Safety of butylphthalide and edaravone in patients with ischemic stroke: a multicenter real-world study
Shu-Xian LYU, Dong-Fang QIAN, Yu-Fei FENG, Cheng-Wu SHEN, Lu-Bo GUO, Jian-Tao LYU, Peng-Fei JIN, Ting LI, Si-Yuan TAN, Zi-Xuan ZHANG, Lin HUANG, Xue ZHONG, Le-Qun SU, Xin HU, Xin HUANG,✉, Xue-Yan CUI,✉
1.Department of Clinical Pharmacy, the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Medicine and Health Key Laboratory of Clinical Pharmacy, Shandong, China; 2.Beijing North Medical Health Economic Research Center, Beijing, China; 3.Department of Pharmacy, Peking University People’s Hospital, Beijing, China; 4.Department of Pharmacy, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Shandong, China; 5.Department of Pharmacy, Central Hospital Affiliated to Shandong First Medical University, Shandong, China; 6.Department of Pharmacy, Yantai Affiliated Hospital of Binzhou Medical University (the Second School of Clinical Medicine of Binzhou Medical University), Shandong, China; 7.Department of Pharmacy, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing Key Laboratory of Assessment of Clinical Drugs Risk and Individual Application (Beijing Hospital), Beijing, China
✉ Correspondence to: 13791120711@126.com (HUANG X); qfscxy@126.com (CUI XY)https://doi.org/10.26599/1671-5411.2023.04.002
ABSTRACT BACKGROUND Butylphthalide (NBP) and edaravone (EDV) injection are common acute ischemic stroke medications in China,but there is a lack of large real-world safety studies on them.This study aimed to determine the incidence of adverse events, detect relevant safety signals, and assess the risk factors associated with these medications in real-world populations.
Ischemic stroke is a type of disease in which local brain tissue suffers from a rapid loss of blood circulation for various reasons, resulting in corresponding neurological deficits; this disease accounts for 87% of all strokes.[1]A collaborative study published inThe Lancet Neurologyrevealed an incidence rate of more than two million new stroke cases in China annually.[2]In 2017, stroke was the top cause of mortality,up from third place in 1990.In China, ischemic stroke is one of the most serious threats to public health.The Chinese guidelines for the diagnosis and treatment of acute ischemic stroke 2018 recommended that the main drug treatments for acute ischemic stroke (AIS) include medications to improve cerebral blood circulation, statins, and neuroprotective agents, such as butylphthalide (NBP), human urea kininogenase, and edaravone(EDV).[3]
NBP is a synthetic racemic N-butylphthalide (3-Nbutylphthalide), approved by the National Medical Products Administration with an indication for the improvement of neurological impairment in AIS patients.NBP can play a therapeutic role by improving cerebral microcirculation, increasing blood flow in ischemic areas, improving mitochondrial function, inhibiting oxidative stress and apoptosis, reducing inflammation, and regulating Ca2+concentration, among other mechanisms.[4]The adverse reactions mentioned in the drug label for NBP injection mainly include increased transaminase Drug-drug interactions are unclear.
EDV is a low-molecular-weight antioxidant medication that has a protective impact on nerves by scavenging reactive oxygen species, hydroxyl radicals, and reactive nitrogen.It is applicable to the improvement of neurological symptoms, activities of daily living, and dysfunction caused by AIS.[5,6]The drug label for EDV injection mentions that the adverse reactions mainly include increased transaminase levels and rash.A metaanalysis showed that after a three-month follow-up, regardless of patient age and treatment duration, EDV reduced neurological impairments and increased patient survival.The reported adverse events included nausea, rash, and abnormal liver function.[7]
Although both medications are routinely used to treat patients with AIS, studies considering extensive data to explore the safety of NBP and EDV in the real world are lacking.Therefore, this study intended to fill in the gap regarding real-world safety studies of NBP and EDV using a retrospective cohort study.Using existing databases, including literature databases and electronic medical record databases from medical institutions,this study aimed to detect safety signals of association for NBP and EDV through big data mining and an evaluation of the safety of NBP and EDV in real-world populations.
METHODS
Study Design
This study is a multicenter, retrospective, real-world study conducted in accordance with the guidelines for good pharmacoepidemiology practice.[8]The cohorts of NBP, EDV, and third users were constructed through routinely collected data from six tertiary hospitals between January 2019 and August 2021.The followup period started at the first detected medication order and ended 28 days after the last medication intake.Statistical descriptions included patient demographic characteristics, medication use patterns, and adverse events.
Baseline conditions as well as liver function, renal function, coagulation and other tests were compared between the two groups of patients receiving NBP and EDV, and multivariate logistic regression analysis and prescription sequence symmetry analysis (PSSA) were used to assess the safety of the two medications.A third group, with patients who did not receive NBP or EDV but could be prescribed other standard of care medications (hereafter referred to as the third group), was used as a reference to evaluate liver function, renal function, and coagulation.
Data Source
Electronic medical record data from six tertiary hospitals in China served as the data source for this study.Patients diagnosed with “stroke, cerebral infarction,or cerebral embolism”were extracted.Patients were identified throughout multiple visits with unique codes.The extracted variables included patient demographics (age, sex, and hospitalization), medical order records (medicine orders, non-drug orders, and operations),and laboratory results.
The diagnosis and drug records in the database were mapped to the International Statistical Classification of Disease and Related Health Problems, 10thRevision,and the Anatomical Therapeutic Chemical drug classification system, respectively.This study adhered to the Declaration of Helsinki.[9]Personal information was desensitized.The Ethics Committee of Shandong Provincial Qianfoshan Hospital, Shandong, China approved this study [No.YXLL-KY-2021 (072)].Informed consent was waived.
