生物质超临界水制氢研究进展
徐功迅,陈 伟,方 真
生物质超临界水制氢研究进展
徐功迅,陈 伟,方 真※
(南京农业大学工学院,南京 210031)
生物质超临界水制氢(supercritical water gasification,SCWG)以超临界水为介质通过热化学方式将生物质中的有机物转化为氢气等能源气体。相较于传统制氢方式,SCWG过程具有反应速度快、氢气选择性好、副产物少等优点,是一种高效、经济、清洁的生物质处理技术。该研究主要围绕SCWG过程中的影响因素进行系统地分析,介绍了超临界水特殊的物理化学性质,详细阐述了生物质主要成分如纤维素、半纤维素和木质素在SCWG过程中的反应机理,以及试验装置、原料类型和浓度、反应温度、停留时间、压力等工艺因素对SCWG的影响。研究发现纤维素占比较高的作物气化效果更好,低浓度的进料有利于气化效率和碳气化效率的提升,提高装置升温速率、适当增加反应温度和停留时间能够增加氢气产率,过大的压力会形成“溶剂笼”效应降低氢气产量。对不同类型反应系统研究表明,间歇式反应装置虽然结构简单、操作方便但也存在物料与催化剂混合不均匀、不能实现连续化生产而不适用于工业化推广,连续式反应装置虽面领着堵塞等问题,但具有性能好、效益高的优点,是工业化推广的发展方向。对SCWG主要应用的催化剂进行讨论发现,均相催化剂虽然具有催化效果但具有较强腐蚀性,非均相催化剂因其具有高催化活性、易回收、稳定性好等优点更适用于大规模SCWG生产过程。同时还研究了金属催化剂酸度在催化过程中的影响,酸度越高,在SCWG过程中积碳会越明显,通过添加Cu、Ce、Co、La等合适的第二金属作为促剂可以改变催化剂性能,增加催化剂使用寿命,提高氢气选择性。未来应针对SCWG的试验装置、高效催化剂及经济性分析等核心技术开展研究,加速SCWG的工业化推广,实现经济、安全、绿色、高效的氢能供给。该研究期望加深对生物质SCWG理解,为日后研究提供理论指导。
生物质;氢气;催化剂;超临界水
0 引 言
当前国内外仍以化石燃料为主要能源[1],造成了严重的环境污染问题。自2015年《联合国气候变化框架公约》提出以来各国纷纷提出碳减排措施。欧盟出台《欧洲气候法》提出在2050年前实现碳中和[2];美国承诺2050年前达到碳净零排放;中国作为碳排放大国,宣布力争在2030年前完成碳达峰,争取2060年实现碳中和[3]。目前世界正处于能源发展的交替时代,急需寻找一种科学技术上可行、环境友好的可替代能源形式[4]。
生物质能是将生物质转化为有用的能源[5],是自然界中唯一一种可再生的碳源,不仅可以用于燃烧还可以作为原料转化为各种液体或气体燃料[6]。氢能作为一种清洁高效的可再生二次能源,可以通过燃烧或者燃料电池的方式利用[7]。氢能在使用中有诸多优点:1)氢在燃烧过程中唯一产物是水,可以做到零碳排放[8];2)氢的热值达到142.3 MJ/kg,是同等质量化石能源的3~4倍[9]; 3)氢是很多化学产品的重要原料,氢能产业发展将推动工业的发展[10]。通过主要制氢方式对比发现,传统化石能源制氢虽成本较低但产生污染较大,工业副产制氢和水电解制氢成本相对较高,而生物质制氢具有能耗低、原料广泛等优点,具有很大发展潜力[7,9,11-13]。
1 生物质超临界水制氢机理研究
1.1 超临界水及性质
当温度超过374 ℃,压力超过22.1 MPa时,水就会变成超临界水(supercritical water,SCW)。当水的温度和压力超过其沸点,但在临界点以下仍然保持液态时称为亚临界水。表1总结了不同条件下水的性质[14-15]:当水处于超(亚)临界状态时物理化学性质会发生巨大变化,主要包括以下几点:
1)随着氢键断裂、介电常数降低,SCW更像非极性溶剂[16],主要表现为可以溶解大多数有机溶剂并且可以与气体互溶,但是无机物溶解度大大降低[17]。SCW能够为反应物提供均相反应条件[18],是一种很好的反应媒介[19];
2)SCW超低的黏度有更好的分子迁移率,可以加速溶质扩散[20],防止碳沉积和催化剂中毒[21];
3)SCW会抑制离子反应,增强自由基反应,有利于气体产物生成[22];
4)在临界点附近,水中H3O+和OH-离子浓度增加,此时非常有利于酸性或者碱性催化反应的进行,SCW本身也可以替代一些酸和碱催化剂促进反应[18,23]。
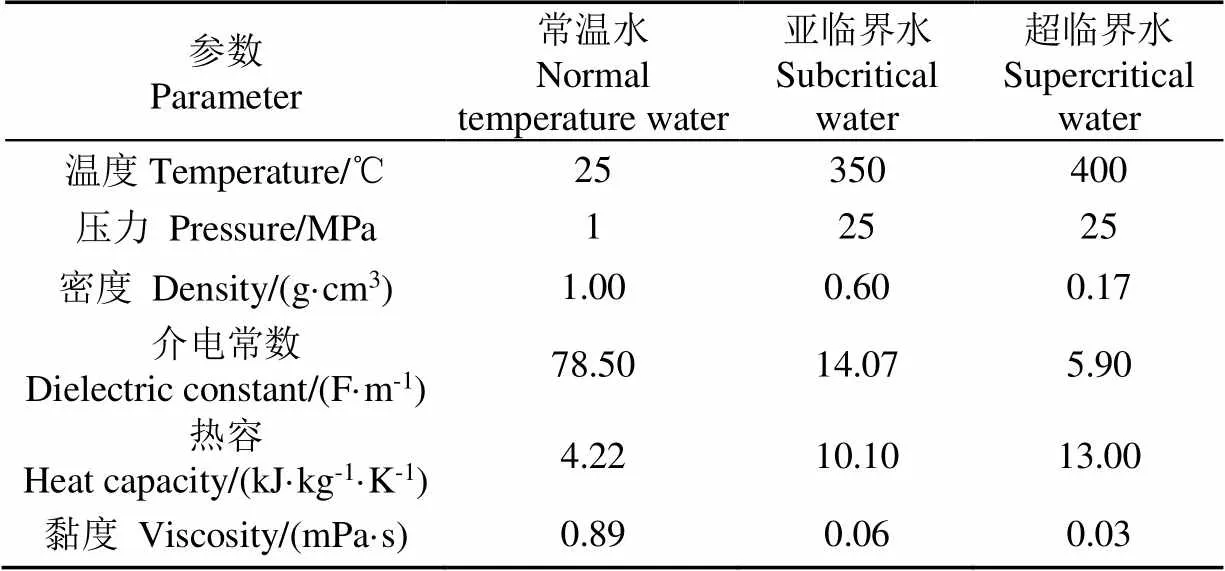
表1 不同条件下水的性质
分子动力学常用于模型的预测,LIEW等[24]用四位柔性模型对亚临界和超临界区域的纯水进行了分子动力学模拟,通过空间分布函数研究了水的三维溶解结构和氢键,发现除了线性氢键的水外,超临界水还包括分叉氢键的水;BOERO等[25]利用Car-Parrinello分子动力学构建出超临界点附近水的氢键网络结构和介电特性,发现在低密度下水主要分裂成三聚体、二聚体和单分子。SASAKI等[26]利用连续流动装置发现纤维素在350 ℃和25 MPa的条件下仅4 s就可以完全溶解和转化为葡萄糖和果糖等小分子物质,并将快速溶解的原因归结于超临界流体形成的均相反应条件,因此可以看出超临界状态非常有利于生物质的溶解和进一步反应。
1.2 生物质超(亚)临界气化反应机理
生物质主要由纤维素、半纤维素和木质素构成,纤维素是一种由葡萄糖单体通过(1,4)糖苷键连接而成的多糖,这使得纤维素分子内和分子间形成强氢键,使其结晶、耐水溶胀[27-28]。半纤维素由木糖、半乳糖和葡萄糖等多种糖单体组成(图1),不同作物糖单体的比例也不同。
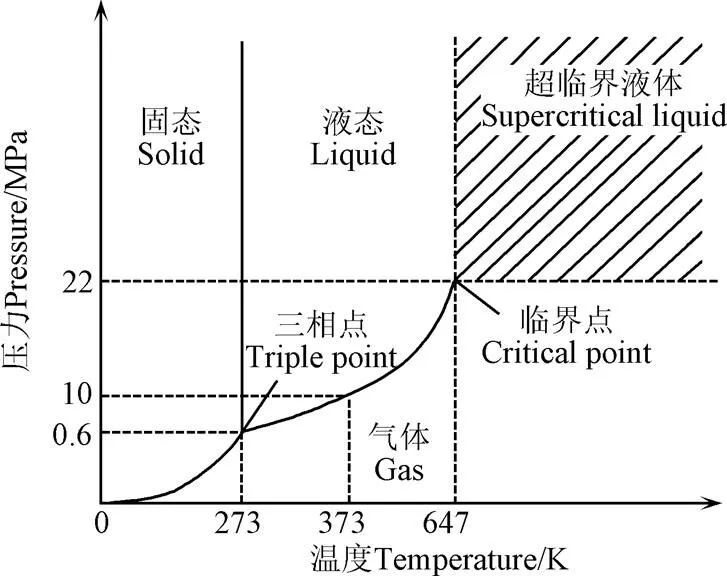
图1 水的状态[29]
半纤维素结构较不稳定,更容易水解[30-31]。木质素是由对香豆醇、松柏醇和芥子醇3种单体组成的一种复杂高分子量化合物,化学性质相对稳定。超临界水气化的过程复杂,涉及水解、热解、焦油重整、水煤气转化、甲烷化反应等[32-33],主要反应方程式[34-36]如下:
CHO+(2-)H2O → CO2+ (2-+/2)H2(1)
CHO+(1-)H2O → CO + (1-+/2)H2(2)
纤维素的水解:
(C6H10O5) +H2O → nC6H12O6(3)
葡萄糖重整反应:
C6H12O6→ 6CO + 6H2(4)
木质素水解:
(C10H10O3)+H2O →C10H12O4→ 酚醛树脂 (5)
蒸汽重整反应:
酚醛树脂+H2O → CO + CO2+ H2(6)
水煤气转化反应:
CO + H2O → CO2+ H2(7)
CO的甲烷化反应:
CO + 3H2→ CH4+ H2O (8)
CO2的甲烷化反应:
CO2+ 4H2→ CH4+ 2H2O (9)
加氢反应:
