镇麦品种相关品质性状基因的分子标记检测分析
郭瑞 姚维成 陈琛 曲朝喜 温明星 刘家俊 邓垚 申雪懿 李东升


摘要: 为了阐明镇麦品种相关品质基因分布特点,以11份镇麦品种为试验材料,分别利用单粒谷物特性测定系统测定籽粒硬度指数,采用分子标记和琼脂糖凝胶电泳分離技术检测籽粒硬度基因、高/低分子量麦谷蛋白亚基(HMW-GS、LMW-GS)、Wx基因以及面粉色泽相关基因等分布特点,并结合十二烷基磺酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)技术明确镇麦材料的HMW-GS类型。结果表明,硬度指数大于60的镇麦品种有9份,均可检测到硬度基因突变。HMW-GS分布特点为:镇麦168等8份材料的组合均为1/7+9/5+10,镇麦11号为1/7+8/2+12,镇麦17和镇麦19为Null/7+8/2+12。LMW-GS有Glu-A3c、Glu-A3d、Glu-B3b、Glu-B3f和Glu-B3g 5种基因型,其中镇麦168、镇麦13和镇麦17为Glu-A3c/Glu-B3g,镇麦11号为Glu-A3d/Glu-B3g,镇麦19为Glu-A3c/Glu-B3b,其余6份镇麦品种均为Glu-A3c/Glu-B3f。Wx基因的检测结果表明,镇麦品种中未检测到Wx基因突变,均为野生型。面粉色泽及相关基因检测结果为:镇麦材料的面粉白度为74.47~78.70,其中镇麦168的白度最小;镇麦168等8份材料的基因型为Ppo-A1b/Ppo-B1a/Ppo-D1b/TaLox-B1b,镇麦11号和镇麦19为Ppo-A1a/Ppo-B1a/Ppo-D1a/TaLox-B1b,镇麦17为Ppo-A1a/Ppo-B1a/Ppo-D1a/b/TaLox-B1b;镇麦168、镇麦12号和镇麦16为Psy-A1b/Psy-B1d/Psy-D1a,镇麦11号和镇麦13为Psy-A1b/Psy-B1b/Psy-D1a,其余6份镇麦品种为Psy-A1b/Psy-B1a/Psy-D1a。
关键词: 小麦;品质;基因;分子标记
中图分类号: S512.1+10.1 文献标识码: A 文章编号: 1000-4440(2023)01-0001-14
Analysis of molecular markers detection for genes related to quality traits in Zhenmai wheat cultivars
GUO Rui1,2, YAO Wei-cheng1, CHEN Chen1,2, QU Chao-xi1, WEN Ming-xing1,2, LIU Jia-jun1, DENG Yao1, SHEN Xue-yi1, LI Dong-sheng1,2
(1.Zhenjiang Institute of Agricultural Sciences in the Hilly Area of Jiangsu Province, Jurong 212400, China;2.Jiangsu Key Laboratory of Crop Genomics and Molecular Breeding/ College of Agriculture, Yangzhou University, Yangzhou 225009, China)
Abstract: This study was designed to elucidate the distribution characteristics of quality-related genes in Zhenmai wheat varieties. Eleven wheat varieties of Zhenmai were used as the tested materials. Grain hardness was measured by single kernel characterization system. The distribution characteristics of hardness gene, high molecular weight glutenin subunit (HMW-GS) gene, low molecular weight glutenin subunit (LMW-GS) gene, Wx gene, and flour color related genes in Zhenmai varieties were detected by molecular markers and agarose gel electrophoresis technology, and HMW-GS was determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The results showed that nine Zhenmai wheat varieties were hard wheats, and the hardness gene mutation could be detected. Distribution characteristics of HMW-GS were analyzed, and it was found that the subunit combinations of eight wheat varieties including Zhenmai 168 were 1/7+9/5+10, the subunit combination of Zhenmai 11 was 1/7+8/2+12, the subunit combinations of Zhenmai 17 and Zhenmai 19 were Null/7+8/2+12. For LMW-GS compositions, there were five genotypes, Glu-A3c, Glu-A3d, Glu-B3b, Glu-B3f and Glu-B3g. LMW-GS compositions of Zhenmai 168, Zhenmai 13 and Zhenmai 17 were Glu-A3c/Glu-B3g, Zhenmai 11 and Zhenmai 19 were Glu-A3d/Glu-B3g and Glu-A3c/Glu-B3b, respectively, other six wheat varieties were Glu-A3c/Glu-B3f. The detecting results of Wx gene indicated that there was no Wx gene mutation in Zhenmai varieties. The flour whiteness of Zhenmai wheat varieties was 74.47-78.70, and the whiteness of Zhenmai 168 was the lowest. Genotypes of eight Zhenmai wheat varieties including Zhenmai 168 were Ppo-A1b/Ppo-B1a/Ppo-D1b/TaLox-B1b, Zhenmai 11 and Zhenmai 19 were Ppo-A1a/Ppo-B1a/Ppo-D1a/TaLox-B1b, Zhenmai 17 was Ppo-A1a/Ppo-B1a/Ppo-D1a/b/ TaLox-B1b. Psy gene compositions of Zhenmai 168, Zhenmai 12 and Zhenmai 16 were Psy-A1b/Psy-B1d/Psy-D1a, Zhenmai 11 and Zhenmai 13 were Psy-A1b/Psy-B1b/Psy-D1a, other six Zhenmai wheat varieties were Psy-A1b/ Psy-B1a/Psy-D1a.