Study Population
Inclusion criteria for the NBP and EDV groups included the following: (1) patients with ischemic stroke who received at least one prescription of NBP or EDV from any research center during the consecutive study period (from January 2019 to August 2021); and (2) patients who had at least one complete period of NBP or EDV and identifiable laboratory results before the first and 28 days after the last prescription.The third group met the following criteria: (1) patients with ischemic stroke who did not receive NBP or EDV from any research center during the consecutive study period (from January 2019 to August 2021); and (2) patients who had at least two laboratory results in the same research center at the time of their visit.
The general exclusion criteria for all three cohorts were set as follows: (1) patients received a malignant tumor diagnosis at the time of their visit; and (2) patients in the NBP or EDV groups who were simultaneously prescribed NBP and EDV.Each function group had different exclusion criteria, as patients with functional abnormalities before medication intake should be excluded.Therefore, the treatment of the liver, renal, and other function groups was as follows: (1) the liver function group excluded patients with the diagnosis of jaundice, liver diseases (not including drug-induced liver injury, liver diagnosis related to traditional Chinese medicine, no abnormal liver function, and no clinical significance in the liver imaging examination); (2) the renal function group excluded patients with the diagnosis of uremia, kidney diseases (not including drug-induced renal injury, renal diagnosis related to traditional Chinese medicine, no abnormal renal function, no clinical significance in the renal imaging examination, and a diagnosis of adrenal glands); and (3) other groups required no unique inclusion or exclusion of functional safety evaluations.
Outcome
The objectives of this study were to evaluate the safety of NBP and EDV in real-world populations through a retrospective cohort study, to collect adverse events and their incidence, and to evaluate the potential risk factors for the two medications.The main objective was to compare the incidence levels of “abnormal liver function-related tests”and drug-induced liver damage; “abnormal renal function tests”and drug-induced renal damage; and other adverse events after administration of NBP and EDV.The secondary objective was to clarify the population characteristics of NBP and EDV, including the sex, age, comorbidities, concomitant medications, number of hospitalizations, and length of stay of patients.
Statistical Analysis
MySQL 5.7 (https://www.mysql.com) was used for data curation and governance.R statistical software 4.1.3(http://www.r-project.org) and SPSS 20.0 (SPSS Inc.,IBM, Chicago, IL, USA) were used for statistical analysis.Propensity score matching was used to match the baseline demographic characteristics of patients.Data fit to a normal distribution were described as mean ± SD,and the independent Student’st-test were used to compare groups.Data not normally distributed as medians(interquartile range), and the Kruskal-Wallis test was used for comparisons between groups.All statistical tests were two-sided, and differences were considered statistically significant forP-value < 0.05.
The incidence of safety occurrences was assessed within each patient visit.Multivariate logistic regression was performed, with abnormal laboratory test results(0 = no, 1 = yes) as the dependent variable and with covariates including sex, age, and concomitant medication.Smoking and drinking were not included as regression factors due to missing values, as their inclusion could have led to information bias.
PSSA was used to determine the association between adverse drug events and medications at the population level.A lower limit of the 95% CI for the adjusted sequence ratio (ASR) that is greater than one signifies a correlation between the drug and adverse drug event (i.e., a positive signal).The magnitude of the value indicates the strength of the correlation.
RESULTS
Patient Baseline, Comorbidities, and Concomitant Medications
After propensity score matching, each group contained 727 patients.Figure 1 depicts the inclusion and exclusion flow chart of eligible patients from six tertiary hospitals.Table 1 lists the baseline characteristics of the study population.There was no significant difference in sex, age, or the top two comorbidities, i.e., essential (primary) hypertension and non-insulin-dependent diabetes mellitus, between the NBP group, the EDV group or the third group.However, there were statistically significant differences between the NBP and EDV groups in the number of hospitalizations per patient(1.69 ± 1.32vs.2.30 ± 5.42 hospitalizations,P< 0.05) and the length of hospitalization per visit (12.84 ± 7.95vs.16.64 ± 12.28 days,P< 0.05).Table 2 reports that the NBP, EDV and the third groups had similar comorbidities in terms of combined diagnosis.The incidence of “other postsurgical states”in the NBP group (n= 103, 14.17%)and in the third group (n= 132, 18.16%) was significantly different from that in the EDV group (n= 207, 28.47%)(P< 0.05).The incidence of “intracerebral hemorrhage”in the NBP group (n= 46, 6.33%) and in the third group(n= 44, 6.05%) was significantly different from that in the EDV group (n= 123, 16.92%) (P< 0.05).Overall, the comorbidities between the two groups were comparable.Only “other postsurgical status”was higher in the EDV group (n= 207, 28.47%) than in the NBP group (n=103, 14.17%).

Figure 1 Flow chart of patient inclusion and exclusion.EDV: edaravone; NBP: butylphthalide.
Tables 3-5 summarize the concomitant drugs of theNBP group, the EDV group, and the third group.Approximately 90% of patients in the NBP group were on both the 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase inhibitors (n= 685, 94.22%) and platelet aggregation inhibitors excl.heparin (n= 655, 90.10%).The concomitant drug used in the EDV group did notadhere to the guidelines for cerebral infarction.Proton pump inhibitors (PPI) were the medicine most prescribed in the EDV group (n= 584, 80.33%).