CO + 4H2→ CH4+ 0.5O2(10)
焦油重整反应:
CHO(tar) + CO2→ 2CO +/2H2(11)
式中和分别表示生物质中H/C和O/C的元素摩尔比,产物中气体组分的比例很大程度上取决于和的大小。生物质在超临界水状态下主要转化为氢气、甲烷、二氧化碳、一氧化碳等气体,在这个过程中超临界水既作为反应介质又作为反应物参与反应过程。
反应中主要过程及主要产物如图2所示。在最初的水解过程中,生物质主要分解为糖类、酚类和醇类物质,其中纤维素和半纤维素水解的主要成分是五碳糖和六碳糖,木质素主要水解为酚类,包括愈创木酚、酚醛树脂等。在超临界水的状态下这些中间产物进一步转化为更简单的化合物,例如酸(羧酸、琥珀酸等)、醇(豆香醇、芥子醇等)、酚类化合物、醛和芳香烃化合物。其中,酸类化合物主要来自于糖类物质的分解,醇、芳烃、醛等物质主要来自于木质素(苯酚)的变性产物[37-38]。
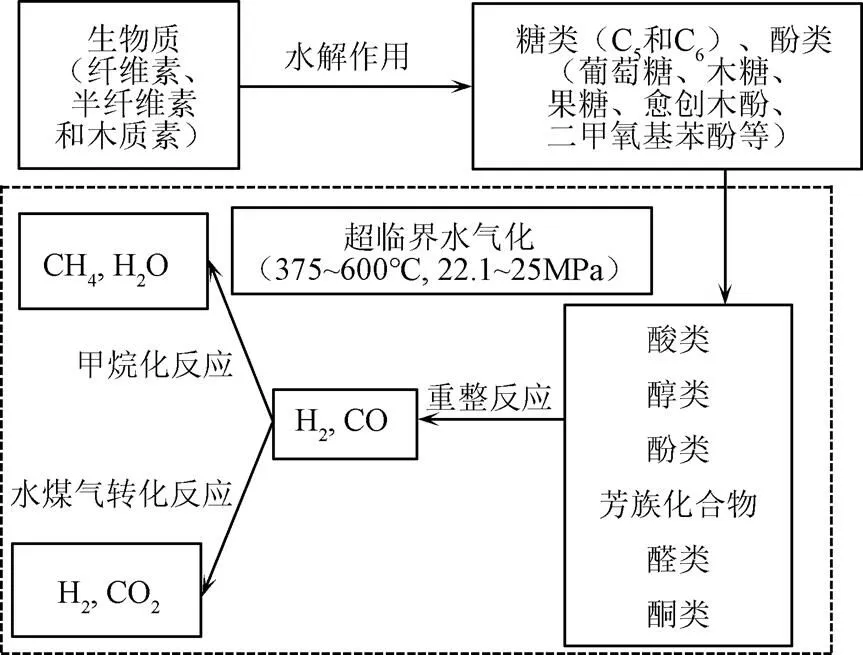
图2 生物质超临界水制氢反应路线图
纤维素有更大的氢含量因此在SCWG过程中比半纤维素对产氢的贡献更大[39],葡萄糖是纤维素水解过程中的主要产物。KABYEMELA等[40]研究发现在水热条件下葡萄糖与木糖会发生互相异构化反应,研究证实果糖在SCWG中具有更高活性。CASTELLO等[41]以不同浓度的葡萄糖和苯酚为模型化合物在400 ℃和25 MPa的连续式反应器中试验提出了反应机理(图3),并指出在400 ℃离子反应条件下葡萄糖主要分解成赤藓糖和乙醇醛。赤藓糖具有呋喃环,进一步反应易形成糠醛,乙醇醛则进一步分解为乙醇;葡萄糖与果糖之间会相互转化,果糖脱水形成5-羟甲基糠醛(5-HMF),5-HMF部分通过缩合形成重质化合物,部分进一步降解为酸类从而形成气体产物。
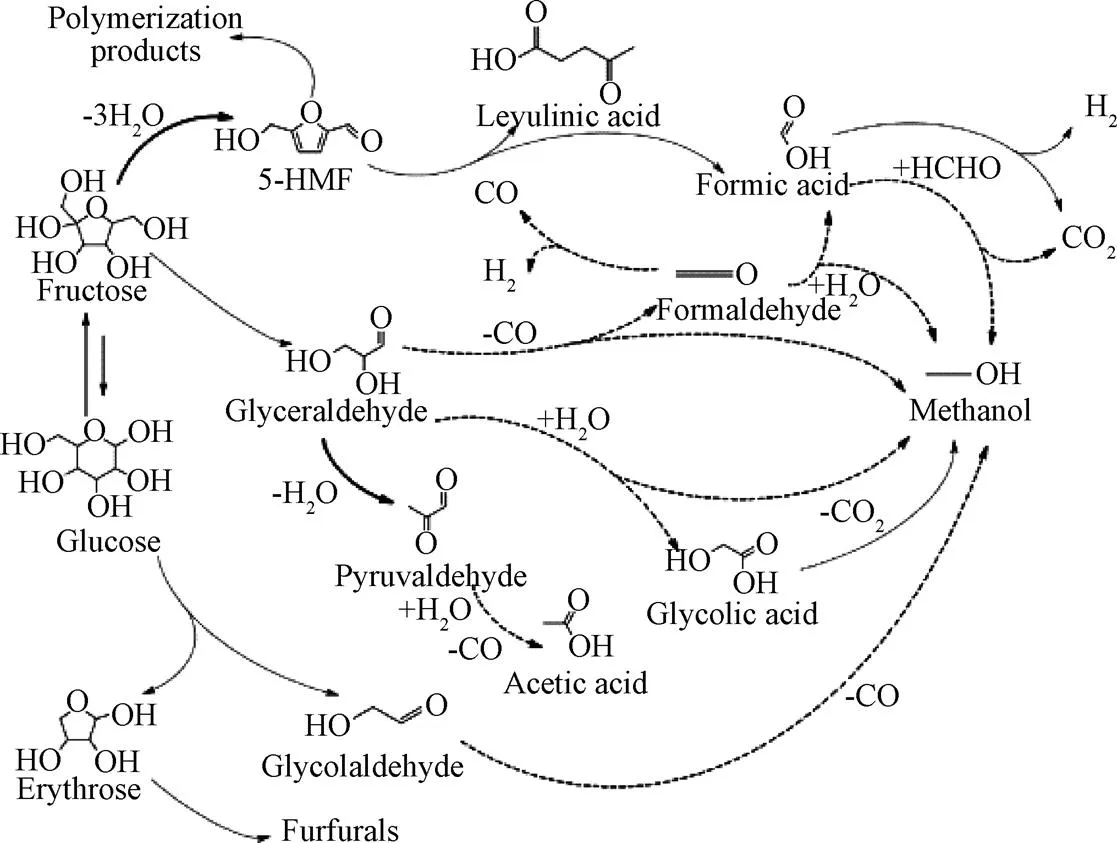
图3 葡萄糖降解机理[41]
CASTELLO等[41]提出苯酚也是葡萄糖的降解产物之一,因为葡萄糖降解产生的糠醛会产生苯酚[42],大量研究表明在水热条件下葡萄糖会分解成5-HMF、苯酚等酚类化合物以及酸类化合物等,这些物质进一步分解为呋喃、芳烃、有机酸、醇、酮等物质,其中有机酸、醇、酮进一步分解为氢气等气体,呋喃、芳烃等化合物更多的转化为焦炭[43-44],而烃类的重整反应和水煤气转化过程中易在催化剂表面发生结焦造成催化剂失活[45]。酚类物质被认为是生物质气化过程中的巨大障碍,因为在超临界状态下酚类物质可以通过加氢脱氧等反应生成难以气化的芳烃,产生的中间产物如呋喃、酚类和芳烃等物质易促进聚合反应转化生成焦炭导致气化效率降低[46]。
半纤维素是由C5和C6糖组成的一种无定型的生物聚合物,GOODWIN等[47]以木糖为模型化合物探究半纤维素在SCWG过程中降解机理(图4),认为木糖反应主要有2条途径:第1种途径是通过脱水生成糠醛,糠醛分解成水溶性腐殖质(water soluble humus, WSHS)和脂类化合物,再进一步分解成气体产物;第2种途径是木糖分解成甲酸甲酯和甘油醛,进一步分解为酸类化合物进而降解为气体产物,在此过程中生成的气体产物还会通过水煤气变换和一氧化碳的甲烷化反应进行转化。
木质素的结构较为复杂,是生物质中最难气化的组分。木质素的存在会抑制气体形成的速率[32],在气化过程中主要产生4个相:油相(如酚类、PAH等)、水相(如醇类、醛类)、气相(如氢气、一氧化碳、二氧化碳、甲烷等)、固相(焦炭等物质)[32,48]。木质素气体产物主要是二氧化碳和甲烷[49],较高的氢气产率需要高温才能实现。在水热降解过程中木质素的醚键断裂形成甲氧基酚等酚类物质,甲氧基苯酚通过脱甲氧基反应产生烷基苯酚和甲醇。其中甲醇通过2种方式转化为气体产品,一种是直接分解成H2和CO,另一种是通过与水反应生成CO2和H2,进一步发生水煤气转化反应和加氢反应生成CH4和氢气等气体[50](图5)。烷基苯酚则通过自由基反应转化为酚和烷烃自由基,烷烃通过自由基反应与氢自由基结合生成甲烷等气体,酚类物质则通过氢化等反应分解,或者通过缩聚反应生成生物炭。木质素在水解过程中还会形成有苯环的高分子化合物,在超临界状态下,苯酚通过脱羟基反应生成苯环,再通过聚合反应生成联苯和苯基苯酚等物质,这两种物质是生成焦炭的主要原料之一[51]。
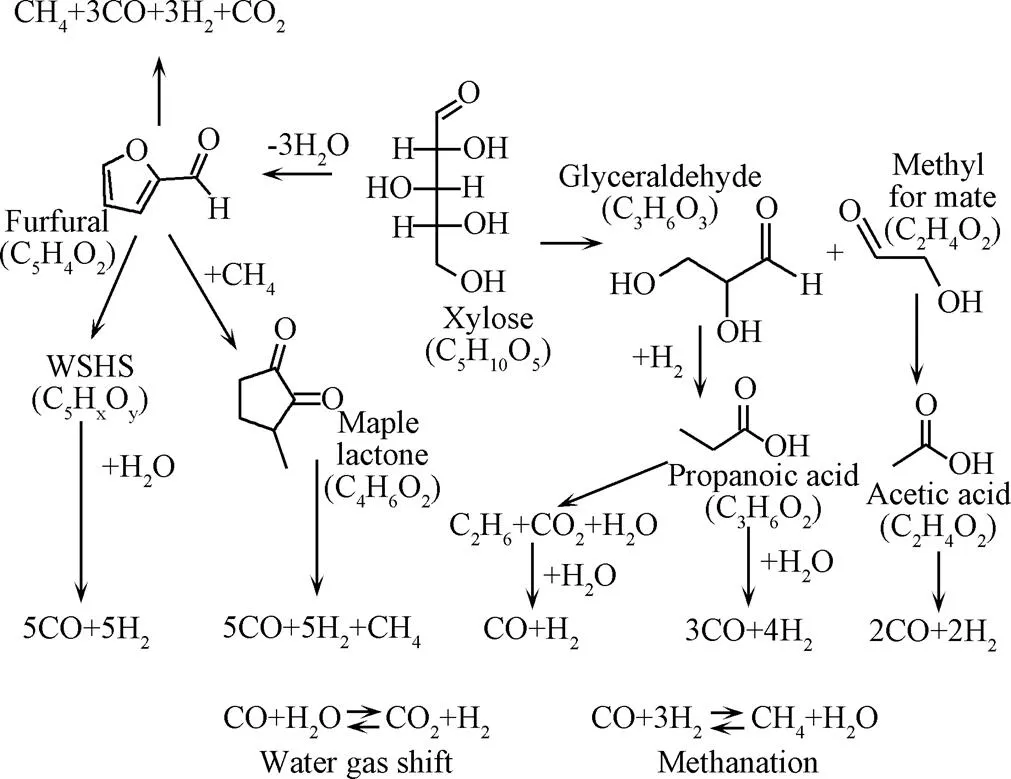
图4 木糖降解机理[47]
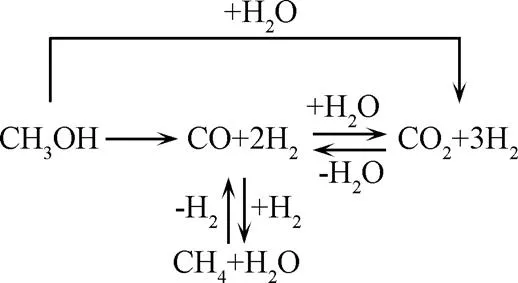
图5 甲醇反应路径图[50]
2 生物质超临界水制氢影响因素
2.1 试验装置
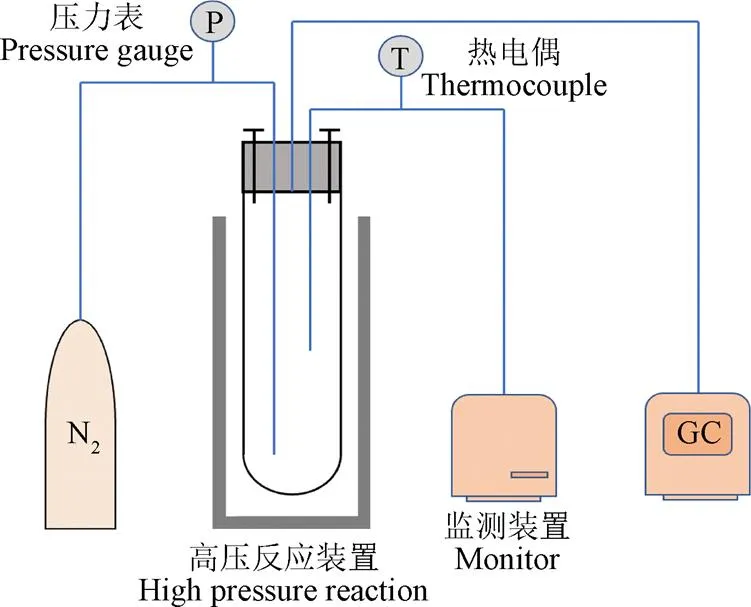
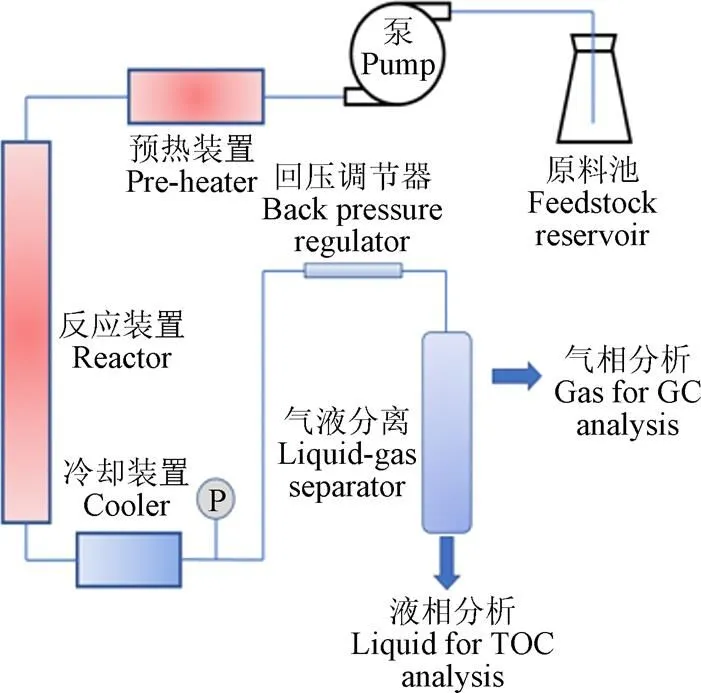
试验装置的升温速率会影响SCWG的产物,在低温区停留过久会导致焦炭等副产物生成[52],高加热速率会促进中间产物(甲酸和乙酸等)形成,抑制酚类化合物形成[53],防止焦油焦炭产生[54-55]。目前应用于SCWG的试验装置主要有间歇式和连续式2种[34],图6展示了典型的间歇式与连续式反应装置。间歇式反应器具有结构简单、操作方便、原料适应性强、周期性使用过程中易于清理反应装置内部产生的焦炭等优点,但温度、压力等因素不易精准控制,易产生较大误差,同时也存在物料与催化剂混合不均匀等缺点[46,56]。连续式反应装置可以较为精准的控制试验参数,并实现连续化商业生产,在气化中生成的焦炭易堵塞反应器是目前面临的主要问题[57]。FANG等[18,37,58]利用可视钻石反应器(图7)发现木质纤维素易溶于高压热水中,并设计出连续装置使生物质在均相条件下快速溶解、水解和分解为小分子化合物,为大规模连续气化快速产氢奠定了试验基础。流化床反应器不仅具有克服反应器堵塞的能力还能够增强传热传质,在过去几年中被认为是解决SCWG焦炭堵塞的有效方案[59-60],YAKABOYLU等[60]以干淀粉为原料使用流化床反应装置在600 ℃条件下反应,发现产生的焦油等副产物量较少并且运行2 h均没有发生堵塞问题。CHEN等[61]对SCWG-Solar系统进行评估提出将太阳能利用于SCWG预热过程可以减少能耗,是未来大规模高效应用SCWG的可行方案之一。
a. 典型间歇式(高压反应釜)反应器
a. Typical batch (high-pressure reactor) reactor
b. 典型连续反应器
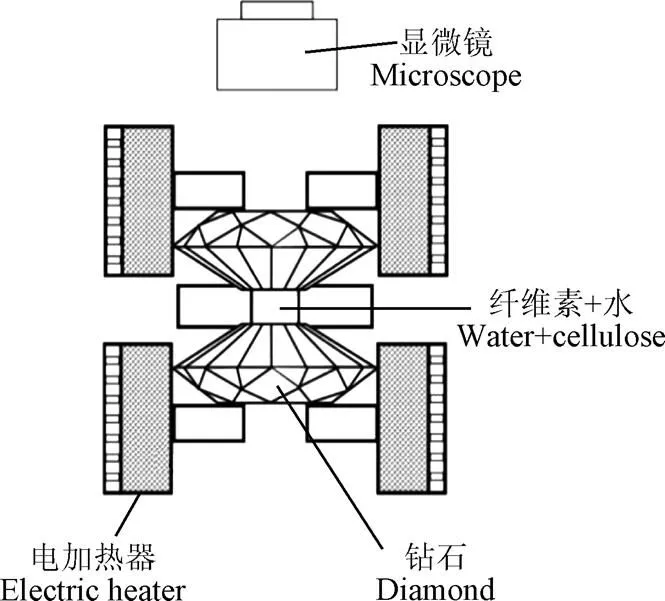
图7 微型可视钻石反应器[18]
2.2 原料类型及浓度