Key words: wheat;quality;gene;molecular markers
镇麦品种因优质红皮中强筋方面的优势,近年来备受关注,并被广泛用作亲本资源。镇麦168是江苏省淮南麦区首个红皮中强筋小麦品种,且后续镇麦品种多以优质中强筋、强筋品种为主,为江苏省淮南麦区开展中强筋、强筋小麦新品种选育奠定了坚实基础。前人对镇麦相关品种的选育和生理特性进行了研究,并形成了相应的栽培技术规程,但对镇麦品质特性的研究相对较少,因此研究其品质遗传规律将为小麦育种及生产应用等方面提供理论支撑,非常必要。
目前,影响小麦品质的硬度、高分子量谷蛋白亚基、低分子量谷蛋白亚基、Wx基因、多酚氧化酶和黄色素等相关品质性状的功能性分子标记已得到广泛应用。籽粒硬度是小麦品质分类的重要依据,主要由Pina、Pinb基因控制[1],Pina-D1b、Pinb-D1b[2-3]基因的功能标记已在小麦辅助筛选中广泛应用。麦谷蛋白是影响小麦面粉加工品质的重要因素,其构成亚基根据分子量大小可分为高分子量麦谷蛋白亚基(HMW-GS)和低分子量麦谷蛋白亚基(LMW-GS)。其中,HMW-GS在改进小麦的面筋品质中有重要作用[4],而LMW-GS影响面团的强度和延伸性,其等位变异对小麦面筋品质和籽粒蛋白组分含量均有显著影响[5]。目前HMW-GS主要有Ax1、Null、Ax2*、Bx7、Bx7OE、By8、By9、By17、Dx2、Dy12、Dx5和Dy10等类型,优质的LMW-GS基因包括Glu-A3d、Glu-B3b、Glu-B3g等[6-12]。编码直链淀粉合成的关键基因Waxy,其等位基因类型决定了直链淀粉的含量,进而影响小麦的品质。普通小麦含有3种类型的Wx基因,即Wx-A1、Wx-B1和Wx-D1[13-15],其中任一基因功能异常,尤其是Wx-B1,都会影响直链淀粉合成,改变糊化温度和膨胀特性[16-17]。面粉及面制品的色泽主要与色素含量相关,是由多酚氧化酶(PPO)、脂肪氧化酶(LOX)等对色素类物质的氧化降解,以及八氢番茄红素合酶(PSY)等对色素类物质的合成积累来调控[18-20]。PPO是导致面制品褐变的主要因素,其蛋白质活性主要由不同基因型决定[21],PPO18[22]、F-8[23]、STS01[24]、PPO16和PPO29[25]等功能标记可用来鉴定各染色体上的控制高/低PPO活性的等位基因。LOX可以促进多聚不饱和脂肪酸释放出高活性氧自由基,氧化类胡萝卜素等色素类物质,从而对面粉颜色起到漂白作用[26-27],可有效检测TaLox-B1基因的显性标记有LOX16和LOX18[28]。黄色素含量与面制品的外观品质显著相关,用于鉴别控制高/低黄色素含量的等位基因的标记主要有YP7A、YP7B和YP7D[29-30]。
镇麦品种品质优良,但其相关品质的遗传特性尚不清楚,相关品质性状基因的研究尚处于起步阶段。本研究拟对镇麦168及镇麦9号等镇麦品种的品质相关性状进行功能标记检测,明确其品质遗传特性,筛选优质亲本资源,探索镇麦品种优异品质形成机理,以期为优质中强筋、强筋小麦品种的选育提供理论依据。
1 材料与方法
1.1 试验材料
试验材料包括镇麦168、镇麦9号、镇麦10号、镇麦11号、镇麦12号、镇麦13、镇麦15、镇麦16、镇麦17、镇麦18和镇麦19(表1),对照材料为扬麦158和扬麦20,共计13份原种材料,其中镇麦相关材料均由江苏丘陵地区镇江农业科学研究所选育,对照品种来源于江苏里下河农业科学研究所。2018-2021年连续3年统一种植在江苏丘陵地区镇江农业科学研究所农业科技创新中心小麦展示示范基地,成熟期按小区收获、脱粒,并进行品质分析。
1.2 硬度测定
采用单粒谷物特性测定仪(Perten公司产品,SKCS 4100型)测定籽粒硬度指数,测定方法按GB/T 2304-2007执行,测定前确保每个样本的纯度。
1.3 白度测定
利用智能白度测定仪(杭州天成光电有限公司产品,WGB-2000L型)分析小麦面粉白度,每个样品设3个重复。
1.4 分子標记鉴定
小麦籽粒DNA的提取采用十六烷基三甲基溴化铵(CTAB)法。PCR反应体系:2×Taq Mix 7.5 μl,10 μmol/L的引物各0.5 μl,模板DNA为1.0 μl,加ddH2O补足至15.0 μl。扩增程序:94 ℃预变性3 min;94 ℃变性30 s,53~62 ℃退火30 s,72 ℃延伸30 s(延伸时间根据产物大小按1 kb/min调整),扩增30~35个循环,72 ℃延伸10 min,16 ℃保温。
PCR反应产物在1%~3%的经溴化乙锭(EB)染色的琼脂糖凝胶中进行电泳,利用凝胶成像仪拍照观察。引物序列见表2。
1.5 SDS-PAGE蛋白质电泳
