Evaluation of metoprolol standard dosing pathway in Chinese patients with acute coronary syndrome: a prospective multicenter single-arm interventional study
Xiao-Yun YIN, Yun-Mei ZHANG, Ai-Dong SHEN, Jing-Ping WANG, Zhe-Xun LIAN,Yi-Bing SHAO, Wen-Qi ZHANG, Shu-Ying ZHANG, Yang ZHENG, Kang CHENG,Biao XU, Cheng-Xing SHEN, Rong-Chong HUANG, Jin-Cheng GUO, Guo-Sheng FU,Dong-Kai SHAN, Dan-Dan LI,✉, Yun-Dai CHEN,✉
1.Senior Department of Cardiology, the Sixth Medical Centre, Chinese PLA General Hospital, Beijing, China; 2.Medical School of Chinese PLA, Beijing, China; 3.Department of Cardiology, Yunnan First People’s Hospital, Kunming, China; 4.Department of Heart Center, Beijing Friendship Hospital, Capital Medical University, Beijing, China; 5.Department of Cardiology, Shanxi Cardiovascular Hospital, Taiyuan, China; 6.Department of Cardiology, the Affiliated Hospital of Qingdao University, Qingdao, China; 7.Department of Cardiology, Qingdao Municipal Hospital, Qingdao, China; 8.Department of Cardiology, China-Japan Union Hospital of Jilin University, Changchun, China; 9.Department of Cardiology, the Affiliated Zhongshan Hospital of Dalian University, Zhongshan, China; 10.Cardiovascular Center, the First Hospital of Jilin University, Changchun, China; 11.Department of Cardiology, the Affiliated Hospital of Northwest University & Xi’an No.3 Hospital, Xi’an, China; 12.Department of Cardiology, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, China; 13.Department of Cardiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai,China; 14.Department of Cardiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China; 15.Department of Cardiology, Beijing Luhe Hospital, Capital Medical University, Beijing, China; 16.Department of Cardiology, School of Medicine, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, China
✉ Correspondence to: cardio_lidandan@163.com (LI DD); cyundai@vip.163.com (CHEN YD)https://doi.org/10.26599/1671-5411.2023.04.001
ABSTRACT OBJECTIVE To evaluate the feasibility and tolerability of metoprolol standard dosing pathway (MSDP) in Chinese patients with acute coronary syndrome (ACS).
Acute coronary syndrome (ACS), including ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction, and unstable angina pectoris, is a serious manifestation of coronary heart disease (CHD)with high mortality.[1]Coronary revascularization, antimyocardial ischemia, anticoagulation, and antiplatelet therapy, as well as heart rate (HR) control, were considered as common effective treatment to restore myocardial oxygen supply, improve cardiac function, relieve symptoms and reduce mortality.Earlier studies revealed that elevated HR was an independent risk factor for long-term cardiovascular events, particularly for heart failure (HF) and all-cause death.[2-5]HR reduction decreases myocyte oxygen consumption, prolongs diastolic perfusion time and improves coronary flow reserve.It may be a key point in improving the long-term prognosis of patients.[6]Thus, a target HR of 55-60 beats/min was recommended as an important therapeutic goal for ACS by the literature and expert consensus.[7,8]
However, there are still regional differences and clinical practice differences in HR control.In clinical practice, target HR was generally not achieved.[9,10]As clinical routine, beta-blockers are essential medicine for controlling HR which can reduce mortality, particularly sudden cardiac death, and re-infarction in the acute phase of ACS.[11,12]To achieve faster HR control, the metoprolol titration method was proposed by the 2013 ACCF/AHA guidelines and was considered to be the optimal treatment strategy in ACS patients.[13]The 2020 ESC guidelines recommend early initiation of beta-blocker treatment in the acute phase of non-ST-segment elevation ACS which was recommended in patients with ongoing ischemic symptoms and without contraindications.[14]However, the usage of metoprolol and the standard titration method were various in clinical practice for ACS patients.A standard and normalizing metoprolol titration method was still lacking.Therefore, the metoprolol standard dosing pathway (MSDP) was proposed that it should be initiated early and titrated to the target dose of metoprolol step-by-step during hospitalization for ACS patients, and continue to titrate to maximum tolerated dose after discharge and maintain.