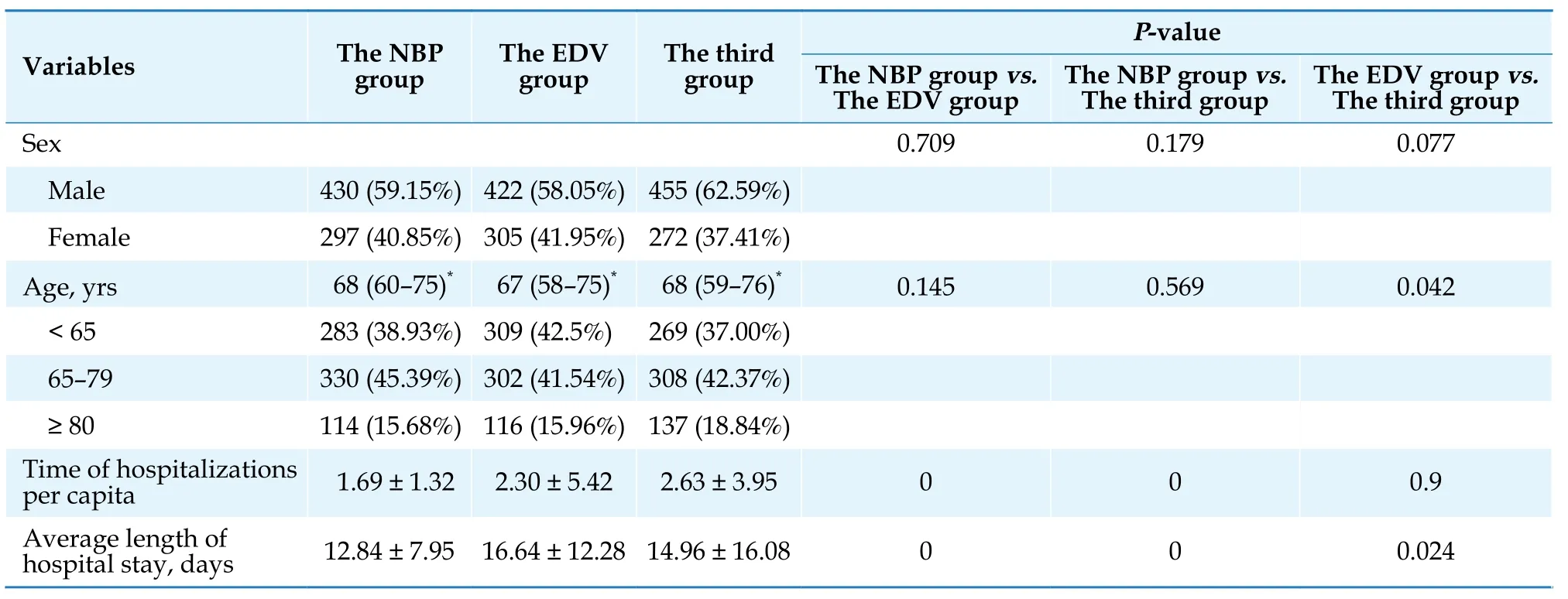
Table 1 Baseline population of patients in the NBP group, the EDV group, and the third group.

Table 2 Combined diagnoses of patients in the NBP group, the EDV group, and the third group.
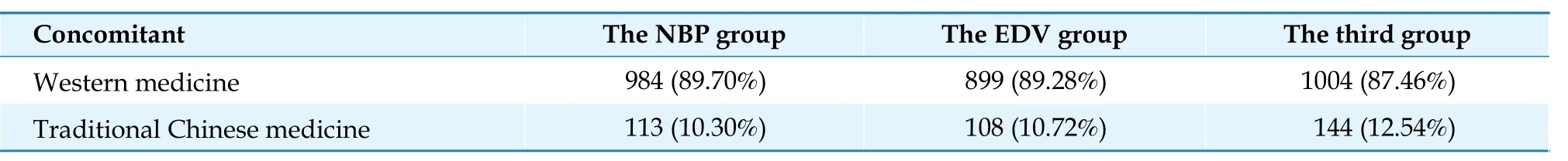
Table 3 Type of concomitant medications used in the NBP group, the EDV group, and the third group.

Table 4 Concomitant medications used in the NBP group, the EDV group, and the third group (by ATC code, except for traditional Chinese medicines).
Incidence of Drug-related Adverse Events
This study included 24 laboratory tests.Table 6 shows the incidence of abnormal laboratory test results in the NBP group, the EDV group, and the third group.Abnormal results were defined as laboratory test results that were above the upper level normal.The incidence of abnormal test results in the liver, kidney, blood lipids, coagulation, and other functional groups was determined separately for the three groups.In the NBP and EDV groups, 24-hour total urine protein and prothrombin time activity were excluded because no abnormal values exceeding the upper level normal were found to support the calculation.Among the remaining 22 items, the incidence of abnormal values for 15 items in the NBP group was significantly lower than that in the EDV group (P< 0.05).The incidence of abnormal values for 13 items in the NBP group was lower than that in the third group, and the incidence of abnormal values for 9 items in the EDV group was higher than that in the third group, with a statistically significant difference (P<0.05).Table 7 shows the incidence of organ damage determined by the laboratory results.Test results over the adverse event threshold suggested by the adverse drug reaction guidelines indicated organ damages.The results showed that the incidence of organ damage in the NBP group was lower than that in the EDV group.Res-ults of the three test subsets were significantly different,including alanine aminotransferase (ALT), which can indicate liver damage, and prothrombin time (PT) and activated partial thromboplastin time, which indicate coagulation dysfunction.

Table 5 Concomitant medications of patients in the NBP group, the EDV group, and the third group (by drug name).