选取无病小麦种子,按照张平平等[31]的方法进行蛋白质提取及SDS-PAGE电泳检测,对试验材料的HMW-GS进行分析。
2 结果与分析
2.1 小麦籽粒硬度指数及硬度基因分子标记检测
利用单粒谷物特性测定系统(SKCS)法测定籽粒硬度,硬度指数≥60的为硬质麦,40~60的为混合麦,<40的为软质麦[42]。硬度指数和分子标记检测结果表明,镇麦品种中硬度指数>60的硬质麦有9份,硬度指数<60的混合麦有2份。镇麦品种及对照品种扬麦158和扬麦20的等位变异类型均为Pina-D1a类型;镇麦9号、镇麦10号、镇麦15、镇麦18和扬麦158为Pinb-D1b类型,镇麦168、镇麦12号、镇麦13和镇麦16为Pinb-D1p类型,且硬度指数大于60,其余材料为Pinb-D1a类型(图1、表3)。利用Pina-N1、Pina-N2和Pina-N3标记引物对Pina-D1a类型的材料进行扩增,结果表明无Pina-D1b、Pina-D1c、Pina-D1l、Pina-D1r和Pina-D1s等变异类型。Pinb-D1的扩增产物测序比对结果进一步验证了镇麦9号等材料为Pinb-D1b类型,而镇麦168、镇麦12号、镇麦13和镇麦16的扩增产物序列缺失单碱基G,为Pinb-D1p类型。
M:DL2 000 DNA marker;1:镇麦168;2:镇麦9号;3:镇麦10号;4:镇麦11号;5:镇麦12号;6:镇麦13;7:镇麦15;8:镇麦16;9:镇麦17;10:镇麦18;11:镇麦19;12:扬麦158;13:扬麦20。
2.2 高/低相对分子质量麦谷蛋白亚基的分子标记检测
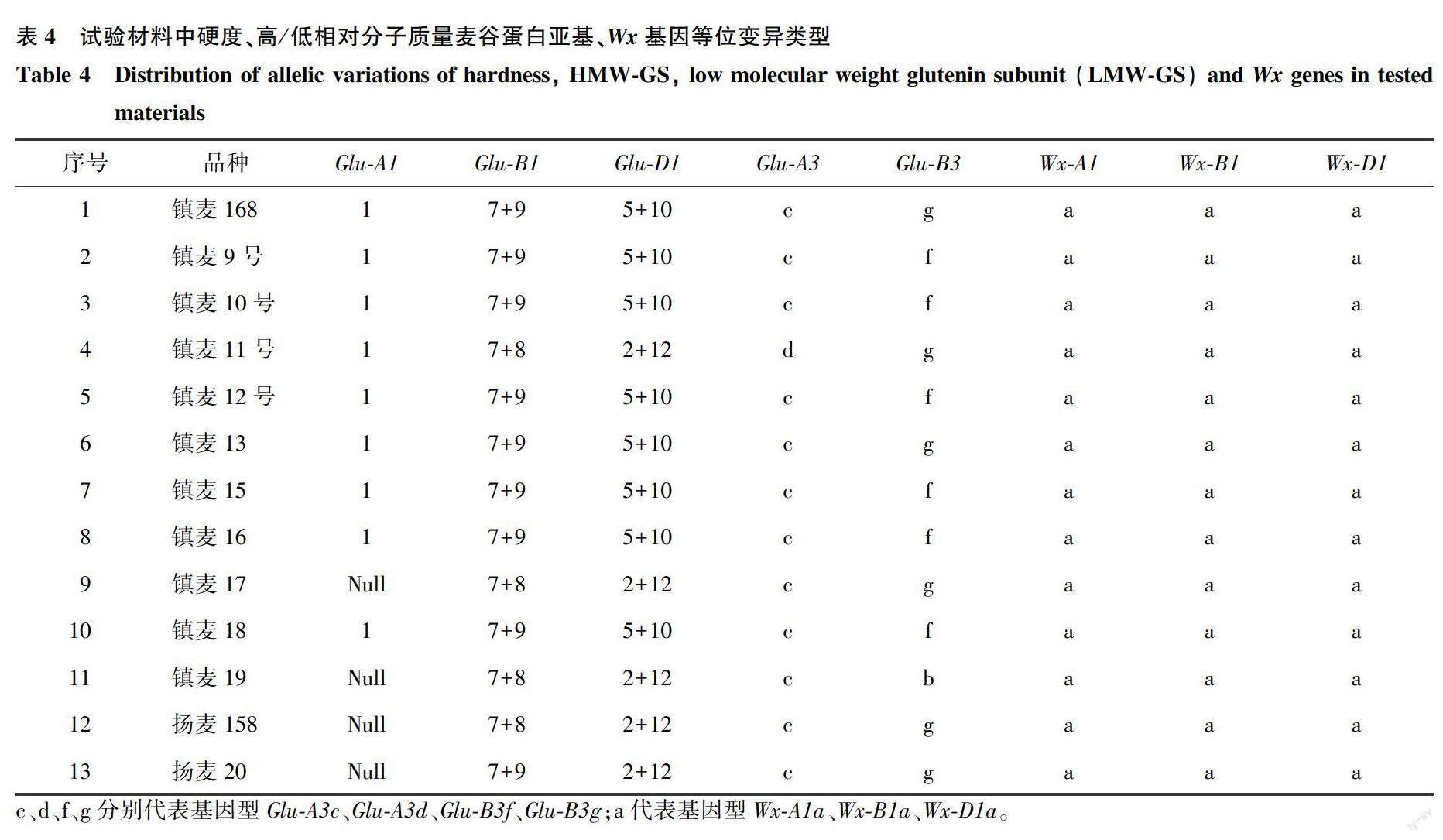
HMW-GS的分子标记检测结果(图2、表4)表明,11份镇麦品种在Glu-A1、Glu-B1和Glu-D1 3个位点上有8种亚基类型,分别为1或Null、7、8、9、2、5、10和12。利用UMN19和Ax2*区分Glu-A1位点上的Ax2*与Ax1或Null,试验材料均为Ax1或Null类型,无Ax2*类型。Glu-B1位点利用bx7、TaBAC1215C06-F517/R964、TaBAC1215C06-F24671/R25515、ZSBy9aF1/R3和ZSBy8F5/R5来区分鉴定,PCR扩增结果表明,试验材料均为Bx7类型。镇麦11号、镇麦17、镇麦19和扬麦158均有By8基因,而镇麦168等9份材料均含有By9基因。利用共显性标记UMN25和UMN26来区分Dx5、Dx2和Dy10、Dy12,其试验材料检测结果为,镇麦11号、镇麦17、镇麦19、扬麦158和扬麦20为2+12等位变异类型,其余镇麦品种均为5+10等位变异类型。综上所述,试验材料Glu-A1位点上的等位变异类型为1或Null,Glu-B1位点上的等位变异类型为7+8和7+9,Glu-D1位点上的等位变异类型为2+12和5+10。进一步利用SDS-PAGE电泳来区分Glu-A1位点上的1和Null(图3),并且验证Glu-B1位点和Glu-D1位点的分子标记可用性。结合分子标记和蛋白质电泳结果,13份材料中检测出1/7+9/5+10、1/7+8/2+12、Null/7+8/2+12和Null/7+9/2+12共4種HMW-GS组合,其中镇麦168、镇麦9号、镇麦10号、镇麦12号、镇麦13、镇麦15、镇麦16和镇麦18均为1/7+9/5+10,镇麦11号为1/7+8/2+12,镇麦17、镇麦19和扬麦158为Null/7+8/2+12,扬麦20为Null/7+9/2+12。
低相对分子质量麦谷蛋白Glu-A3位点的各个标记检测结果为:镇麦11号为Glu-A3d类型,其余12份材料均为Glu-A3c类型;Glu-B3位点上,镇麦19为Glu-B3b,其余材料均为Glu-B3f或Glu-B3g类型。进一步利用SB7F/SB7R标记扩增,结果表明镇麦168、镇麦11号、镇麦13、镇麦17、扬麦158和扬麦20为Glu-B3g类型,镇麦9号、镇麦10号、镇麦12号 、镇麦15、镇麦16和镇麦18为Glu-B3f类型(图4、表4)。
M、1~13见图1注。
2.3 Wx基因分子标记检测
MAG264、BDFL/BRC1和BFC/BRC2、Wx-D1分子标记可分别鉴定Wx-A1、Wx-B1和Wx-D1等位基因的分布特点。上述标记在Wx-A1、Wx-B1和Wx-D1位点上,在野生材料和突变材料中分别对应的目标条带大小为336 bp和317 bp、778 bp和668 bp、840 bp和260 bp,2种条带同时出现的为杂合型材料,对应的基因型分别为Wx-A1a和Wx-A1b、Wx-B1a和Wx-B1b、Wx-D1a和Wx-D1b [13-15]。以上3个位点的分子标记检测结果表明,13份试验材料均为Wx-A1a、Wx-B1a、Wx-D1a野生型,无突变型和杂合型(图5、表4)。