Therefore, completing the MSDP is considered to be a crucial factor in achieving the target HR.Up to now,the feasibility and tolerability of the pathway in Chinese ACS patients have not been verified.Thus, the study aimed to evaluate the feasibility and tolerability of this clinical pathway of Chinese ACS patients.The hypothesis was that part of Chinese ACS patients could complete this clinical pathway to achieve the target dose (95 mg/d), and achieve good HR control with only a few serious adverse event (SAE).
METHODS
Study Design and Participants
This was a multicenter, prospective, open label, single-arm and interventional study.[15]The study protocol was approved by the Institutional Review Board of Chinese PLA General Hospital (No.S2017-112-01) and the Clinical Trial Number: NCT03413410.Patients aged ≥ 18 years who were diagnose as ACS and admitted to the cardiology departments of fifteen Chinese hospitals distributed in various regions from February 2018 to April 2019 were enrolled.Exclusion criteria: (1) betaagonists use; (2) adverse events such as cardiac shock, unstable HF, hypotension [blood pressure (BP) < 90/60 mmHg], and bradycardia (HR < 50 beats/min), sick sinus syndrome, atrioventricular block II-III, Killip class >II, suspected acute myocardial infarction with HR < 45 beats/min, P-R interval > 0.24 s or systolic BP (SBP) < 100 mmHg; (3) patients allergic or contraindicated for metoprolol; (4) cancer patients; (5) patients at a risk of developing cardiogenic shock; and (6) pregnant or lactating women.The study cohort creation process is illustrated in Figure 1.
MSDP
All participants received standard treatment recommended by international guidelines, and on this basis,they received guidance of the MSDP.According to the ACCF/AHA guidelines, ACS patients who do not have contraindications should be prescribed to use beta-blockers and achieve the maximum tolerated dose subsequently.[13,16]For the present study, clinical signs were evaluated before beta-blocker administration and within 48 h of enrollment, and up-titration (the amount of which was determined by the clinician based on clinical experience) occurred only after the following parameters were met: HR ≥ 45 beats/min without symptomatic bradycardia, SBP ≥ 100 mmHg, and no signs of acute HF.After the first 48 h, evaluation was performed daily, and the dose increased when appropriate.[15]Metoprolol tartrate(25 mg) and metoprolol succinate (47.5 mg) were both acceptable for prescribing to all patients without any randomization every 6-12 h within the first 48 h following admission.The dose was then increased to 95-190 mg/d and decreased or discontinued when necessary.[15]If the patient was intolerant, the dose was reduced or the administration was stopped altogether.