Risk Factors for Drug-related Adverse Events
The construction of the multivariate logistic regression model used the occurrence of abnormal laboratory test index results (0 = no, 1 = yes) as the dependent variable and sex, age, and concomitant medications as covariates.According to the regression results, NBP was not a promoting factor for the abnormalities of the 24 tests included in this study (P> 0.05).In contrast, EDV exerted significant effects on aspartate aminotransferase (AST) (OR = 2.00, 95% CI: 1.07-3.71,P= 0.029), low-density lipoprotein cholesterol (OR = 12.59, 95% CI: 2.07-76.58,P= 0.006), and total cholesterol (OR = 4.06, 95% CI:1.09-15.12,P= 0.037).The detailed results are shown in Tables 8-10.
Correlation Evaluation Using PSSA
Tables 11 & 12 provide the PSSA results for the NBP and EDV groups.A weak correlation existed between NBP and abnormal platelet count (PLT) (ASR = 1.50,95% CI: 1.11-2.04).For other adverse events, PSSA sho-wed no positive signals.For EDV, PSSA demonstrated positive signals on gamma-glutamyl transferase (GGT)(ASR = 1.71, 95% CI: 1.22-2.48), ALT (ASR = 1.65, 95% CI:1.14-2.48), AST (ASR = 1.42, 95% CI: 1.07-1.91) from the liver function group, PT (ASR = 1.36, 95% CI: 1.11-1.69)from the coagulation group, and PLT (ASR = 1.62, 95%CI: 1.30-2.05).

Table 6 Incidence of abnormal test results in the NBP group, the EDV group, and the third group.
DISCUSSION
Baseline, Comorbidities, and Concomitant Medications
The NBP group and the EDV group did not differ significantly statistically in terms of age or sex follow-ing propensity score matching, but there were significant differences in the number of hospitalizations per person (1.69 ± 1.32vs.2.30 ± 5.42 hospitalizations,P<0.05) and the length of hospitalization per visit (12.84 ±7.95vs.16.64 ± 12.28 days,P< 0.05).Patient visits and hospitalization may have been affected because the data extraction period fell within the COVID-19 outbreak period.Regarding comorbidities, the common diseases of patients in the NBP group and the EDV group were similar, and only “other postsurgical status”and “intracerebral hemorrhage”in the EDV group were significantly higher than those in the NBP group (P< 0.05).Given the higher number of hospitalizations per capita and the average stay of patients in the EDV group, there may have been more intracerebral hemorrhage, postoperative bed rest, or mobility problems in the EDV group.The influence of prescribing habits also cannot be ruled out.The available data do not yet support the extrapolation of disease severity associated with ischemic stroke.
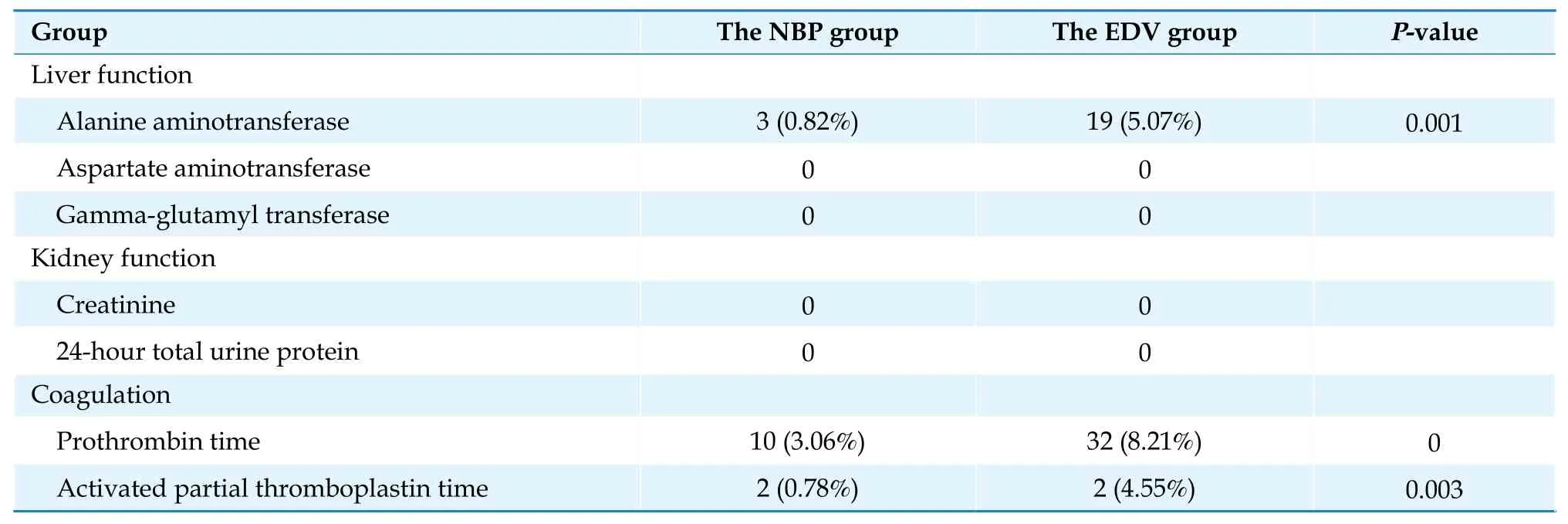
Table 7 Incidence of organ damage in the NBP group and the EDV group.