2.4 面粉色泽相关基因分子标记检测
共显性标记PPO18,可用于区分控制高/低PPO活性的等位基因Ppo-A1a和Ppo-A1b,其对应的目的片段大小分别为685 bp和876 bp[22]。标记F-8可以区分Ppo-B1a和Ppo-B1b,扩增出双条带(400 bp和600 bp)的与低PPO活性材料相关,而只扩增出单条带(400 bp)的与高PPO活性材料相关[35]。标记PPO16和PPO29分别能扩增出713 bp和490 bp的靶片段,分别对应的是Ppo-D1a(低PPO活性)和Ppo-D1b(高PPO活性)[6, 36]。以上4个分子标记的检测结果(图6、表5)表明,Ppo-A1位点上,镇麦11号、镇麦17和镇麦19为Ppo-A1a,对照扬麦158和扬麦20均为Ppo-A1a,其余8份材料为Ppo-A1b;Ppo-B1位点上,所有材料均为Ppo-B1a;Ppo-D1位点上,镇麦11号、镇麦19、扬麦158和扬麦20为Ppo-D1a,镇麦17为杂合型,其余材料均为Ppo-D1b。另外一个Ppo-D1的功能标记STS01,与低PPO活性密切相关[24],本研究中在Ppo-D1位点上表现为低PPO活性的材料有镇麦11号、镇麦17、镇麦19、扬麦158和扬麦20,这与PPO16的扩增结果一致。LOX16和LOX18能有效地检测TaLox-B1基因,在高/低LOX活性材料中能扩增出的靶片段大小分别为489 bp和791 bp,分别对应TaLox-B1a和TaLox-B1b[28],标记检测结果(图6)表明,镇麦品种均为TaLox-B1b,无TaLox-B1a及杂合型。
八氢番茄红素合酶(PSY)基因Psy-A1对应的标记为YP7A-1、YP7A-2,Psy-B1对应的标记为YP7B-1、YP7B-2、YP7B-3、YP7B-4,Psy-D1对应的标记为YP7D-1、YP7D-2。其中YP7A-1在高、低黄色素含量的Psy-A1a/c和Psy-A1b上对应的目标片段大小分别为194 bp和231 bp,YP7A-2可进一步区分Psy-A1a/b和Psy-A1c,其目标片段分别为1 686 bp和1 001 bp。YP7B-1、YP7B-2、YP7B-3、YP7B-4可分别用于区分Psy-B1a和Psy-B1b、Psy-B1c、Psy-B1d和Psy-B1e,扩增的片段分别为151 bp/156 bp、428 bp、884 bp和717 bp。YP7D-1和YP7D-2可以用来区分Psy-D1a和Psy-D1g,扩增片段为1 074 bp/1 093 bp和967 bp/1 064 bp[41]。本研究检测结果(图7、表5)表明,13份试验材料均为Psy-A1b类型,镇麦168、镇麦12号和镇麦16为Psy-B1d,镇麦11号和镇麦13为Psy-B1b,镇麦9号、镇麦10号、镇麦15、镇麦17、镇麦18、镇麦19、扬麦158和扬麦20为Psy-B1a,Psy-D1位点均为Psy-D1a。
白度分析结果表明供试材料的白度值为74.47~79.03(表5),其中11份镇麦材料中,在Ppo-D1位点上含有低PPO活性的镇麦11号、镇麦17和镇麦19面粉白度显著高于镇麦168等Psy-B1d基因型的品种。虽然镇麦13和镇麦18在Ppo-D1位点上含有高PPO活性,但其面粉白度为77.90和78.20,相对较高。对照品种扬麦158含有Ppo-D1a,但其面粉白度相对较小。因此,面粉白度不仅与相关基因的基因型有关,还受其他因素的影响,面粉色泽相关基因类型及其他因素与面粉白度的相关性,还需要进一步研究。
3 讨论
镇麦品种的亲本来源相对简单,其中镇麦168、镇麦9号和镇麦10号均来源于同一亲本组合,镇麦12号来源于镇麦168系选株系,镇麦18是以镇麦168为母本选育而来。籽粒硬度基因Pina或Pinb中有一个发生突变都会导致小麦籽粒硬度变硬,影响小麦品质[1-3]。镇麦9号、镇麦10号、镇麦15和镇麦18中可检测到Pinb-D1b突变型,结合硬度指数测定结果来看,镇麦168等硬度指数大于60的品种虽未检测Pinb-D1b突变型,但通过测序比对发现其属于Pinb-D1p突变型。苏麦6号中可检测到Pinb-D1b,因此,镇麦9号和镇麦10号的Pinb-D1基因型可能来源于苏麦6号,在利用苏麦6号等材料构建的DH群体中,需加强Pinb-D1b的筛选,加强品质育种中硬度指数高、有硬度基因突变的材料利用。