农作物秸秆中纤维素约占质量的35%~55%,半纤维素占20%~40%,木质素占10%~25%[62-63]。不同农作物类型的纤维素、半纤维素和木质素含量不同,在SCWG中产物也有很大不同,通常来说纤维素的生物可降解性大于木质素,因此纤维素占比高的作物气体转化率大于木质素占比高的作物[64]。生物质中还有蛋白质、矿物质、灰分等其他组分,其中蛋白质的存在会降低气体产率,因为蛋白质降解形成氨基酸与葡萄糖水解反应会相互干扰。蛋白质分解过程中的丙氨酸会产生自由基清除剂,抑制自由基反应,而SCWG过程中大部分气体通过自由基反应生成[65],此外生物质中部分矿物质可以充当催化剂的效果[66]。
YANIK等[67]以玉米秸秆、棉花秸秆等8种不同生物质作为反应原料,使用间歇式高压反应釜在500 ℃进行气化,氢气产量在4.05~4.65 mol/kg生物质,发现不同作物的氨基酸、蛋白质、油脂含量等都会影响氢气产量。WILLIAMS等[68]以纤维素、淀粉、葡萄糖和木薯废弃物为研究对象,在间歇式高压反应装置中试验发现葡萄糖的氢气产率最高,木薯废弃物的氢气产率最低。淀粉和纤维素虽然都是葡萄糖的聚合物,但在气化过程中纤维素产出的烃类化合物产出较多,淀粉产出的氢气、一氧化碳和油类物质较多,试验表明相同单体的聚合物连接方式不同在SCWG中效果也不同。郭烈锦院士团队以不同种陆生植物、水生植物以及生物质废料等为原料气化,发现陆生禾本植物具有较高的活性,其次为水生植物、有机废料等[69]。YOSHIDA等[70]使用木质素、纤维素及其混合物在400 ℃、25 MPa条件下利用镍催化剂进行气化,发现当原料中有软木质素时气化效率较低,纤维素与软木质素在超临界状态下相互作用会生成焦油使催化剂失活,相较于木质素、纤维素等模型化合物,真实生物质在气化过程中各组分之间也会相互作用影响气化效果。
进料浓度也会影响气化效果,物料浓度高会导致物料与水的接触面积减小,阻碍蒸汽重整过程,过高的进料浓度会使生物质形成较多焦炭、焦油等中间副产物,容易导致反应器堵塞等问题[57]。GUO等[71]使用连续流动管式反应器以甘油为原料进行研究,在567 ℃下甘油浓度由10%增加至25%过程中,氢气产率和气化效率均降低;WANG等[72]使用间歇式反应器在600 ℃条件下以碱性木质素为原料进行试验,发现当进料浓度由1%增加到6%时气化效率由79.86%下降到42.20%,表明增加木质素原料浓度抑制了气化反应。提高物料浓度反而会降低气化效率,低浓度的进料反而有益于气化率和碳气化效率的提高[49],然而高浓度的进料有利于对污水、废弃生物质的批量处理,能有效提高超临界水处理废弃物的效率,实现高浓度进料水平下高氢气产率是未来的研究方向。
2.3 反应温度
提高温度可以增加气化效率和氢气产率,但在实际应用过程中也就意味着能耗增加[73],对试验器材也提出了更高要求,不符合绿色生产理念。因此在较低温度下实现较高气化效率是未来研究方向。
温度在SCWG过程中起着至关重要的作用。从热力学角度分析,生物质分解需要大量热量,因此高温对SCWG非常有利[74]。随着温度升高生物质会加速分解,较高的温度能提高碳气化效率并且增加氢气产量[57]。升高温度会增加自由基的浓度,促进离子积反应向自由基转化,有利于气体产物生成[43]。OSADA等[75]将SCWG分为3个温度区域:1)500 ~700 ℃的超临界水区域,生物质分解迅速,可以使用活性炭催化剂避免结焦或碱性催化剂促进水煤气转化;2)374 ~500 ℃的超临界水区域,可以利用金属催化剂促进生物质水解;3)当温度低于374 ℃时,为亚临界水状态,此时生物质水解非常缓慢,需要催化剂才能促进气体形成。PROMDEJ等[76]利用连续式流化床管式反应装置在300 ~460 ℃、25 MPa条件下,以1.5%葡萄糖为原料进行试验,发现在亚临界水状态中产生的焦炭量明显比超临界水状态下要多,在亚临界条件下葡萄糖的分解以离子积反应为主,在超临界水状态下以自由基反应为主。KARAGOZ等[77]使用间歇式高压反应釜分别在180、250 和280 ℃条件下气化锯末,发现会产生2-呋喃甲醛、2-甲氧基苯酚等中间产物,这些产物不能气化会导致反应器堵塞,而较快的加热速度能缓解这种现象的出现。大量研究表明,在较低温度下气化主要产物为甲烷,随着温度升高氢气成为主要产物。从反应机理上来解释,随着温度升高,重整反应的吸热特性有利于氢气和二氧化碳的生成,水煤气转化反应的微弱放热性质会倾向于产生氢气和二氧化碳,从而抑制一氧化碳的产生[38,78-80]。
2.4 停留时间
反应物在反应器内停留的时间或者持续的时间称为停留时间,在一定时间范围内氢气产率会随着停留时间的增加而增加[81]。YOUSSEF等[82]以猪粪为原料气化,发现反应时间为60 min时甲烷和二氧化碳的产量最高,反应时间由30 min变化到60 min时氢气产量几乎不变,当反应时间达到90 min时,氢气产量反而降低。OSADA等[83]以甘蔗渣废料为原料气化,发现反应时间为15 min时气体产物的碳产量为10%,且不随着时间的增加而增加,反应30 min时甘蔗渣完全气化气且氢气产量几乎恒定。RESENDE等[80]建立了纤维素和木质素SCWG的动力学模型,发现短时间内氢气主要通过重整反应生成,较长时间主要通过水煤气转化反应产生,而一氧化碳、二氧化碳和甲烷等气体会由中间产物水热反应产生。CHEN等[84]利用石英管反应器将食品废弃物用于SCWG,通过建立模型发现碳气化效率随反应温度的升高而升高,反应活性在气化初期急剧升高,随后随反应时间的延长而降低。
2.5 压 力
在超临界水状态下,氢气产量和气化效率与压力之间的关系非常复杂。为达到超临界状态,反应压力需要大于22 MPa,在反应过程中适合的反应压力范围为22~30 MPa[85]。
溶质在溶剂中的扩散速率取决于溶剂黏度,溶剂黏度是压力和温度的函数,因此压力可以影响溶质的溶解程度进而影响反应速率。在SCW状态下溶剂的“笼效应”得到增强,此时溶剂笼可以通过隔离反应物来降低溶质与溶质之间的反应速率,加强溶质与溶剂之间的反应速率。因此高压有利于水煤气转化,但是阻碍了原料的分解速率。GOKKAYA等[86]在500 ℃条件下对苯酚进行气化,发现当压力增大时碳气化效率反而下降。LU等[87]使用流化床间歇式高压反应器对生物质模型化合物(纤维素和葡萄糖)和真实生物质(稻秆、麦秆、花生壳等)进行气化,发现高压有利于水煤气转化进而促进氢气产量。MADENOGLU等[88]在间歇式高压反应釜中以葡萄糖为原料气化,发现相同温度下增大压力会促进葡萄糖分解称为醛和酮类化合物,随着压力增加碳气化效率和氢气产量均减小。
2.6 催化剂