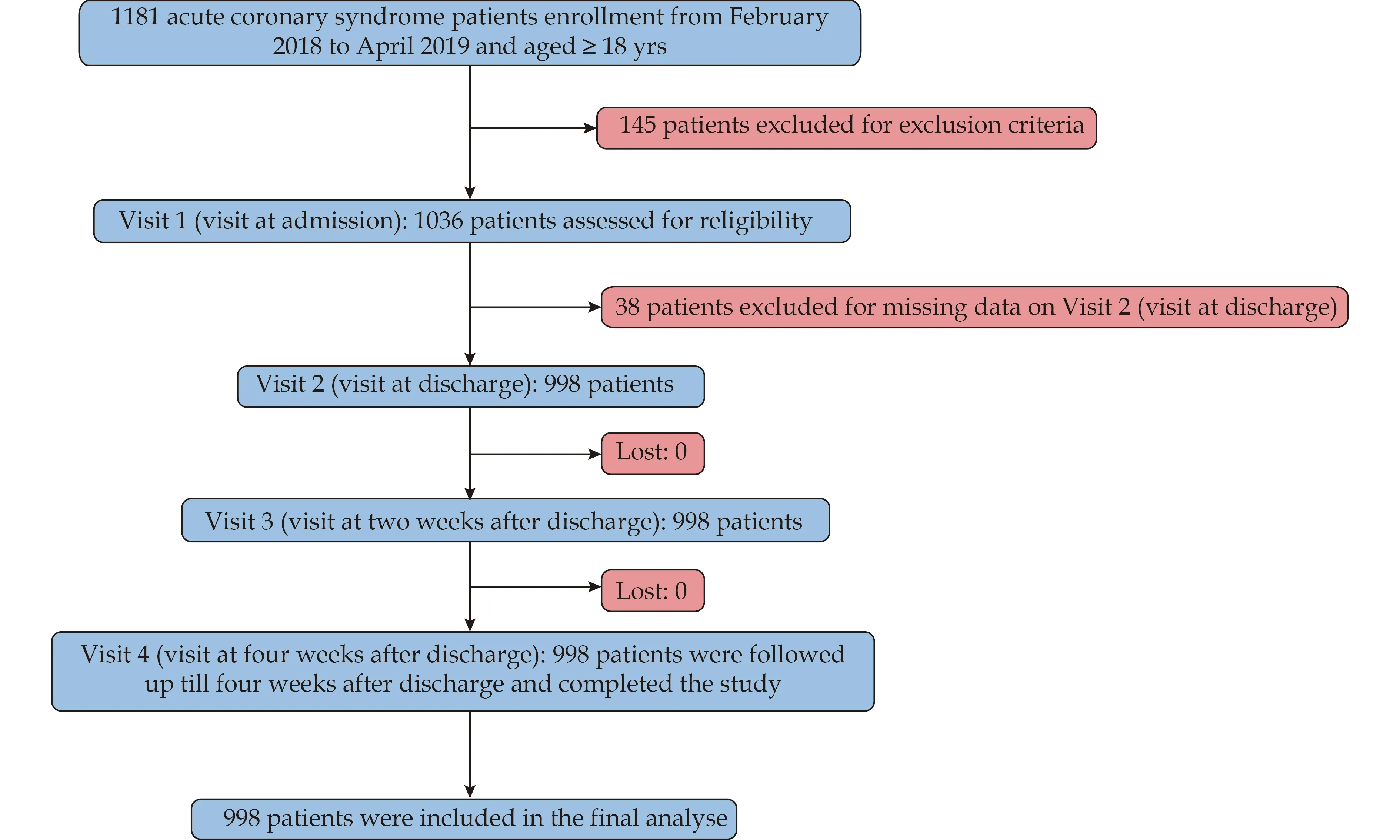
Figure 1 Flow chart of the enrollment process.
Target dose was defined as metoprolol succinate with 95 mg/d or higher.Standard-reaching rate of dose referred to the ratio of patients with a dose of metoprolol ≥95 mg at corresponding time.Target HR was defined as HR of 55-60 beats/min, bradycardia as HR < 50 beats/min, and hypotension as BP < 90/60 mmHg.Standard-reaching rate of HR was defined as the ratio of patients with HR in 55-60 beats/min at corresponding time.
HR and BP Monitor
In acute phase, BP was monitored using an electronic device (Cardiocloud Medical Technology, Beijing, China).The devices were calibrated before the first use and would be routinely calibrated every month thereafter.After the acute phase and during hospitalization, electrocardiogram was used to acquire HR parameter; and before discharge, patients were instructed how to self-administer the BP device.After discharge, patients self-monitored their HR and BP parameters.The HR and BP were assessed three times each day both during hospitalization and after discharge.[15]
Visit
The main flow chart of research procedures is demonstrated previously.[15]All patients were visited according to the four time points: admission (V1), discharge (V2),two weeks after discharge (V3) and four weeks after discharge (V4).Data for V1 and V2 were acquired from the inpatient data and those for V3 and V4 were acquired through telephone calls.
Study Endpoints
The primary endpoint was the percentage of patients achieving target metoprolol dose at V2.The secondary endpoints included the mean HR and BP at V2 and V4, and the percentage of patients experiencing bradycardia, hypotension and transient cardiac dysfunction at V2 and V4.Figure 2 elaborates on the study procedure including the visit schedules of patients and dose titration of metoprolol.
According to the administered dose of metoprolol at V2, the patients were divided into two groups: target group included patients achieving the target dose at V2,and non-target group included patients not achieving the target dose at V2.We performed data analysis on the difference of outcome indicators between the two groups.

Figure 2 Study procedure with visit schedules of patients and dose titration of metoprolol.