HMW-GS和LMW-GS在强筋小麦品种选育中能改进小麦面筋品质[43-44]。胡琳等[45]研究结果表明不同位点优异HMW-GS的品質效应可以累加,对于强筋小麦选育而言,1/7+8/5+10组合的面筋指数、形成时间和面筋强度均处于较高水平,此外,1/7+9/5+10、Null/7+8/2+12等组合的面筋强度也较好。强筋小麦的面团弹性和延展性可以通过LMW-G的优异组合来改善和提高[5],其中Glu-3位点的不同LMW-GS对面团弹性和加工品质影响最大的是Glu-B3,其次是Glu-A3[46],而Glu-D3位点差异对小麦品质的影响不大。优质的LMW-GS主要有Glu-A3d、Glu-B3b、Glu-B3g和Glu-B3i [47-48],因此在优质小麦品质育种中,可以加强这些优质亚基的应用。本研究使用的镇麦品种中可以检测到部分优质的HMW-GS和LMW-GS的亚基,如7+8、5+10、Glu-A3d、Glu-B3b,但没有检测到1/7+8/5+10优异组合,镇麦168等中强筋、强筋小麦品种的HMW-GS组合类型均为1/7+9/5+10。而含HMW-GS和LMW-GS相对优质亚基的材料有镇麦168和镇麦13(1/7+9/5+10,Glu-A3c/Glu-B3g),这可能是镇麦168和镇麦13在长江中下游麦区种植时品质稳定的原因。因此,后续小麦新品种选育中可以通过聚合7+8和5+10组合及优质的LMW-GS亚基,优化小麦品种的面筋特性。扬麦158和扬麦20的HMW-GS和LMW-GS检测结果及本研究中其他品质相关基因的检测结果均与已发表文章中的结果一致[49]。
镇麦品种中均未检测到Wx基因的突变型,想要选育出低直链淀粉含量面条专用小麦品种可通过聚合Wx-B1突变基因降低面粉中的直链淀粉含量,降低面粉糊化温度,改善面条品质[50-52]。小麦籽粒中PSY、PPO、LOX等氧化酶降解色素类物质影响面粉色泽、磨粉品质等品质性状[53-55]。PPO含量低,对面粉、面制品加工和保存过程中色泽褐变有重要作用[56]。镇麦168等8份镇麦品种在Ppo-A1、Ppo-B1位点均含有低PPO活性基因片段,而在Ppo-D1位点,这些材料则含有高PPO活性基因片段。镇麦11号、镇麦17和镇麦19在Ppo-A1位点则可以检测到高PPO活性基因片段,而在Ppo-D1位点则可以检测到低PPO活性基因片段。结合面粉白度分析结果,Ppo-D1位点上的高PPO活性,可能是造成镇麦168等品种的面粉白度值偏小的重要原因。因此,在小麦品种选育过程中加强Ppo-A1和Ppo-D1位点上低PPO活性基因聚合,对面粉白度大的强筋小麦品种选育有重要作用。LOX催化不饱和脂肪酸的氧化,通过氧化降解色素类物质来影响面粉及面制品颜色[57]。本研究中镇麦品种材料均为低LOX活性品种,其面粉白度小及面制品颜色偏深也可能与LOX活性低有直接关系。PSY是影响黄色素合成的限速酶,抑制Psy基因的功能对改良面粉及面粉制品颜色具有推动作用[58]。镇麦品种在Psy-A1/D1位点上均为Psy-A1b/Psy-D1a基因型,为低黄色素含量的材料,而镇麦168、镇麦12号和镇麦16在Psy-B1位点为高黄色素含量的基因型Psy-B1d。我们在利用镇麦品种面粉制作面包、面条等面制品的时候也观察到镇麦168、镇麦12号和镇麦16相应的面制品的颜色相对较深,而这3份材料的面粉白度值也较小。面粉色泽相关基因的基因型以及PPO和黄色素等的含量对面粉白度的影响,还需要进一步研究。
4 結论
在11份镇麦品种中,镇麦9号、镇麦10号、镇麦15和镇麦18均可检测到Pinb-D1b硬度突变基因,镇麦168、镇麦12号、镇麦13和镇麦16均可检测到Pinb-D1p硬度突变基因,且上述材料的硬度指数均大于60。HMW-GS组合类型:镇麦168等8份中强筋、强筋小麦品种均为1/7+9/5+10组合,镇麦11号、镇麦17和镇麦19分别为1/7+8/2+12、Null/7+8/2+12、Null/7+8/2+12组合。LMW-GS组合类型:镇麦11号的组合为Glu-A3d/Glu-B3g,镇麦19为Glu-A3c/Glu-B3b,镇麦168、镇麦13和镇麦17为Glu-A3c/Glu-B3g,其余镇麦品种均为Glu-A3c/Glu-B3f。镇麦品种中未检测到Wx基因突变,均为野生型。面粉色泽相关基因分布特点:镇麦168等8份材料表现为Ppo-A1b/Ppo-B1a/Ppo-D1b/TaLox-B1b,镇麦11号和镇麦19为Ppo-A1a/Ppo-B1a/Ppo-D1a/TaLox-B1b,镇麦17为Ppo-A1a/Ppo-B1a/Ppo-D1a/b/TaLox-B1b;镇麦168、镇麦12号和镇麦16为Psy-A1b/Psy-B1d/Psy-D1a,镇麦11号和镇麦13为Psy-A1b/Psy-B1b/Psy-D1a,其余6份镇麦品种为Psy-A1b/Psy-B1a/Psy-D1a。镇麦材料的面粉白度值为74.47~78.70,整体偏小。镇麦品种品质优良,但Wx蛋白、面粉色泽相关的部分品质仍需进一步改良,对已存在的优异基因片段,在后续品种改良中要充分利用,硬度、色泽等相关性状需进一步加强研究。而在强筋小麦品质育种中,需注重7+8、5+10亚基和Pinb-D1b变异位点,以及低PPO和低黄色素含量基因等标记的筛选与聚合,优化镇麦品种品质特性。