传统的SCWG过程需要高温高压条件,不仅功耗大而且对于反应装置要求也非常高。向反应体系中加入催化剂可以降低反应的活化能,使反应在较低温度下进行,能有效减少运营成本、提高氢气选择性[89]、减少SCWG过程焦油和焦炭产生[90]。催化剂主要是通过催化生物质水解、脱水等形成中间体,快速实现C-C键断裂,尤其对于酚类化合物而言,气化过程必须足够快速才能避免产生的中间体聚合形成焦炭等物质。同时催化剂会解离H2O,在催化剂表面产生活性自由基,这些自由基将与分解的小分子CHO结合,最终释放出CO和CO2,从水分解和裂解的CHO中吸附的氢原子将结合形成H2。催化剂主要分为均相催化剂和非均相催化剂2种。
2.6.1 均相催化剂
均相催化剂便于操作,能够与原料均匀混合。碱类催化剂具有低成本和高活性的特性,是最广泛应用的均相催化剂。碱性催化剂可以吸收CO2促使水煤气转化向生成氢气方向移动[91-92]。CHEN等[93]使用流化床反应装置对污水污泥SCWG进行了研究,得到催化剂的活性顺序为由大到小依次为:KOH、K2CO3、NaOH、Na2CO3、KOH,通过对比发现碱性催化剂不会显著改变产物中碳的分布情况,而氢气产率增加主要是碱性催化剂促进超临界水状态下的水煤气转化反应。ONWUDILI等[94]用间歇式高压反应釜在550 ℃、36 MPa下以葡萄糖为原料进行研究,发现高浓度的NaOH对氢气选择性更高。FENG等[95]通过分析模拟轨迹并跟踪目标原子,指出C-H-O自由基是产生氢气或氢离子的主要反应物,解释了碱金属盐催化剂(NaOH和KOH)可以提高氢气产量的原因。虽然碱性催化剂降低了纤维素降解的起始温度,有利于气体产物生成[96],但在实际操作过程中需要持续添加新鲜催化剂,并且反应过后产生的含碱催化剂废液难以处理[17,97],因此使用范围受到限制。
2.6.2 非均相催化剂
非均相催化剂具有易于回收、化学性质稳定等优点而被广泛研究,主要包括有金属催化剂、金属氧化物催化剂和碳基催化剂等。
ELLIOTT等[98]发现在SCWG过程活性最好的金属是Ru、Rh、Ni,它们可以改善甲烷化反应。Ni基催化剂因催化活性与贵金属相当,但是价格较为低廉因而得到大量研究[99]。Ni能有效促进C-O键断裂,同时促进生物质分解的中间体(苯酚、酸类、醛类)降解,进而增加气化效率[100-101]。ADAMU等[102]发现Ni基催化剂具有金属/载体协同效应,特别是当浸渍在金属氧化物载体(如Al2O3)上。Ni具有很强的催化C-C键断裂能力,从而提高碳转化率[101,103],还能有效催化水溶性产物的蒸汽重整反应和甲烷化反应[104]。SINAG等[100]提出在葡萄糖SCWG过程中沉积的碳主要通过2个路径实现,一是通过中间液体产物分解,二是副产气;在超临界状态下葡萄糖分解为糠醛和有机酸,糠醛容易分解为焦炭沉积,Ni基催化剂能够促进有机酸分解为气体产物。ZHU等[105]以不同浓度的硝酸镍和硝酸锆为原料,采用超临界水合成法成功制备了一系列ZrO2负载的Ni纳米催化剂(Ni、0.8Ni-0.2ZrO2、0.6Ni-0.4ZrO2、0.4Ni-0.6ZrO2和ZrO2),以甘油为原料试验表明0.4Ni-0.6ZrO2获得了最高的氢气产率,因为它在水煤气转化反应中具有将CO转化为H2的气体的高活性,表征发现超临界水合成法制备的Ni/ZrO2催化剂表现出优异的晶体结构和形态稳定性,以及对甘油的SCWG具有良好的抗结焦能力。
尽管Ni基催化剂具有很多优点,但是不可避免的面临着易烧结、水热性质不稳定、形成的焦油焦炭等会影响Ni的选择性和稳定性等问题[17,106-107]。改变载体材料和选择合适的促进剂可以有效提高Ni基催化剂的稳定性,良好的载体材料能够为催化剂保证机械强度、提供活化中心并增加活性金属的分散性[108](图8)。研究表明-Al2O3作为催化剂载体会在高温水热条件下发生相变转化为-Al2O3,这种相变会导致表面积和孔体积损失以及平均孔径增加[109],使活性元素溶解在水中从而造成催化剂失活[110]。LI等[111]利用溶胶-凝胶法制备了ZrO2、TiO2和Ta2O5负载的Ni基催化剂(分别表示为Ni-Zr、Ni-Ti和Ni-Ta),以甘油为原料在连续流动反应装置中进行SCWG试验发现Ni-Zr和Ni-Ta利用SCW的结晶环境可以实现“催化剂原位活化”效果,是用于长期催化SCWG的良好候选者,LI认为Ni与载体的烧结和催化剂材料的浸出是SCW环境中可能会导致催化剂失活的原因。XIE等[112]采用浸渍法制备了一系列不同沸石的镍催化剂,使用高压间歇式反应器以微藻为原料进行SCWG试验发现沸石的强酸性位点可以增加碳气化效率,证明沸石也可以作为SCWG的催化剂载体。
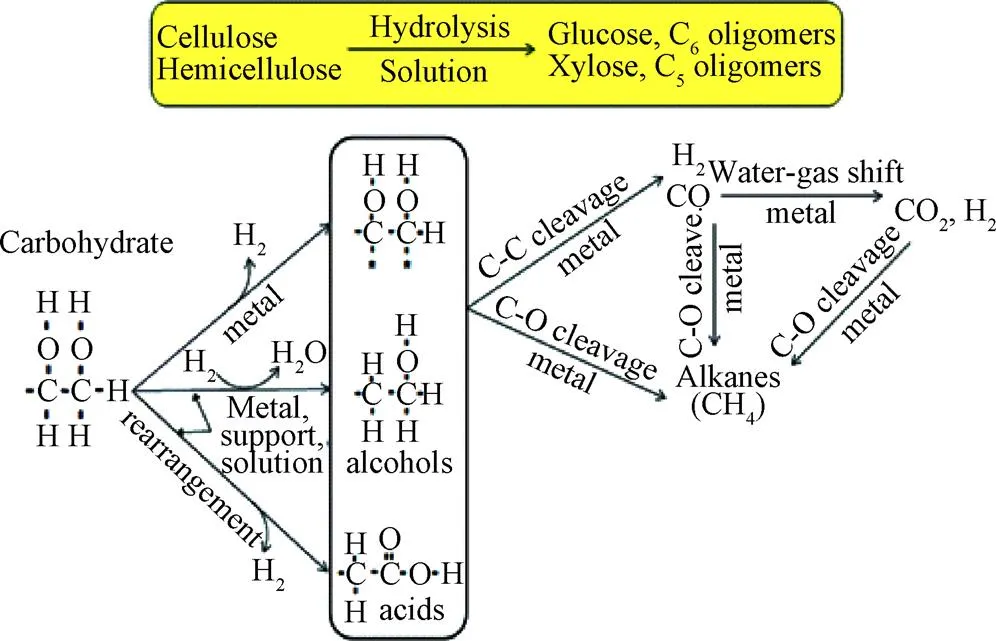
图8 镍基催化剂SCWG催化纤维素生物质反应路径图[109]
酸度是决定催化剂活性、稳定性和抗积碳能力的重要参数,因为糖类化合物会在催化剂酸性位点上发生脱水反应,使用具有氢化活性金属和强酸性位点的双功能催化剂会导致连续脱水/氢化,因此会产生高级烷烃。酸性位点还会促进聚合反应导致焦油状物质的形成[109]。催化剂的酸度越高在SCWG中积碳会越明显[113],Ni基催化剂的强酸性加速了焦炭形成[114]。虽然焦炭对催化剂活性位点覆盖会导致的催化剂失活,但可以通过高温煅烧和还原的方式恢复催化剂活性。而大量试验表明在催化剂使用过程中失活是不可逆的,因此推断在SCWG催化剂催化过程中烧结是导致催化剂失活最主要的原因,尤其集中在高负载的金属催化剂上。催化剂烧结将导致微晶长大、催化剂表面孔隙和孔径分布发生变化、比表面积和活性位点减少、氢气选择性降低[45,115]。选择合适的促剂将会提高Ni基催化剂氢气选择性和抗烧结能力。表2总结了不同金属元素作为Ni基催化剂促进剂对气化效率的影响。
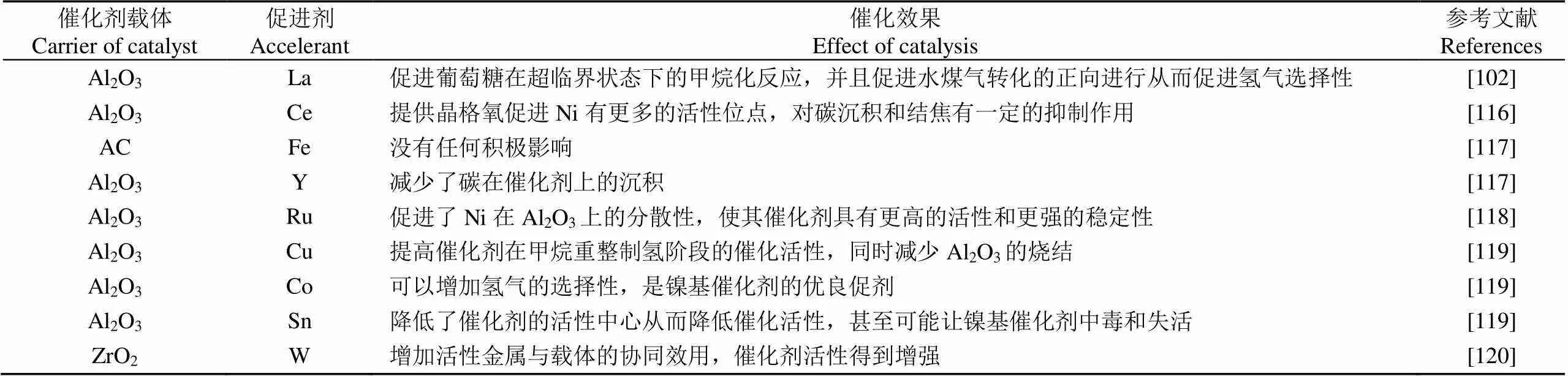
表2 不同金属促进剂对Ni基催化剂影响
活性炭(activated carbon,AC)催化剂可以使用作物秸秆、贝壳等天然生物质在惰性气体条件下制备。AC可以作为催化剂载体或者单独作为催化剂使用,在SCWG过程中AC可以促进碳气化效率。MATSUMURA等[121]使用填充床式反应器以葡萄糖为原料,加入椰壳AC在600 ℃条件下几乎可以实现100%的碳气化效率。LEE等[122]以葡萄糖为原料在575 ~725 ℃、28 MPa条件下通过对使用前后的催化剂进行表征发现AC的结构几乎没有改变,证明AC是一种不受超临界水状态影响的稳定材料。QUAN等[123]发现将Ce引入催化剂显著提高了炭基催化剂的稳定性,表征发现在使用过的催化剂上形成了无定形碳和丝状碳两种碳,无定形碳比丝状碳更容易氧化,Ce的加入可以减少反应过程中非晶碳的生成,增强碳基催化剂稳定性。催化剂的负载量和载体材料不同都会影响气体的组分比率和产率[124]。YAO等[125]以麦秸、稻壳和棉秆为原料在500 ℃管式炉中快速热解得到3种生物炭用作催化剂载体,采用工业品AC进行对照试验,当气化温度为800 ℃,Ni负载量为15%时,Ni/AC催化剂的性能最佳,棉花生物炭为载体的Ni气化产氢活性最高,为64.02%,而水稻生物炭为载体的Ni气化产氢活性最低。