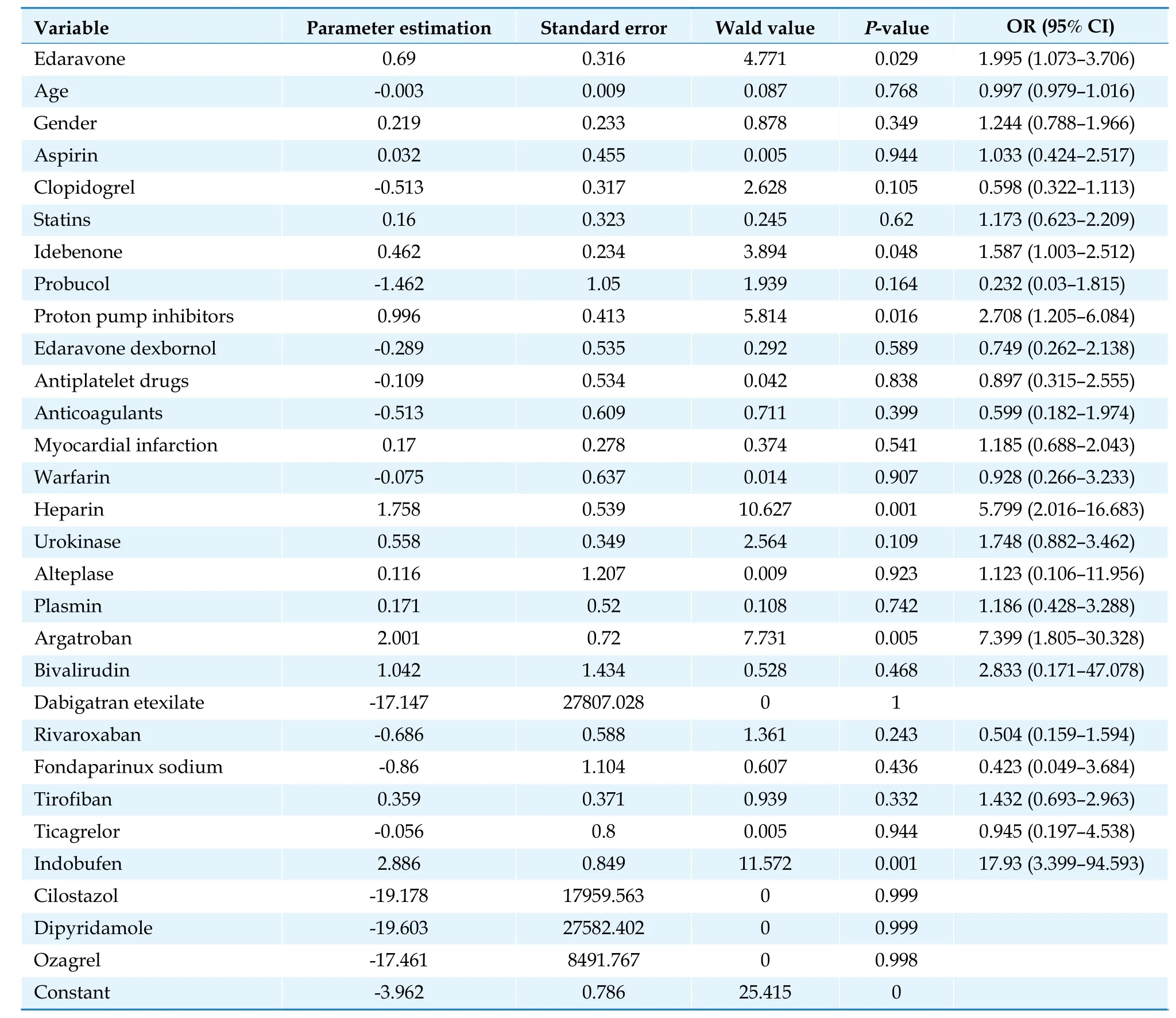
Table 8 Multivariate logistic regression analysis of abnormal aspartate aminotransferase results.
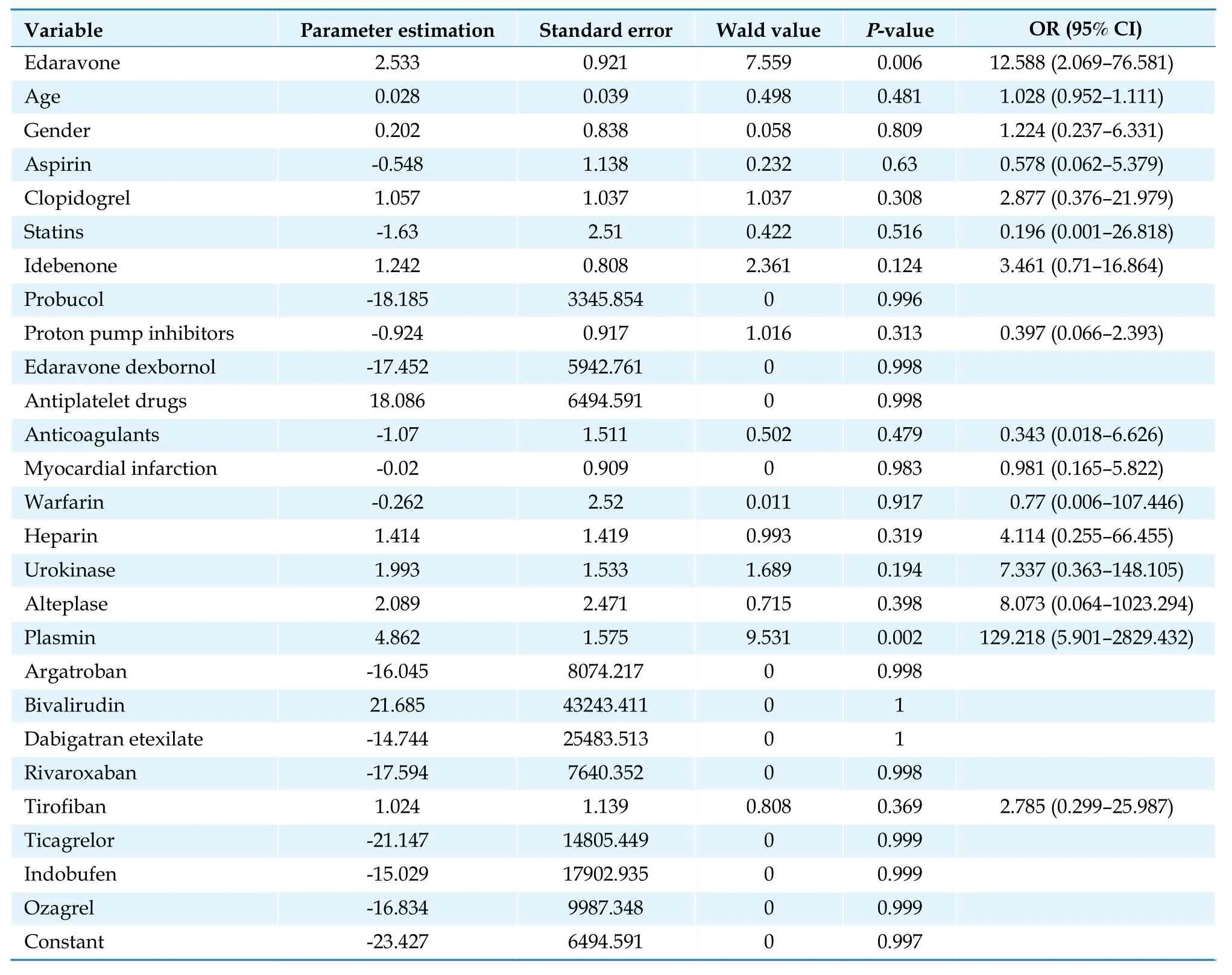
Table 9 Multivariate logistic regression analysis of abnormal low-density lipoprotein cholesterol results.