参考文献:
[1] GIROUX M J, MORRIS C F. A glycine to serine change in puroindoline b is associated with wheat grain hardness and low levels of starch-surface friabilin[J]. Theoretical & Applied Genetics, 1997, 95: 857-864.
[2] CHEN F, ZHANG F, CHENG X Y, et al. Association of puroindoline b-2 variants with grain traits, yield components and flag leaf size in bread wheat (Triticum aestivum L.) varieties of the Yellow and Huai Valleys of China[J]. Journal of Cereal Science, 2010, 52(2): 247-253.
[3] MORRIS C F, BHAVE M. Reconciliation of D-genome puroindoline allele designations with current DNA sequence data[J]. Journal of Cereal Science, 2008, 48(2): 277-287.
[4] SONG W F, REN Z Y, ZHANG Y B, et al. Effects of allelic variation in glutenin subunits and gliadins on baking-quality in near-isogenic lines of common wheat cv. Longmai 19[J]. Cereal Research Communications, 2015, 43: 284-294.
[5] VERAVERBEKE W S, DELCOUR J A. Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality[J]. Critical Review in Food Techology, 2002, 42(3): 179-208.
[6] LIU S, CHAO S, ANDERSON J A. New DNA markers for high molecular weight glutenin subunits in wheat[J]. Theoretical & Applied Genetics, 2008, 118(1): 177-183.
[7] D′OVIDIO R, ANDERSON O D. PCR analysis to distinguish between alleles of a member of a multigene family correlated with wheat bread-making quality[J]. Theoretical & Applied Genetics 1994, 88(6/7):759-763.
[8] LEI Z S, GALE K R, HE Z H, et al. Y-type gene specific markers for enhanced discrimination of high-molecular weight glutenin alleles at the Glu-B1 locus in hexaploid wheat[J]. Journal of Cereal Science, 2006, 43(1): 94-101.
[9] BUTOW B J, GALE K R, IKEA J, et al. Dissemination of the highly expressed Bx7 glutenin subunit (Glu-B1al allele) in wheat as revealed by novel PCR markers and RP-HPLC[J]. Theoretical & Applied Genetics, 2004, 109(7): 1525-1535.