碳纳米管(carbon nanotubes,CNT)具有比表面积大、热稳定性好、机械强度高等优点而被广泛应用于催化剂载体[126]。RASHIDI等[127]利用间歇式高压反应装置以甘蔗渣为原料,采用浸渍法制备具有不同镍负载量的Ni/CNT纳米催化剂,发现由于CNT巨大的内表面,在SCWG过程中使用Ni/CNTs纳米催化剂可显着提高氢气产量。LI等[126]采用沉淀法制备了Ni负载在Mg促进的-Al2O3、α-Al2O3和CNT催化剂,使用连续式流动装置以甘油为原料进行SCWG试验,发现在SCW的高压下,CNT表现出优异的晶体稳定性,减少了活性金属流失,能在长期SCWG过程中保证机械稳定。
金属氧化物催化剂具有耐高温、价格低廉、易于氧化再生、易于运输储存等优点。CAO等[128]在600 ℃条件下以葡萄糖为模型化合物,对12种金属氧化物(V2O5、Cr2O3、MnO2、Fe2O3、Co2O3、CuO、ZnO、ZrO2、MoO3、SnO2、WO3和CeO2)进行SCWG,发现大多数氧化物对氢气形成的影响很小,但对CO形成的影响很大,其中Cr2O3,CuO、WO3和V2O5是最有效的制氢催化剂。ZHANG等[129]研究了不同价态的铁基催化剂在催化木质素方面的表现,发现铁基催化剂在SCWG中有得天独厚的优势,尤其零价态的铁H2产量明显高于不同价态的氧化铁催化体系,低价态的铁基催化剂(Fe和FeO)具有低配位数活性铁原子,有利于CO产生,高价态的铁(Fe2O3和Fe3O4)有足够的晶格氧,容易将CO氧化成CO2。CAO等[130]以黑液为原料测试了14种金属氧化物在SCWG中效果,发现金属氧化物不仅可以提供催化活性位点并且氧含量也是影响气体组成的重要因素。
3 总结与展望
本文通过对生物质SCWG(supercritical water gasification)机理总结和影响因素系统的梳理得到以下结论:1)在SCWG过程中有机酸、醛类、酮类等小分子中间体是形成气体的主要来源,而酚类和呋喃则主要是通过聚合反应生成固体碳颗粒;2)间歇式反应器适用于研究相行为和反应机制,而连续式反应装置适合参数研究,提高升温速率可以抑制焦油、焦炭等副产物产生;3)适当提高进料浓度有利于提高氢气产量,但进料浓度过高易形成较多的焦炭、焦油等中间产物导致氢气产量降低,还易导致反应器堵塞等问题;4)低温高压水热环境有利于离子反应,随着温度升高氢键数目减少,自由基反应增强,气化效率会提高;5)在高压下溶剂笼会导致分解反应速率降低,有利于碳的形成,不利于氢气产生,选择合适的压力对于SCWG过程十分重要;6)催化剂的酸性是影响SCWG过程的重要因素,酸性强会加速焦炭的形成造成催化剂失活,Ce、La、Ru等金属元素作为合适的促剂可以增强催化剂活性。
为了能够进一步加深对生物质SCWG的理解并投入于日常生产生活,未来还需要在以下几个方向加强研究:1)现阶段对于反应路径的研究主要集中在对于生物质模型化合物,对于实际生物质的反应路径和反应机理还需要进一步研究;2)高温高压的反应环境对反应装置提出了更高要求,目前需要解决反应装置腐蚀、堵塞、盐沉积等问题;3)需要进一步优化催化剂的添加量,探索催化机理,同时进一步探究催化剂失活原因;4)为进一步降低成本,要对催化剂的稳定性和回收利用性进行研究,开发高选择性可重复使用的催化剂;5)目前没有实际应用的超临界水制氢系统,因此对于实际生产中的能耗建模、经济性分析等相关工作较少。尽管以上的诸多问题还需要不断的研究和探索,但是生物质SCWG能够清洁高效的将生物质废弃物转化为清洁的氢能,既实现了废弃物的无害化处理,又能生产能源,是一种非常有前景的技术。
[1] 王伟兴,康庆华,董帆,等. 国内外能源利用现状分析[J]. 云南化工,2019,46(6):48-49.
WANG Weixing, KANG Qinghua, DONG Fan, et al. Analysis of energy utilization situation at home and abroad[J]. Yunnan Chemical Industry, 2019, 46(6): 48-49. (in Chinese with English abstract)
[2] 兰莹,秦天宝. 《欧洲气候法》:以“气候中和”引领全球行动[J].环境保护,2020,48(9):7.
LAN Ying, QIN Tianbao. European Climate Law: Leading global action with “climate neutrality”[J]. Environmental Protection, 2020, 48(9): 7. (in Chinese with English abstract)
[3] 刘宇,羊凌玉,李欣蓓,等. 碳中和目标实现下中国转型发展路径研究[J]. 北京理工大学学报(社会科学版),2022,24(4):27-36.
LIU Yu, YANG Lingyu, LI Xinbei, et al. Research on China’s transformation development path under the carbon neutrality goal[J]. Transactions of Beijing Institute of Technology (Social Sciences Edition), 2022, 24(4): 27-36. (in Chinese with English abstract)
[4] DRESSELHAUS M S, THOMAS I L. Alternative energy technologies[J]. Nature, 2001, 414(6861): 332-337.
[5] ELLABBAN O, ABU-RUB H, BLAABJERG F. Renewable energy resources: Current status, future prospects and their enabling technology[J].Renewable and Sustainable Energy Reviews, 2014, 39: 748-764.
