2015, 20(4): 814-823.
[31] Smejkal K, Slapetová T, Krmenčík P,. Evaluation of cytotoxic activity oflignans [J]., 2010, 76(15): 1672-1677.
[32] Chen M, Xu X M, Xu B,. Neglschisandrins E-F: Two new lignans and related cytotoxic lignans from[J]., 2013, 18(2): 2297-2306.
[33] Song Z H, Houa Y, Han N,. Research on the biotransformation of fourlignans [J]., 2015, 10(6): 129-133.
[34] Baek N I, Lee Y G, Wang R B,. Anwulignan from the fruits ofand its cytotoxicity against human cancer cell lines [J]., 2020, 16(72): 695-699.
[35] 郭超, 童希洋, 秦伟伟, 等. 苏黄止咳胶囊改善哮喘豚鼠气道重塑的作用及其机制 [J]. 中成药, 2021, 43(4): 893-901.
[36] Tong X Y, Liang R Y, Jia Y N,. Suhuang antitussive capsules-ameliorative effects on LPS-induced sputum obstruction in mice through promoting HGF secretion [J]., 2019, 10: 1422-1436.
[37] Qin W W, Tong X Y, Liang R Y,. Preservation of mitochondrial homeostasis is responsible for the ameliorative effects of Suhuang antitussive capsule on non-resolving inflammation via inhibition of NF-κB signaling and NLRP3 inflammasome activation [J]., 2021, 271: 113827-113844.
[38] Qin W W, Wu X D, Jia Y N,. Suhuang antitussive capsule inhibits NLRP3 inflammasome activation and ameliorates pulmonary dysfunction via suppression of endoplasmic reticulum stress in cough variant asthma [J]., 2019, 118: 109188-109202.
[39] Jiang H, Bai Z Y, Ou Y Y,. β-Hydroxybutyric acid upregulated by Suhuang antitussive capsule ameliorates cough variant asthma through GSK3β/AMPK-Nrf2 signal axis [J]., 2023, 307: 116013-116025.
[40] Liang R Y, Tong X Y, Dong Z K,. Suhuang antitussive capsule ameliorates post-infectious cough in mice through AhR-Nrf2 pathway [J]., 2022, 283: 114664-114676.
A new dibenzocyclooctadiene lignan from
DI Tong-lian1, LIU Xiao-qing2, WU Nan1, CHEN Xiao-yuan1, FAN Jun-ting3, TAN Ning-hua1
1. School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, China 2. Beijing Haiyan Pharmaceutical Co., Ltd., Yangtze River Pharmaceutical Group, Beijing 102206, China 3. School of Pharmacy, Nanjing Medical University, Nanjing 211116, China
To study the lignans in.The petroleum ether extract mainly contained lignans ofwas isolated and purified by silica gel column chromatography, medium pressure reversed phase column chromatography, semi-prepared and prepared liquid chromatography. The structures of compounds were identified by spectral data.Twenty-six compounds were obtained, in which 24 were dibenzocyclooctadiene lignans. They were identified as schiviridin B (1), sphaerandrin A (2), sieverlignan E (3), arisanschinin G (4), widdaranal F (5), gomisin A (6), gomisin B (7), gomisin D (8), gomisin G (9), (−)-gomisin K1(10), (+)-gomisin K2(11), (−)-gomisin L1(12), (+)-gomisin M1(13), gomisin N (14), angeloylgomisin H (15), angeloylgomisin O (16), angeloylgomisin Q (17), benzoylgomisin Q (18), schisandrin (19), schisandrin A (20), γ-schisandrin (21), schisandrin C (22), schisantherin C (23), neglschisandrin E (24), 7(18)-dehydroschisandro A (25), and anwulignan (26).Compound 1 is new and compounds 2—5 are isolated from this plant for the first time.
(Turcz.) Baill.; schiviridin B; dibenzocyclooctadiene lignans; sphaerandrin A; sieverlignan E; arisanschinin G; widdaranal F; schisandrin
R284.1
A
0253 - 2670(2023)12 - 3759 - 12
10.7501/j.issn.0253-2670.2023.12.002
2023-04-02
国家自然科学基金项目(32070356)
邸同莲,女,硕士研究生,研究方向为中药物质基础和质量控制研究。E-mail: 1282046265@qq.com
通信作者:谭宁华,教授,博士生导师,主要从事含环肽中药和止咳中药系统研究。E-mail: nhtan@cpu.edu.cn
范君婷,副教授,主要从事天然药物化学与作用机制研究。E-mail: juntingfan@njmu.edu.cn
[责任编辑 王文倩]