[10]RAGUPATHY R, NAEEM H A, REIMER E, et al. Evolutionary origin of the segmental duplication encompassing the wheat GLU-B1 locus encoding the overexpressed Bx7 (Bx7OE) high molecular weight glutenin subunit[J]. Theoretical & Applied Genetics, 2008, 116(2): 283-296.
[11]WANG L H, LI G Y, PENA R J, et al. Development of STS markers and establishment of multiplex PCR for Glu-A3 alleles in common wheat (Triticum aestivum L.) [J]. Journal of Cereal Science, 2010, 51(3): 305-312.
[12]WANG L H, ZHAO X L, HE Z H, et al. Characterization of low-molecular-weight glutenin subunit Glu-B3 genes and development of STS markers in common wheat (Triticum aestivum L.) [J]. Theoretical & Applied Genetics, 2009, 118(3): 525-539.
[13]刘迎春,朱惠兰,程顺和,等. 小麦Wx-A1和Wx-D1位点的PCR分子标记[J]. 麦类作物学报, 2005, 25(1): 1-5.
[14]SAITO M, VRINTEN P, ISHIKAWA G, et al. A novel codominant marker for selection of the null Wx-B1 allele in wheat breeding programs[J]. Molecular Breeding, 2009, 23: 209-217.
[15]NAKAMURA T, VRINTEN P, SAITO M, et al. Rapid classification of partial waxy wheats using PCR-based markers[J]. Genome, 2002, 45(6): 1150-1156.
[16]周淼平,任丽娟,蔡士宾,等. 小麦糯质基因的分子标记辅助选择[J]. 麦类作物学报,2006, 26(5): 10-15.
[17]陈东升, KIRIBUCHI-OTOBE C, 徐兆华, 等. Waxy蛋白缺失对小麦淀粉特性和中国鲜面条品质的影响[J]. 中国农业科学, 2005, 38(5): 865-873.
[18]LINTIG J V, WELSCH R, BONK M, et al. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings[J]. The Plant Journal, 1997, 12(3): 625-634.
[19]ZILIC S, DODIG D, SUKALOVIC H T, et al. Bread and durum wheat compared for antioxidants contents, and lipoxygenase and peroxidase activities[J]. International Journal of Food Science & Technology, 2010, 45(7): 1360-1367.
[20]張福彦,陈 锋,张建伟,等. 逆境胁迫下小麦脂肪氧化酶基因表达的qRT-PCR分析[J]. 麦类作物学报, 2016, 36(9): 1153-1158.
[21]RAMAN R, RAMAN H, JOHNSTONE K, et al. Genetic and in silico comparative mapping of the polyphenol oxidase gene in bread wheat (Triticum aestivum L.) [J]. Functional & Integrative Genomics, 2005, 5(4): 185-200.
[22]SUN D J, HE Z H, XIA X C, et al. A novel STS marker for polyphenol oxidase activity in bread wheat[J]. Molecular Breeding, 2005, 16(3):209-218.
[23]SI H Q, ZHOU Z L, WANG X B, et al. A novel molecular marker for the polyphenol oxidase gene located on chromosome 2B in common wheat[J]. Molecular Breeding, 2012, 30(3):1371-1378.
[24]王晓波,马传喜,何克勤,等. 小麦2D染色体上多酚氧化酶(PPO)基因STS标记的开发与应用[J]. 中国农业科学, 2008, 41(6): 1583-1590.
[25]HE X Y, HE Z H, ZHANG L P, et al. Allelic variation of polyphenol oxidase (PPO) genes located on chromosomes 2A and 2D and development of functional markers for the PPO genes in common wheat[J]. Theoretical and Applied Genetics,2007, 115(1): 47-58.
[26]SERPEN A, GKMEN V. Effects of β-carotene on soybean lipoxygenase activity: kinetic studies[J]. European Food Research & Technology, 2007, 224(6): 743-748.
[27]MERCIER M, GELINAS P. Effect of lipid oxidation on dough bleaching[J]. Cereal Chemistry, 2001, 78(1): 36-38.
[28]GENG H W, XIA X C, ZHANG L P, et al. Development of functional markers for a lipoxygenase gene TaLox-B1 on chromosome 4BS in common wheat[J]. Crop Science, 2012,52(2):568-576.
[29]HE X Y, ZHANG Y L, HE Z H, et al. Characterization of phytoene synthase 1 gene (Psy1) located on common wheat chromosome 7A and development of a functional marker[J]. Theoretical and Applied Genetics, 2008, 116(2): 213-221.
[30]HE X Y, HE Z H, MA W, et al. Allelic variants of phytoene synthase 1 (Psy1) genes in Chinese and CIMMYT wheat cultivars and development of functional markers for flour colour[J]. Molecular Breeding, 2009, 23(4): 553-563.
[31]張平平,周淼平,马鸿翔. 小麦种子高分子量谷蛋白亚基的高通量检测方法[J]. 分子植物育种, 2015, 13(11):2593-2598.
[32]MA W, ZHANG W, GALE K R. Multiplex-PCR typing of high molecular weight glutenin alleles in wheat[J]. Euphytica, 2003, 134(1): 51-60.
[33]CHEN F, ZHANG F Y, MORRIS C, et al. Molecular characterization of the Puroindoline a-D1b allele and development of an STS marker in wheat (Triticum aestivum L.) [J]. Jouunal of Cereal Science, 2010, 52(1): 80-82.