[6] HALL D O, SCRASE J I. Will biomass be the environmentally friendly fuel of the future?[J]. Biomass Bioenerg, 1998, 15(4/5): 357-367.
[7] 岳国君,林海龙,彭元亭,等. 以生物质为原料的未来绿色氢能[J]. 化工进展,2021,40(8):7.
YUE Guojun, LIN Hailong, PENG Yuanting, et al. Future green hydrogen energy using biomass as raw material[J]. Chemical Industry Progress, 2021, 40(8): 7. (in Chinese with English abstract)
[8] BALAT M. Potential importance of hydrogen as a future solution to environmental and transportation problems[J]. International Journal of Hydrogen Energy, 2008, 33(15): 4013-4029.
[9] PARTHASARATHY P, NARAYANAN K S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield-A review[J]. Renewable Energy, 2014, 66: 570-579.
[10] 邹才能,李建明,张茜,等. 氢能工业现状,技术进展,挑战及前景[J]. 天然气工业,2022,42(4):20.
ZOU Caineng, LI Jianming, ZHANG Qian, et al. Current situation, technological progress, challenges and prospects of hydrogen energy industry[J]. Natural Gas Industry, 2022, 42(4): 20. (in Chinese with English abstract)
[11] 曹军文,张文强,李一枫,等. 中国制氢技术的发展现状[J]. 化学进展,2021,33(12):30.
CAO Junwen, ZHANG Wenqiang, LI Yifeng, et al. Development status of hydrogen production technology in China[J]. Advances in Chemistry, 2021, 33(12): 30. (in Chinese with English abstract)
[12] 刘翠伟,裴业斌,韩辉,等. 氢能产业链及储运技术研究现状与发展趋势[J]. 油气储运,2022,41(5):17.
LIU Cuiwei, PEI Yebin, HAN Hui, et al. Research status and development trend of hydrogen energy industry chain and the storage and transportation technologies [J]. Oil and Gas Storage and Transportation, 2022, 41(5): 17. (in Chinese with English abstract)
[13] YAO J G, KRAUSSLER M, BENEDIKT F, et al. Techno-economic assessment of hydrogen production based on dual fluidized bed biomass steam gasification, biogas steam reforming, and alkaline water electrolysis processes[J]. Energy Conversion & Management, 2017, 145: 278-292.
[14] KRUSE A, DINJUS E. Hot compressed water as reaction medium and reactant-properties and synthesis reactions[J]. The Journal of Supercritical Fluids, 2007, 39(3): 362-380.
[15] KRAMMER P, VOGEL H. Hydrolysis of esters in subcritical and supercritical water[J].The Journal of Supercritical Fluids, 2000, 16(3): 189-206.
[16] CHEN J, WANG Q, XU Z, et al. Process in supercritical water gasification of coal: A review of fundamentals, mechanisms, catalysts and element transformation [J]. Energy Conversion & Management, 2021, 237: 114122.
[17] OKOLIE J A, RANA R, NANDA S, et al. Supercritical water gasification of biomass: A state-of-the-art review of process parameters, reaction mechanisms and catalysis[J]. Sustainable Energy & Fuels, 2019, 3(3): 578-598.
[18] FANG Z, MINOWA T, SMITH R L, et al. Liquefaction and gasification of cellulose with Na2CO3and Ni in subcritical water at 350 degrees C[J]. Industrial & Engineering Chemistry Research, 2004, 43(10): 2454-2463.
[19] ABDPOUR S, SANTOS R M. Recent advances in heterogeneous catalysis for supercritical water oxidation/ gasification processes: Insight into catalyst development[J]. Process Safety and Environmental Protection, 2021, 149: 169-184.
[20] KRITZER P, DINJUS E. An assessment of supercritical water oxidation (SCWO) : Existing problems, possible solutions and new reactor concepts[J].Chemical Engineering Journal, 2001, 83(3): 207-214.
[21] HU Y, GONG M, XING X, et al. Supercritical water gasification of biomass model compounds: A review [J]. Renewable and Sustainable Energy Reviews, 2020, 118: 109529.
[22] 张丽莉,陈丽,赵雪峰,等. 超临界水的特性及应用[J]. 化学工业与工程,2003(1):33-38,54.
ZHANG Lili, CHEN Li, ZHAO Xuefeng, et al. Characteristics and application of supercritical water[J]. Chemical Industry and Engineering, 2003(1): 33-38, 54. (in Chinese with English abstract)
[23] MARINO F , BOVERI M , BARONETTI G , et al. Hydrogen production via catalytic gasification of ethanol. A mechanism proposal over copper-nickel catalysts[J]. International Journal of Hydrogen Energy, 2004, 29(1): 67-71.
[24] LIEW C C, INOMATA H, ARAI K, et al. Three-dimensional structure and hydrogen bonding of water in sub- and supercritical regions: A molecular simulation study[J]. Journal of Supercritical Fluids, 1998, 13(1/2/3): 83-91.
[25] BOERO M, TERAKURA K, IKESHOJI T, et al. Hydrogen bonding and dipole moment of water at supercritical conditions: A first-principles molecular dynamics study [J]. Physical Review Letters, 2000, 85(15): 3245.
[26] SASAKI M, KABYEMELA B, MALALUAN R, et al. Cellulose hydrolysis in subcritical and supercritical water [J]. The Journal of Supercritical Fluids 1998, 13(1/2/3): 261-268.
[27] KARIMI K, TAHERZADEH M J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity[J]. Bioresource Technology, 2016, 200: 1008-1018.
[28] PETERSON A A, VOGEL F, LACHANCE R P, et al. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies[J]. Energy & Environmental Science, 2008, 1(1): 32-65.
[29] 郑豪,鱼涛,屈撑囤,等. 超临界水的基本特性及应用进展 [J]. 化工技术与开发,2020,49(Z1):62-66.
ZHENG Hao, YU Tao, QU Chengdun, et al. Basic characteristics and application progress of supercritical water[J]. Chemical Technology and Development. 2020, 49(Z1): 62-66. (in Chinese with English abstract)
[30] GARROTE G, DOMINGUEZ H, PARAJO J C. Hydrothermal processing of lignocellulosic materials[J]. Holz Als Roh-Und Werkstoff, 1999, 57(3): 191-202.
[31] BOBLETER O. Hydrothermal degradation of polymers derived from plants [J]. Progress in Polymer Science, 1994, 19(5): 797-841.
[32] OSADA M, SATO T, WATANABE M, et al. Low-temperature catalytic gasification of lignin and cellulose with a ruthenium catalyst in supercritical water[J]. Energy Fuels, 2004, 18(2): 327-333.
[33] CHEN J, LIANG J, XU Z, et al. Assessment of supercritical water gasification process for combustible gas production from thermodynamic, environmental and techno-economic perspectives: A review[J]. Energy Conversion and Management, 2020, 226: 113497.
[34] REDDY S N, NANDA S, DALAI A K, et al. Supercritical water gasification of biomass for hydrogen production[J]. International Journal of Hydrogen Energy, 2014, 39(13): 6912-6926.
[35] FERREIRA-PINTO L, PARIZI M P S, DE ARAUJO P C C, et al. Experimental basic factors in the production of H2via supercritical water gasification[J]. International Journal of Hydrogen Energy, 2019, 44(47): 25365-25383.
[36] 赵小燕,汤文,曹景沛,等. 炭载金属催化剂在生物质焦油重整中的研究进展[J]. 燃料化学学报,2022,50(12):1547-1563.
ZHAO Xiaoyan, TANG Wen, CAO Jingpei, et al. Research progress of carbon supported metal Catalyst in reforming biomass tar[J]. Journal of Fuel Chemistry and Technology 2022, 50(12): 1547-1563. (in Chinese with English abstract)
[37] FANG Z, SATO T, SMITH R L, et al. Reaction chemistry and phase behavior of lignin in high-temperature and supercritical water[J]. Bioresource Technology, 2008, 99(9): 3424-3430.
[38] KRUSE A. Supercritical water gasification [J]. Biofuels Bioproducts & Biorefining, 2010, 2(5):415-437.
[39] YOSHIDA T, MATSUMURA Y. Gasification of cellulose, xylan, and lignin mixtures in supercritical water[J].Engineering Chemistry Research, 2001, 40(23): 5469-5474.
[40] KABYEMELA B M, ADSCHIRI T, MALALUAN R M, et al. Kinetics of glucose epimerization and decomposition in subcritical and supercritical water[J]. Industrial & Engineering Chemistry Research, 1997, 36(5): 1552-1558.
[41] CASTELLO D, KRUSE A, FIORI L. Low temperature supercritical water gasification of biomass constituents: Glucose/phenol mixtures[J]. Biomass and Bioenergy, 2015, 73: 84-94.
[42] KRUSE A, HENNINGSEN T, SMAG A, et al. Biomass gasification in supercritical water: influence of the dry matter content and the formation of phenols[J]. Industrial & Engineering Chemistry Research ,2003, 42(16): 345-360.
[43] KRUSE A, DINJUS E. Hot compressed water as reaction medium and reactant-2. Degradation reactions[J]. The Journal of Supercritical Fluids, 2007, 41(3): 361-379.
[44] KLINGLER D, VOGEL H. Influence of process parameters on the hydrothermal decomposition and oxidation of glucose in sub- and supercritical water[J]. The Journal of Supercritical Fluids, 2010, 55(1): 259-270.
[45] LU Y, JIN H, ZHANG R. Evaluation of stability and catalytic activity of Ni catalysts for hydrogen production by biomass gasification in supercritical water[J]. Carbon Resources Conversion, 2019, 2(1): 95-101.
[46] 陆柒安,张军,赵亮,等. 生物质超临界水气化制氢研究进展[J]. 现代化工,2018,38(12):29-33.
LU Qi’an, Zhang Jun, Zhao Liang, et al. Research progress on hydrogen production through biomass gasification in supercritical water [J]. Modern Chemical Industry, 2018, 38(12): 29-33. (in Chinese with English abstract)
[47] GOODWIN A K, RORRER G L. Reaction rates for supercritical water gasification of xylose in a micro-tubular reactor[J]. Chemical Engineering Journal, 2010, 163(1): 10-21.
[48] WATANABE M, INOMATA H, OSADA M, et al. Catalytic effects of NaOH and ZrO2for partial oxidative gasification of n-hexadecane and lignin in supercritical water[J]. Fuel, 2003, 82(5): 545-552.
[49] RESENDE F L P, FRALEY S A, BERGER M J, et al. Noncatalytic gasification of lignin in supercritical water[J]. Energy Fuels, 2008, 22(2): 1328-1334.
[50] van BENNEKOM J G, VENDERBOSCH R H, ASSINK D, et al. Reforming of methanol and glycerol in supercritical water[J]. The Journal of Supercritical Fluids, 2011, 58(1): 99-113.
[51] HUELSMAN C M, SAVAGE P E. Reaction pathways and kinetic modeling for phenol gasification in supercritical water[J]. The Journal of Supercritical Fluids, 2013, 81: 200-209.