Statistical Analysis
Data were presented as counts (percentages) for categorical variables and mean ± SD for continuous variables.For numerical variables, in group comparison, two independent samplest-test was used with normal distributions; otherwise, the Mann-WhitneyUtest was used for non-normal distribution.Within the group, pairedttests were used for normal distribution; otherwise, the Wilcoxon-Mann-Whitney test was used for non-normal distribution.Differences between categorical variables were analyzed using the Pearson’s chi-squared test or Fisher’s exact probability test.Two-sidedP-value < 0.05 were considered statistically significant.Statistical analysis was performed using SPSS 24.0 (SPSS Inc., IBM,Armonk, NY, USA).
RESULTS
Baseline Characteristics
A total of 998 patients were included, with a mean age of 60.11 ± 10.61 years.294 patients achieved the target dose at V2 and 704 patients did not.Demographic and baseline characteristics of the study patients are shown in Table 1.Patients in the target group were younger, had higher baseline HR and higher frequency of previous diabetes mellitus and chronic kidney disease (allP< 0.05).There were no significant differences in sex, body mass index, BP, ACS classification, surgical history, or left ventricular ejection fraction (LVEF) between the two groups.
Feasibility of the MSDP
The proportion of the entire study population achieving the target dose at V2 was 29.46%.The proportion of doses of 190 mg or more was only 0.90%.The dose of betablocker was 63.22 ± 29.71 mg/d at V2 and 61.92 ± 29.65 mg/d at V4.At V4 visit point, 705 patients (70.64%)maintained the same dose as that at V2, 73 patients (7.31%)increased, 107 patients (10.72%) reduced and 113 patients (11.32%) stopped taking metoprolol.The main reasons for patients’drug reduction or increase were following the doctors’advice (38.42%), or self-adjustment(35.56%).
The values of HR and BP of patients at each visit point are shown in Figure 3.In target group, HR at V4 was significant lower than that of V2 (69.87 ± 9.33 beats/minvs.72.21 ± 8.40 beats/min,P< 0.0001); SBP at V4 was not significantly different from that at V2 (125.84 ± 16.18 mmHgvs.123.64 ± 13.15 mmHg,P= 0.606), but diastolic BP at V4 was significantly higher than that at V2 (78.28 ±10.90 mmHgvs.74.27 ± 9.17 mmHg,P< 0.0001).For inter-group comparison, the SBP at V2 was significantly lower in target group than that in non-target group.There was no significant difference in diastolic BP at V2 and no significant difference in both SBP and diastolic BP at V4 between two groups.BP in target group was not lower than normal criteria at any visit point.The distributions of HR and BP parameters at each visit point is shown in Figure 4.Surprisingly, only 6.48% of the overall study population achieved the target HR of 55-60 beats/min atV2, and the proportion of patients with HR of 55-60 beats/min increased continuously after discharge and was 17.99% at V4.However, most patients’HR were above normal criteria standard at each visit point.

Table 1 Demographic and baseline characteristics of the study cohort.
Of the overall patients, the mean HR decreased from 74.52 beats/min at baseline to 71.15 beats/min at discharge (mean difference: -3.37,P< 0.0001) (Table 2).In target group, the mean HR decreased from 77.17 beats/min at baseline to 72.21 beats/min at discharge (mean difference: -4.97,P< 0.0001).In non-target group, the mean HR decreased from 73.41 beats/min at baseline to 70.70 beats/min at discharge (mean difference: -2.70,P< 0.0001).Moreover, there was a statistical difference between target group and non-target group in the reduction of HR(P= 0.034) (Figure 5).
Tolerability of MSDP
Focusing on the tolerability of MSDP, no patient suffered bradycardia at V2 (Figure 4).The rate of bradycardia at V4 was 0.810% (2/247) in target group and 0.334%(2/598) in non-target group (P= 0.715).The proportion of patients with hypotension at V2 was 0.004% (1/276)in target group and 0.004% (3/682) in non-target group(P= 1.000).No hypotension occurred in target group and 0.005% (3/606) in non-target group at V4 (P= 0.560).Regardless of group, no patient suffered transient cardiac dysfunction at V2 or V4.