Regarding concomitant medications, the NBP group’s concomitant medications complied with the Chinese guidelines for the diagnosis and treatment of acute ischemic stroke published in 2018.[3]Approximately 90% of the patients in the NBP group concomitantly used HMGCoA reductase inhibitors (n= 685, 94.22%) and platelet aggregation inhibitors excl.heparin (n= 655, 90.10%).However, the medications concomitantly used with EDV showed inconsistency with medications advised by guidelines for ischemic stroke.Among these, PPI (n= 584,80.33%) was the most commonly used concomitant medication.While HMG-CoA reductase inhibitors (n= 470,64.65%) and platelet aggregation inhibitors excl.heparin(n= 450, 61.90%), accounted for only approximately 60%of the concomitant medications.Heparin use amounted to 45.12% (n= 328), excluding the use of blocked tubes,which is discordant with the recommended medications in the guidelines for patients with AIS.However,this result may be related to the EDV group having higher percentages of patients with the diagnosis of “intracerebral hemorrhage”, “other postoperative states”.These patients do not use antiplatelet medications in acute episodes or perioperative periods but are often prescribed PPIs.Influenced by the lower unit cost of EDV, prescriptions were more likely to be based on dependency than on the patient’s actual needs.Careful consideration should be given to whether a safety event can arise from clinically irrational drug use.

Table 10 Multivariate logistic regression analysis of abnormal total cholesterol results.
Incidence of Drug-related Adverse Events
This study solely excluded patients with malignant tumors to reflect the real-world scenario.Patients with a confirmed diagnosis of non-drug-induced liver or kidney diseases were excluded from the liver and renal function group.The exclusion was not based on abnormal laboratory results prior to drug use.No exclusion criteria were set for the blood coagulation or other function groups.Therefore, abnormal PT might be associated with disease progression in patients with AIS.Since NBP is mainly metabolized by the liver,[10]and EDV is metabolized in liver and kidney microsomes;[11]the incidence of abnormal liver and kidney function indicators reflects the safety of both medications.The incidence of abnormal liver function indicators in the NBP group was considerably lower than that in the EDV group and the third group.Due to the limited availability of laboratory tests reflecting renal function, this study did not find a difference between NBP and EDV in the incidence of abnormal test results reflecting renal function.
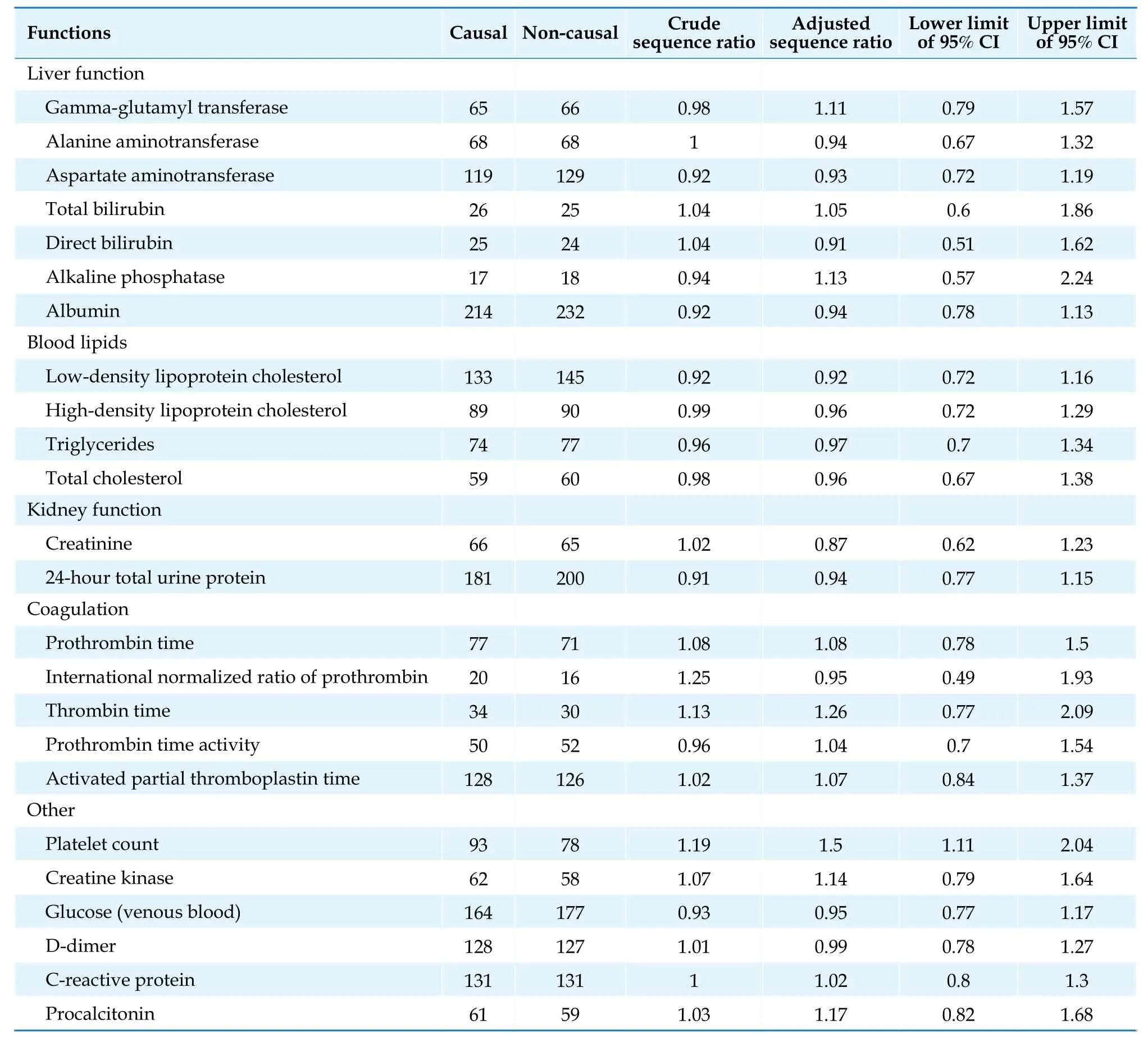
Table 11 Prescription sequence symmetry analysis result of butylphthalide and abnormal laboratory tests results.
The incidence of abnormal liver function tests in the NBP group (including GGT, ALT, AST, and alkaline phosphatase) was significantly lower than that in the third group.According to the 2018 guidelines,[3]patients with AIS can be treated with medications to improve circulation and neuroprotection.While patients in the third group did not use NBP or EDV, they could be prescribed other circulation improving medications.The use of ligustrazine hydrochloride injection, ginkgo biloba extract (the third group:n= 49, 6.74%; the NBP group:n= 23,3.16%), and poppy biloba hydrochloride injection (the third group:n= 54, 7.43%; the NBP group:n= 22, 3.03%)were higher in the third group, and the difference was statistically significant (P< 0.05) as compared to the NBP group.Thus, the effect of these medications on liver fu nction could not be disregarded.