[34]CHEN F, ZHANG F Y, XIA X C, et al. Distribution of puroindoline alleles in bread wheat cultivars of the Yellow and Huai Valley of China and discovery of a novel puroindoline a allele without PINA protein[J]. Molecular Breeding, 2012, 29(2): 371-378.
[35]CHEN F, LI H H, CUI D C. Discovery, distribution and diversity of Puroindoline-D1 genes in bread wheat from five countries (Triticum aestivum L.) [J]. BMC Plant Biology, 2013, 13: 125.
[36]CHEN F, HE Z H, XIA X C, et al. Molecular and biochemical characterization of puroindoline a and b alleles in Chinese landraces and historical cultivars[J]. Theoretical and Applied Genetic,2006, 112: 400-409.
[37]SCHWARZ G, FELSENSTEIN F G, WENZEL G. Development and validation of a PCR-based marker assay for negative selection of the HMW glutenin allele Glu-B1-1d (Bx-6) in wheat[J]. Theoretical and Applied Genetic, 2004, 109(5): 1064-1069.
[38]LIU S, CHAO S, ANDERSON J A. New DNA markers for high molecular weight glutenin subunits in wheat[J]. Theoretical and Applied Genetic, 2008,118(1): 177-183.
[39]SI H Q, ZHOU Z L, WANG X B, et al. A novel molecular marker for the polyphenol oxidase gene located on chromosome 2B in common wheat[J]. Molecular Breeding, 2012, 30(3): 1371-1378.
[40]HE X Y, HE Z H, ZHANG L P, et al. Allelic variation of polyphenol oxidase (PPO) genes located on chromosomes 2A and 2D and development of functional markers for the PPO genes in common wheat[J]. Theoretical and Applied Genetic, 2007, 115(1): 47-58.
[41]WANG J W, HE X Y, HE Z H, et al. Cloning and phylogenetic analysis of phytoene synthase 1 (Psy 1) genes in common wheat and related species[J]. Hereditas, 2010, 146(5): 208-256.
[42]周艷华,何中虎,阎 俊,等. 中国小麦硬度分布及遗传分析[J]. 中国农业科学, 2002, 35(10) :1177-1185.
[43]SONG W F, REN Z Y, ZHANG Y B, et al. Effects of allelic variation in glutenin subunits and gliadins on baking-quality in near-isogenic lines of common wheat cv. Longmai 19[J]. Cereal Research Communication, 2015, 1(1): 1-11.
[44]HE Z H, LIU L, XIA X C, et al. Composition of HMW and LMW glutenin subunits and their effects on dough properties, Pan bread, and noodle quality of Chinese bread wheats[J]. Cereal Chemistry, 2005, 82(4): 345-350.
[45]胡 琳,盖钧镒,许为钢,等. 小麦不同高分子量谷蛋白亚基对面筋数量和质量的影响[J]. 麦类作物学报, 2007, 26(6): 57-58.
[46]王林海. 普通小麦及其近缘种低分子量麦谷蛋白基因克隆与STS标记开发[D]. 北京:中国农业科学院, 2009.
[47]ZHANG X F, JIN H, ZHANG Y, et al. Composition and functional analysis of low-molecular-weight glutenin alleles with Aroona near-isogenic lines of bread wheat[J]. Bmc Plant Biology, 2012, 12(1): 229-243.
[48]周 阳,刘建军,何中虎,等. Glu-1和Glu-3等位变异及1BL/1RS易位与面包和面条品质关系的研究[J]. 中国农业科学, 2004, 37(9): 1265-1273.
[49]张 晓,张伯桥,江 伟,等. 扬麦系列品种品质性状相关基因的分子检测[J]. 中国农业科学, 2015, 48(19): 3779-3793.
[50]WICKRAMASINGHE H A M, MIURA H. Gene dosage effect of the wheat Wx alleles and their interaction on amylose synthesis in the endosperm[J]. Euphytica, 2003, 132(3):303-310.
[51]翟红梅,田纪春. 小麦Wx基因突变体的建立及其淀粉特性的研究[J]. 作物学报, 2007, 33(7): 1059-1066.
[52]MIURA H, WICKRAMASINGHE M H A, SUBASINGHE R M, et al. Development of near-isogenic lines of wheat carrying different null Wx alleles and their starch properties[J]. Euphytica, 2002,123(3): 353-359.
[53]CRAWFORD A C, STEFANOVA K, LAMBE W, et al. Functional relationships of phytoene synthase 1 alleles on chromosome 7A controlling flour colour variation in selected Australian wheat genotypes[J]. Theoretical and Applied Genetics, 2011, 123(1): 95-108.
[54]CHANG C, ZHANG H P, XU J, et al. Variation in two PPO genes associated with polyphenol oxidase activity in seeds of common wheat[J]. Euphytica, 2007, 154(1/2): 181-193.
[55]WANG X M, LU Z X. Research advance on lipoxygenase application in the agri-food[J]. Journal of Nucelar Agricultural Sciences, 2013, 27(1): 1547-1552.
[56]ANDERSON J V, MORRIS C F. An improved whole-seed assay for screening wheat germplasm for polyphenol oxidase activity[J]. Crop Science, 2001, 41(6): 1697-1705.
[57]MARES D J. Mapping components of flour and noodle colour in Australian wheat[J]. Australian Journal of Agricultural Research, 2001, 52(12): 1297-1309.
[58]孫建喜. 小麦籽粒多酚氧化酶活性和黄色素含量性状的基因检测[D]. 郑州:河南农业大学, 2014.
(责任编辑:张震林)