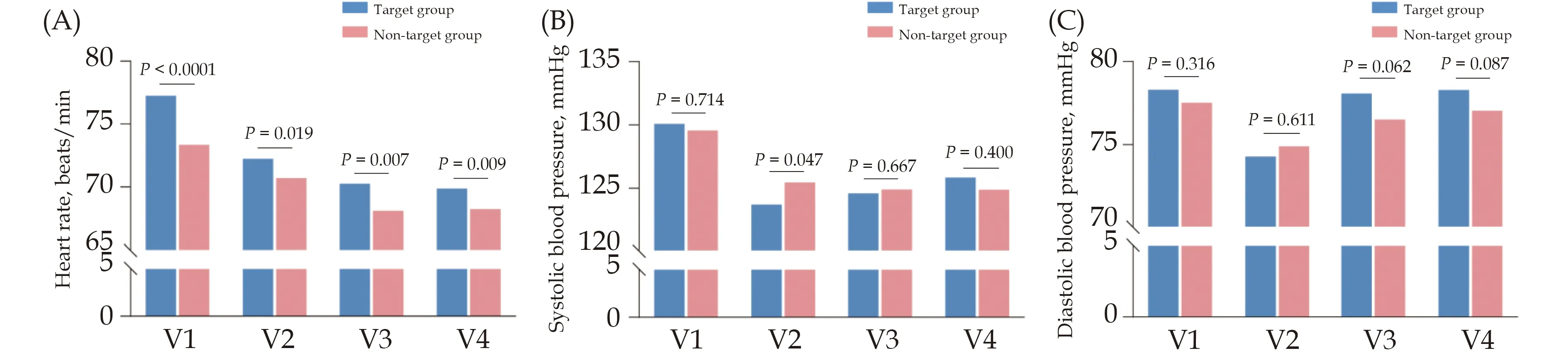
Figure 3 Heart rate and blood pressure values of target group and non-target group at V1, V2, V3 and V4.(A): Heart rate values of target group and non-target group at V1, V2, V3 and V4; (B): systolic blood pressure values of target group and non-target group at V1,V2, V3 and V4; and (C): diastolic blood pressure values of target group and non-target group at V1, V2, V3 and V4.Target group referred to patients achieving target dose at discharge.Non-target group referred to patients not achieving target dose at discharge.V1:admission; V2: discharge; V3: two weeks after discharge; V4: four weeks after discharge.

Figure 4 Heart rate and blood pressure distribution of target group and non-target group at V1, V2, V3 and V4.(A): Heart rate distribution of target group and non-target group at V1, V2, V3 and V4; (B): systolic blood pressure distribution of target group and nontarget group at V1, V2, V3 and V4; and (C): diastolic blood pressure distribution of target group and non-target group at V1, V2, V3 and V4.Target group referred to patients achieving target dose at discharge.Non-target group referred to patients not achieving target dose at discharge.V1: admission; V2: discharge; V3: two weeks after discharge; V4: four weeks after discharge.

Table 2 Primary efficacy outcomes.
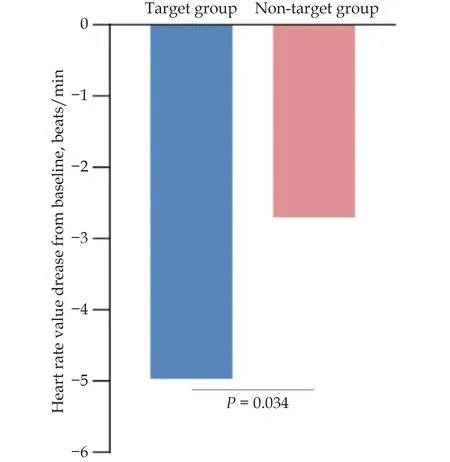
Figure 5 The decrease of heart rate value from baseline to discharge in target group and non-target group.Target group referred to patients achieving target dose at discharge.Non-target group referred to patients not achieving target dose at discharge.
Safety situation is shown in Table 3.A total of five adverse events (1.70%) and one SAE (0.34%) were determined to be related to the MSDP in target group.Only one SAE lead to death due to cardiovascular disease but was not related to MSDP in target group.There was no significant difference in the incidence of adverse events and SAEs between the two groups.The most frequently reported adverse events in the two groups were general disorders and administration site conditions (4.42%vs.3.13%,P= 0.310), respiratory system, chest and mediastinum diseases (2.72%vs.4.12%,P= 0.310), neurological adverse events (2.38%vs.3.84%,P= 0.310), cardiac diseases (1.70%vs.1.14%,P= 0.310), metabolic and nutritional diseases (1.70%vs.0.43%,P= 0.310), and gastrointestinal adverse events (1.02%vs.1.70%,P= 0.310).