The incidence of blood lipids (low-density lipoprotein cholesterol and total cholesterol) and albumin abnormalities in the NBP group was significantly lower than that in the third group, which may be related to the significantly higher use of fatty emulsion in the third group (the third group:n= 101, 13.89%; the NBP group:n= 68, 9.35%), and the effect of these medications on liver function and blood lipids cannot be omitted.In addition, higher use of fatty emulsion nutritional drug in the third group indicated a higher nutritional risk in the th-ird group, possibly related to more patients with low albumin.

Table 12 Prescription sequence symmetry analysis results of edaravone and abnormal laboratory tests results.
The incidence of abnormal PT and PLT in the NBP group was significantly lower than that in the third group, which could be attributed to the higher usage of lowmolecular-weight heparin and cefoperazone sodium/sulbactam sodium in the third group than in the NBP group (P< 0.05).Both the low-molecular-weight heparin sodium injections (the third group:n= 53, 7.29%;the NBP group:n= 23, 3.16%) and the cefoperazone sodium/sulbactam sodium (the third group:n= 57, 7.84%;the NBP group:n= 34, 4.7%) may have affected coagulation function and platelet counts.
However, since the EDV group was not the main subject of this study, it will not be particularly addressed in this article.It is possible that the EDV group also experienced abnormal results as a result of variations in medication patterns.Moreover, the EDV group contained higher “intracerebral hemorrhage”, “other postoperative states”and was prescribed more PPI, and the incidence of abnormal test results for patients with acute episodes and perioperative status may be higher than that in the general patient population.
Risk Factors for Drug-related Adverse Events
Adverse drug reactions can arise for a variety of complicated causes, including patient individuality, pharmacological compatibility, and various aspects of drug use.The results of multivariate logistic regression showed that NBP was not a promoting factor for any of the 24 laboratory tests included in this study.The use of EDV was a statistically significant risk promoting factor for AST (OR = 2.00, 95% CI: 1.07-3.71,P= 0.029), lowdensity lipoprotein cholesterol (OR = 12.59, 95% CI: 2.07-76.58,P= 0.006), and total cholesterol (OR = 4.06, 95% CI:1.09-15.12,P= 0.037) when compared to the use of NBP.The relative risk coefficients associated with EDV, abnormal low-density lipoprotein cholesterol, and the total cholesterol results could not be exempted from selection bias.
In the EDV group, only 64.65% of patients (n= 470)used HMG-CoA reductase inhibitor (statin) concurrently, whereas in the NBP group, the percentage was significantly higher (n= 685, 94.22%).However, the number of patients with dyslipidemia did not significantly differ between the two groups.
Therefore, the higher relative risk of abnormal blood lipids in the EDV group may result from the fact that some patients using EDV did not concomitantly use lipid-controlling medications, resulting in a higher number of lipid abnormalities, while patients using NBP showed superior lipid control as a result of concomitant lipid-controlling medication use.