[52] CHEN Y, YI L, WEI W, et al. Hydrogen production by sewage sludge gasification in supercritical water with high heating rate batch reactor [J]. Energy, 2022, 238: 121740.
[53] LI S, GUO L. Stability and activity of a co-precipitated Mg promoted Ni/Al2O3catalyst for supercritical water gasification of biomass [J]. International Journal of Hydrogen Energy, 2019, 44(30): 15842-15852.
[54] ZÖHRER H, VOGEL F. Hydrothermal catalytic gasification of fermentation residues from a biogas plant [J]. Biomass and Bioenergy, 2013, 53: 138-148.
[55] KARAYILDIRIM T, SINAG A, KRUSE A. Char and coke formation as unwanted side reaction of the hydrothermal biomass gasification[J]. Chemical Engineering & Technology, 2008, 31(11): 1561-1568.
[56] VENKITASAMY C, HENDRY D, WILKINSON N, et al. Investigation of thermochemical conversion of biomass in supercritical water using a batch reactor[J]. Fuel, 2011, 90(8): 2662-2670.
[57] GUO L J, LU Y J, ZHANG X M, et al. Hydrogen production by biomass gasification in supercritical water: A systematic experimental and analytical study[J]. Catal Today, 2007, 129(3/4): 275-286.
[58] FANG Z, FANG C. Complete dissolution and hydrolysis of wood in hot water[J]. Aiche Journal, 2008, 54(10): 2751-2758.
[59] LU Y J, JIN H, GUO L J, et al. Hydrogen production by biomass gasification in supercritical water with a fluidized bed reactor[J]. International Journal of Hydrogen Energy, 2008, 33(21): 6066-6075.
[60] YAKABOYLU O, ALBRECHT I, HARINCK J, et al. Supercritical water gasification of biomass in fluidized bed: First results and experiences obtained from TU Delft/Gensos semi-pilot scale setup[J]. Biomass and Bioenergy, 2018, 111: 330-342.
[61] CHEN J, XU W, ZUO H, et al. System development and environmental performance analysis of a solar-driven supercritical water gasification pilot plant for hydrogen production using life cycle assessment approach[J].Energy Conversion & Management, 2019, 184: 60-73.
[62] MCKENDRY P. Energy production from biomass (part 1): Overview of biomass[J]. Bioresour Technol, 2002, 83(1): 37-46.
[63] NANDA S, MOHANTY P, PANT K K, et al. Characterization of north american lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels[J]. Bioenergy Reserach, 2013, 6(2): 663-677.
[64] MANKAR A R, PANDEY A, MODAK A, et al. Pretreatment of lignocellulosic biomass: A review on recent advances[J]. Bioresour Technol, 2021, 334: 12.
[65] KRUSE A, MANIAM P, SPIELER F. Influence of proteins on the hydrothermal gasification and liquefaction of biomass. 2. Model compounds[J]. Industrial & Engineering Chemistry Research, 2007, 46(1): 87-96.
[66] 张清叶,李好学. 生物质气化制氢产氢率影响因素研究[J]. 湖北农业科学,2012(23):5442-5444.
ZHANG Qingye, LI Haoxue. Research on influencing factors of hydrogen production rate from biomass gasification[J]. Hubei Agricultural Sciences, 2012(23): 5442-5444. (in Chinese with English abstract)
[67] YANIK J, EBALE S, KRUSE A, et al. Biomass gasification in supercritical water: Part 1. Effect of the nature of biomass[J]. Fuel, 2007, 86(15): 2410-2415.
[68] WILLIAMS P T, ONWUDILI J. Subcritical and supercritical water gasification of cellulose, starch, glucose, and biomass waste[J]. Energy Fuels, 2006, 20(3): 1259-1265.
[69] 郝小红,郭烈锦. 超临界水生物质催化气化制氢实验系统与方法研究[J]. 工程热物理学报,2002, 23(2):143-146.
HAO Xiaohong, GUO Liejin. Experimental system and method for hydrogen production by catalytic gasification of supercritical aquatic materials[J]. Journal of Engineering Thermophysics, 2002, 23(2):143-146. (in Chinese with English abstract)
[70] YOSHIDA T, OSHIMA Y, MATSUMURA Y. Gasification of biomass model compounds and real biomass in supercritical water [J]. Biomass and Bioenerg, 2004, 26(1): 71-78.
[71] GUO S M, GUO L J, CAO C Q, et al. Hydrogen production from glycerol by supercritical water gasification in a continuous flow tubular reactor[J]. International Journal of Hydrogen Energy, 2012, 37(7): 5559-5568.
[72] WANG H M, MIAO R R, YANG Y, et al. Study on the catalytic gasification of alkali lignin over Ru/C nanotubes in supercritical water[J]. Journal of Fuel Chemistry and Technology, 2015, 43(10): 1195-1201.
[73] YANG C, WANG S Z, YANG J Q, et al. Hydrothermal liquefaction and gasification of biomass and model compounds: A review[J]. Green Chemistry, 2020, 22(23): 8210-8232.
[74] SU H, KANCHANATIP E, WANG D, et al. Production of H2-rich syngas from gasification of unsorted food waste in supercritical water[J]. Waste Management, 2020, 102: 520-527.
[75] OSADA M, SATO T, WATANABE M, et al. Catalytic gasification of wood biomass in subcritical and supercritical water[J]. Combustion Science and Technology, 2006, 178(1/2/3): 537-552.
[76] PROMDEJ C, MATSUMURA Y. Temperature effect on hydrothermal decomposition of glucose in sub- and supercritical water[J]. Industrial & Engineering Chemistry Research, 2011, 50(14): 8492-8497.
[77] KARAGOZ S, BHASKAR T, MUTO A, et al. Low-temperature hydrothermal treatment of biomass: Effect of reaction parameters on products and boiling point distributions[J]. Energy Fuels, 2004, 18(1): 234-241.
[78] SUSANTI R F, DIANNINGRUM L W, YUM T, et al. High-yield hydrogen production from glucose by supercritical water gasification without added catalyst[J]. International Journal of Hydrogen Energy, 2012, 37(16): 11677-11690.
[79] KERSTEN S R A, PRINS W, VAN DER DRIFT B, et al. Principles of a novel multistage circulating fluidized bed reactor for biomass gasification[J]. Chemical Engineering Science, 2003, 58(3/4/5/6): 725-731.
[80] RESENDE F L P, SAVAGE P E. Kinetic model for noncatalytic supercritical water gasification of cellulose and lignin[J]. Aiche Journal, 2010, 56(9): 2412-2420.
[81] SUSANTI R F, VERIANSYAH B, KIM J D, et al. Continuous supercritical water gasification of isooctane: A promising reactor design[J]. International Journal of Hydrogen Energy, 2010, 35(5): 1957-1970.
[82] YOUSSEF E A, ELBESHBISHY E, HAFEZ H, et al. Sequential supercritical water gasification and partial oxidation of hog manure[J]. International Journal of Hydrogen Energy, 2010, 35(21): 11756-11767.
[83] OSADA M, YAMAGUCHI A, HIYOSHI N, et al. Gasification of sugarcane bagasse over supported ruthenium catalysts in supercritical water[J]. Energy Fuels, 2012, 26(6): 3179-3186.
[84] CHEN J, FAN Y, E J, et al. Effects analysis on the gasification kinetic characteristics of food waste in supercritical water[J]. Fuel, 2019, 241: 94-104.
[85] 罗威,廖传华,陈海军,等. 生物质超临界水气化制氢技术的研究进展[J]. 天然气化工:C1化学与化工,2016,41(01):84-90.
LUO Wei, LIAO Chuanhua, CHEN Haijun, et al. Research progress in hydrogen production from biomass via supercritical water gasification [J]. Natural Gas Chemical Industry: C1 Chemistry and Chemical Industry, 2016,41(01):84-90. (in Chinese with English abstract)
[86] GOKKAYA D S, SAGLAM M, YILKSEL M, et al. Supercritical water gasification of phenol as a model for plant biomass[J]. International Journal of Hydrogen Energy, 2015, 40(34): 11133-11139.
[87] LU Y J, GUO L J, JI C M, et al. Hydrogen production by biomass gasification in supercritical water: A parametric study[J]. International Journal of Hydrogen Energy, 2006, 31(7): 822-831.
[88] MADENOGLU T G, SAGLAM M, YUKSEL M, et al. Simultaneous effect of temperature and pressure on catalytic hydrothermal gasification of glucose[J]. The Journal of Supercritical Fluids, 2013, 73: 151-160.
[89] LENG L, ZHANG W, LENG S, et al. Bioenergy recovery from wastewater produced by hydrothermal processing biomass: Progress, challenges, and opportunities[J]. Science of the Total Environment, 2020, 748: 142383.
[90] ELLIOTT D C . Catalytic hydrothermal gasification of biomass[J]. Biofuels Bioproducts & Biorefining, 2010, 2(3):254-265
[91] ELIF D, NEZIHE A. Hydrogen production by supercritical water gasification of fruit pulp in the presence of Ru/C [J]. International Journal of Hydrogen Energy, 2016, 41(19): 8073-8083.
[92] SU W, ZHAO M, XING Y, et al. Supercritical water gasification of hyperaccumulators for hydrogen production and heavy metal immobilization with alkali metal catalysts[J]. Environmental Research, 2022, 214: 114093.
[93] CHEN Y A, GUO L J, CAO W, et al. Hydrogen production by sewage sludge gasification in supercritical water with a fluidized bed reactor [J]. International Journal of Hydrogen Energy, 2013, 38(29): 12991-12999.
[94] ONWUDILI J A, WILLIAMS P T. Hydrogen and methane selectivity during alkaline supercritical water gasification of biomass with ruthenium-alumina catalyst[J]. Applied Catalysis B, 2013, 132: 70-79.
[95] FENG H, SUN J, WU Z, et al. Investigation of recycled phenol effects on supercritical water gasification of coal using ReaxFF MD simulation[J]. Fuel, 2021, 303: 121177.
[96] DING N, AZARGOHAR R, DALAI A K, et al. Catalytic gasification of cellulose and pinewood to H2in supercritical water[J]. Fuel, 2014, 118: 416-425.
[97] TAUFIQ-YAP Y H, SIVASANGAR S, SURAHIM M. Catalytic supercritical water gasification of empty palm fruit bunches using ZnO-doped Ni-CaO catalyst for hydrogen production[J]. Bioenergy Research, 2019, 12(4): 1066-1076.
[98] ELLIOTT D C, SEALOCK L J, BAKER E G. Chemical- processing in high-pressure aqueous environments.2. development of catalysts for gasification[J]. Industrial & Engineering Chemistry Research, 1993, 32(8): 1542-1548.
[99] NGUYEN H T, YODA E, KOMIYAMA M. Catalytic supercritical water gasification of proteinaceous biomass: Catalyst performances in gasification of ethanol fermentation stillage with batch and flow reactors[J]. Chemical Engineering Science, 2014, 109: 197-203.