However, the multivariate regression results of the abnormal AST tests were consistent with the results of PSSA, suggesting a positive signal for abnormal liver function-related results in the EDV group.NBP and EDV are both regularly prescribed concomitantly with many medications.Adverse responses could result from a variety of frequently used medication combinations.Highrisk drug combinations should be avoided, and the use of multiple medications should be minimized.
Correlation Evaluation Based on PSSA
PSSA is a drug safety signal detection method suitable for routinely collected medical data.It was proposed by Petri,et al.[12]in 1988, and Hallas,et al.[13]improved the method and named it PSSA.However, novel signals identified by PSSA needs further studies for verification.[14,15]Several large multicenter studies have shown that PSSA gives consistent results for the same adverse drug reaction studies across different sources and populations.[16,17]
There was a weak correlation between NBP and abnormal PLT laboratory results, with an ASR signal intensity range between 1.11 and 2.04.Antiplatelet medications are commonly used concomitant medications in stroke patients.A total of 90.1% (n= 655) of patients included in this study concomitantly used platelet aggregation inhibitors excl.heparin, according to the Anatomical Therapeutic Chemical classification.As a cohort study of AIS patients, it is impossible to exclude participants who may have coagulation problems due to the shared condition.Therefore, the signals of PLT abnormalities indicated by the PSSA results might not reflect any safety issues of NBP.PSSA found no positive signal that demonstrated correlations with other adverse events, suggesting that the abnormal laboratory results were less likely to be associated with NBP or that the association was not statistically significant.
PSSA showed a positive signal between EDV and abnormal laboratory results in GGT (ASR = 1.71, 95% CI:1.22-2.48), ALT (ASR = 1.65, 95% CI: 1.14-2.48), AST(ASR = 1.42, 95% CI: 1.07-1.91), PT (ASR = 1.36, 95% CI:1.11-1.69), and PLT (ASR = 1.62, 95% CI: 1.30-2.05).EDV was related to higher ASR values for abnormal PLT results, suggesting a stronger signal of association.Overall, the PSSA results and estimates of the incidence of drug-related adverse events were generally congruent.However, it should be noted that PSSA is merely a signal mining method that implies correlation rather than causation.The positive signals found by the PSSA should be further validated by prospective studies.Furthermore, because PSSA may produce false negative results,the negative signals found in this study cannot yet be disregarded.
STRENGTHS AND LIMITATIONS
This study has the major advantage of having a sufficient sample size of real-world patients, allowing for relatively accurate incidence estimations.To acquire population characteristics, drug use patterns, and safety data closely resembling the actual drug use scenarios, particular populations, such as elderly individuals, were included in the study, and the follow-up period was longer than that considered in typical prospective studies.Patient data were extracted from six tertiary hospitals in two provinces, improving representativeness and extrapolation of the results.In addition, an innovative aspect of this study is the use of multiple methods to detect adverse events, ensuring thorough and accurate identification of outcomes.This study also provides a methodological reference for drug safety research using real-world retrospective data.
However, there are certain limitations to this study.Firstly, we adopted a retrospective design, and the information about adverse events that could be extracted from electronic medical record data was constrained and was thus not as comprehensive as the data collected in prospective studies.For example, no treatment for adverse reactions could be obtained, and the patient’s smoking and drinking histories were largely missing.Limited by the data quality of some centers, the admission and discharge diagnoses were indistinguishable.Secondly, the limited number of patients with test results before and after drug administration was insufficient to conduct PSSA for some adverse events.Last but not least, the amount of data did not support the exclusion of patients from the EDV group whose discharge diagnosis included intracerebral hemorrhage.A larger retrospective study or one covering a larger geographic area would have provided a stronger basis for evaluating the safety and efficacy of the drug in a real-world setting.
CONCLUSIONS
The NBP group had significantly lower incidence levels of the following 15 laboratory tests compared to the EDV group: GGT, ALT, and AST, which are relatedto liver function; low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and total cholesterol, which are related to blood lipids; total bilirubin, alkaline phosphatase, PT, C-reactive protein, D-dimer, creatine kinase, procalcitonin, glucose (venous blood), and PLT.The NBP group resembled a lower incidence of abnormal values for 13 tests in comparison to the third group, with statistically significantly different results.The results of the multivariate logistic regression equations revealed that NBP was not a risk factor for abnormal laboratory test results.PSSA demonstrated that no safety signal was found for NBP.In conclusion, within the context of this study, NBP is safer than EDV when administered to a large population in the real world.
ACKNOWLEDGMENTS
This study was supported by the China Society for Drug Regulation (CSDR) Project: exploration of criteria for post-marketing risk assessment of medications using real-world data (DRM2021008), and the Special Clinical Pharmacy Program of Shandong Medical Association (YXH2022ZX004).All authors had no conflicts of interest to disclose.
 Journal of Geriatric Cardiology2023年4期
Journal of Geriatric Cardiology2023年4期
- Journal of Geriatric Cardiology的其它文章
- Characteristics and in-hospital mortality of elderly patients with heart failure in Spanish hospitals
- Evaluation of metoprolol standard dosing pathway in Chinese patients with acute coronary syndrome: a prospective multicenter single-arm interventional study
- Feasibility and clinical benefits of the double-ProGlide technique for hemostasis after cryoballoon atrial fibrillation ablation with uninterrupted oral anticoagulants
- Minimally invasive valve surgery: pushing boundaries over the eighty
- Prevalence and incidence of heart failure among community in China during a three-year follow-up
- How to effectively manage the refractory coronary thrombus? A systemic mini-review