[100] SINAG A, KRUSE A, RATHERT J. Influence of the heating rate and the type of catalyst on the formation of key intermediates and on the generation of gases during hydropyrolysis of glucose in supercritical water in a batch reactor[J]. Industrial & Engineering Chemistry Research, 2004, 43(2): 502-508.
[101] SHEIKHDAVOODI M J, ALMASSI M, EBRAHIMI-NIK M, et al. Gasification of sugarcane bagasse in supercritical water; evaluation of alkali catalysts for maximum hydrogen production[J]. Journal of the Energy Institute, 2015, 88(4): 450-458.
[102] ADAMU S, BINOUS H, RAZZAK S A, et al. Enhancement of glucose gasification by Ni/La2O3-Al2O3towards the thermodynamic extremum at supercritical water conditions[J]. Renewable Energy, 2017, 111: 399-409.
[103] AZADI P, FARNOOD R. Review of heterogeneous catalysts for sub- and supercritical water gasification of biomass and wastes[J]. International Journal of Hydrogen Energy, 2011, 36(16): 9529-9541.
[104] MINOWA T, ZHEN F, OGI T. Cellulose decomposition in hot-compressed water with alkali or nickel catalyst[J]. Journal of Supercritical Fluids, 1998, 13(1): 253-259.
[105] ZHU B, LI S, WANG W, et al. Supercritical water synthesized Ni/ZrO2catalyst for hydrogen production from supercritical water gasification of glycerol[J]. International Journal of Hydrogen Energy, 2019, 44(59): 30917-30926.
[106] PINKARD B R, GORMAN D J, TIWARI K, et al. Supercritical water gasification: Practical design strategies and operational challenges for lab-scale, continuous flow reactors[J]. Heliyon, 2019, 5(2): e01269.
[107] GUO Y, WANG S Z, XU D H, et al. Review of catalytic supercritical water gasification for hydrogen production from biomass[J]. Renewable and Sustainable Energy Reviews, 2010, 14(1): 334-343.
[108] SU H, YAN M, WANG S. Recent advances in supercritical water gasification of biowaste catalyzed by transition metal-based catalysts for hydrogen production[J]. Renewable and Sustainable Energy Reviews, 2022, 154: 111831.
[109] AZADI P, AFIF E, AZADI F, et al. Screening of nickel catalysts for selective hydrogen production using supercritical water gasification of glucose[J]. Green Chemistry, 2012, 14(6): 1766-1777.
[110] ELLIOTT D C. Catalytic hydrothermal gasification of biomass[J]. Biofuels Bioproducts & Biorefining-Biofpr, 2008, 2(3): 254-265.
[111] LI S, SAVAGE P E, GUO L. Stability and activity maintenance of sol-gel Ni-MxOy (M=Ti, Zr, Ta) catalysts during continuous gasification of glycerol in supercritical water[J]. Journal of Supercritical Fluids, 2019, 148: 137-147.
[112] XIE L F, DUAN P G, JIAO J L, et al. Hydrothermal gasification of microalgae over nickel catalysts for production of hydrogen-rich fuel gas: Effect of zeolite supports[J]. International Journal of Hydrogen Energy, 2019, 44(11): 5114-5124.
[113] HOSSAIN M Z, CHOWDHURY M B I, CHARPENTIER P A. Effect of surface acidity of Al2O3supported metal catalysts on catalytic activity and carbon deposition during SCWG of glucose[J]. Biomass and Bioenergy, 2019, 124: 142-150.
[114] SU H, KANCHANATIP E, WANG D, et al. Catalytic gasification of food waste in supercritical water over La promoted Ni/Al2O3catalysts for enhancing H2production[J]. International Journal of Hydrogen Energy, 2020, 45(1): 553-564.
[115] LI S, LU Y, GUO L, et al. Hydrogen production by biomass gasification in supercritical water with bimetallic Ni-M/γAl2O3catalysts (M=Cu, Co and Sn)[J]. International Journal of Hydrogen Energy, 2011, 36(22): 14391-14400.
[116] LU Y J, LI S, GUO L J, et al. Hydrogen production by biomass gasification in supercritical water over Ni/gamma Al2O3and Ni/CeO2-gamma Al2O3catalysts[J]. International Journal of Hydrogen Energy, 2010, 35(13): 7161-7168.
[117] LEE I G. Effect of metal addition to Ni/activated charcoal catalyst on gasification of glucose in supercritical water[J]. International Journal of Hydrogen Energy, 2011, 36(15): 8869-8877.
[118] ZHANG L H, XU C B, CHAMPAGNE P. Activity and stability of a novel Ru modified Ni catalyst for hydrogen generation by supercritical water gasification of glucose[J]. Fuel Guildford, 2012. 96(1):541-545
[119] LI S, LU Y J, GUO L J, et al. Hydrogen production by biomass gasification in supercritical water with bimetallic Ni-M/gamma Al2O3catalysts (M=Cu, Co and Sn) [J]. International Journal of Hydrogen Energy, 2011, 36(22): 14391-14400.
[120] YAN B, WU J Z, XIE C, et al. Supercritical water gasification with Ni/ZrO2catalyst for hydrogen production from model wastewater of polyethylene glycol[J]. Journal Supercrit Fluids, 2009, 50(2): 155-161.
[121] MATSUMURA Y, XU X, ANTAL M J. Gasification characteristics of an activated carbon in supercritical water[J]. Carbon, 1997, 35(6): 819-824.
[122] LEE I G, IHM S K. Catalytic gasification of glucose over Ni/Activated charcoal in supercritical water[J]. Industrial & Engineering Chemistry Research, 2009, 48(3): 1435-1442.
[123] QUAN C, WANG H H, GAO N B. Development of activated biochar supported Ni catalyst for enhancing toluene steam reforming [J].International Journal of Energy Research, 2020, 44(7): 5749-5764.
[124] SHAN Y Q, YIN L X, DJANDJA O S, et al. Supercritical water gasification of waste water produced from hydrothermal liquefaction of microalgae over Ru catalyst for production of H2rich gas fuel[J]. Fuel, 2021,292(1):120288.
[125] YAO D D, HU Q, WANG D Q, et al. Hydrogen production from biomass gasification using biochar as a catalyst/ support[J]. Bioresour Technol, 2016, 216: 159-164.
[126] LI S, SAVAGE P E, GUO L. Stability and activity maintenance of Al2O3- and carbon nanotube-supported Ni catalysts during continuous gasification of glycerol in supercritical water[J]. The Journal of Supercritical Fluids, 2018, 135: 188-197.
[127] RASHIDI M, TAVASOLI A. Hydrogen rich gas production via supercritical water gasification of sugarcane bagasse using unpromoted and copper promoted Ni/CNT nanocatalysts[J]. The Journal of Supercritical Fluids, 2015, 98: 111-118.
[128] CAO C Q, ZHANG Y, CAO W, et al. Transition metal oxides as catalysts for hydrogen production from supercritical water gasification of glucose[J]. Catalysis Letters, 2017, 147(4): 828-836.
[129] ZHANG H, CHEN F, ZHANG J, et al. Supercritical water gasification of fuel gas production from waste lignin: The effect mechanism of different oxidized iron-based catalysts[J]. International Journal of Hydrogen Energy, 2021, 46(59): 30288-30299.
[130] CAO C, XIE Y, MAO L, et al. Hydrogen production from supercritical water gasification of soda black liquor with various metal oxides[J]. Renewable Energy, 2020, 157: 24-32.
Research progress of supercritical water hydrogen production from biomass
XU Gongxun, CHEN Wei, FANG Zhen※
(,,210031,)
Green, safe, and reliable clean energy is ever increasing under the "double carbon" goal. Among them, the waste biomass can be converted into green fuel, particularly with the application of many thermochemical and biochemical technologies. Supercritical Water Gasification (SCWG) can be a promising potential to convert the organic matter in the biomass into hydrogen using supercritical water as a medium. The feedstock can be used as a resource for biomass and the waste. SCWG process shares the fast reaction speed, excellent hydrogen selectivity, and fewer by-products, compared with traditional hydrogen production. In addition, water as the reactant in the SCWG process can avoid high energy consumption during drying, and thus reduce the cost. Previous systematic analysis has been made on the SCWG influence factors. In this review, the special physical and chemical properties of supercritical water were introduced to expound the main components (such as cellulose, hemicellulose, and lignin biomass) in the SCWG process reaction, and the experimental device, reaction temperature, residence time, and pressure in the influence factors. It was found that the batch reactor was suitable for the phase behavior and reaction mechanism, due to the simple structure and strong applicability of raw materials. By contrast, the continuous reaction device was used to more accurately control the experimental parameters, and then to realize the continuous commercial production, thus suitable for the parameter research. The hydrogen yield was improved to increase the heating rate of the device during operation, the reaction temperature, and residence time, but to reduce the feed concentration within a certain range. However, there was the complicated influence of pressure on the hydrogen yield. The solvent cage was often used under high pressure, leading to the reduction of the decomposition reaction rate unsuitable for the production of hydrogen. It was necessary to select the appropriate pressure, according to the actual situation. The homogeneous and heterogeneous catalysts were utilized in the SCWG. Specifically, the homogeneous catalyst performed a better catalytic effect on the water gas conversion was reaction, but the strong corrosion was caused the equipment to clog. The heterogeneous catalyst presented high catalytic activity, easy recovery, and excellent stability, more suitable for large-scale SCWG production. At the same time, there were some influences of the acidity of the metal catalyst in the catalytic process. The strong acidity of the catalyst accelerated the formation of carbon deposition, resulting in the catalyst deactivation. Appropriate secondary metals were added, such as Ce, La, and Ru. The performance of the catalyst was accelerated to increase the service life of catalyst for the better hydrogen selectivity. Future research can be focused on the equipment with corrosion resistance and salt deposition resistance, or constantly optimizing operating parameters, while the deactivation mechanism of catalysts, even to optimize the number of catalysts, and the catalysts with high activity and reusable. The existing technical barriers and development prospects of SCWG were analyzed to combine with the current technical development of SCWG. The finding can also provide theoretical guidance for the biomass SCWG in the future.
biomass; hydrogen; catalysts; supercritical water
2023-01-11
2023-02-15
国家自然科学基金面上项目(21878161);南京农业大学高层次人才引进项目(68Q-0603)
徐功迅,研究方向为生物质能源转化。Email:wsywxns@126.com
方真,博士,教授,博士生导师,研究方向为生物燃料及在内燃机中的利用。Email:zhenfang@njau.edu.cn
10.11975/j.issn.1002-6819.202301053
S216
A
1002-6819(2023)-07-0024-12
徐功迅,陈伟,方真. 生物质超临界水制氢研究进展[J]. 农业工程学报,2023,39(7):24-35. doi:10.11975/j.issn.1002-6819.202301053 http://www.tcsae.org
XU Gongxun, CHEN Wei, FANG Zhen. Research progress of supercritical water hydrogen production from biomass[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2023, 39(7): 24-35. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.202301053 http://www.tcsae.org