DISCUSSION
In the present study, of the included ACS patients,29.46% of them achieved the target dose and 6.48% of them achieved the target HR at discharge by following the MSDP.To the best of our knowledge, this is the first time to evaluate this MSDP clinical pathway in Chinese ACS patients.After MSDP treatment, HR of all ACS patients decreased significantly from baseline to discharge.In addition, HR in target group decreased more significantly than that in non-target group.At four weeks after discharge, the proportion of patients whom HR achieved the standard increased to 17.99%.A small part of patients continued to follow this pathway for dose titration after discharge, achieving effective HR control.In target group, the incidence of bradycardia, hypotension, and transient cardiac dysfunction was indeed low;and only a small few SAEs were related to the MSDP during the study period.Our results indicated that in the Chinese ACS patients, the feasibility and tolerability of the clinical pathway are acceptable.
HR is one of the independent predictors of cardiovascular mortality in patients with CHD.[17-20]Elevated HR may increase the mortality of ACS patients in the percutaneous coronary intervention era.[21]Substantialstudies confirmed that poor HR management was associated with cardiovascular morbidity and mortality of ACS patients.[21-24]Therefore, it is necessary to implement strict HR control in these patients.Beta-blocker,which is considered as one of the most commonly used drugs to control HR, could lead to decreased myocardial contractility, reduction in myocardial oxygen demand and decreased frequency of life-threatening ventricular arrhythmias.[9,25]The lower HR allows for a prolonged diastolic phase, which subsequently could improve left ventricular diastolic function and coronary blood supply.[26]The clinical practice guidelines of the American Heart Association and the European Heart Association strongly recommended beta-blockers in patients with acute myocardial infarction with LVEF ≤ 40%(class IA).[14,16,27]In the current study, the LVEF of ACS patients is about 58%, which is normal.Although recognizing the gap in evidence and the need for further research, the two scientific groups also generally advocate the use of beta-blockers in patients with LVEF > 40%.[14,27]The recent REBOOT (tREatment with Beta-blockers after myOcardial infarction withOut reduced ejection fracTion) trial, which is a practical, controlled, prospective, randomized, open label, blind end clinical trial, tests the benefits of blocker treatment for myocardial infarction patients with LVEF > 40% after discharge and managed according to state-of-the-art practice.It is expected that the results could clarify the efficacy of beta-blockers in these patients.[28]The ACS guidelines and Chinese expert consensus emphasized that beta-blockers should be initiated early, titrated to the maximum tolerated dose, and maintained for long-term in ACS patients.[8,13,14,16,27]However, in clinical practice, HR control in ACS patients remains unsatisfied.[9,10]

Table 3 Safety assessment of acute coronary syndrome patients treated according to metoprolol standard dosing pathway.
In recent decades, metoprolol has always been recommended by international guidelines for ACS, and the utilization rate of metoprolol in ACS patients has been significantly improved.Oral beta-blocker therapy was administered to 56.5% of STEMI patients within 24 h after admission.[29]In another study, beta-blocker utilization rates following myocardial infarction were 93.2%at discharge.[30]Notably, the focus on the utilization rate of beta-blockers has shifted to the focus on the using methods recent years.The 2013 ACCF/AHA guidelines for STEMI stated that the oral dosage of metoprolol tartrate is 25-50 mg/6-12 h.After 2-3 days, metoprolol tartrate (twice a day) or metoprolol succinate (once a day)could be administered.If this dose is well tolerated, it can be titrated up to 200 mg/d (metoprolol tartrate) or 190 mg/d (metoprolol succinate).[13]The 2020 ESC guidelines for non-ST-segment elevation myocardial infarction has indicated that oral beta-blockers should be initiated in the first 24 h after admission and continued after discharge in patients with STEMI without contraindications.[14]However, due to lack of a specific clinical pathway as a guide, the specific usage of metoprolol still was a practical challenge for clinicians, since the use of beta-blockers was not standardized in terms of dosage and titration method.[31-33]
The lack of recognition and acceptance of the specific MSDP may result in different practice patterns in different institutions, often fostering potential sub-optimal HR management with clinicians’conservative betablocker titration.The reasonable dosage of metoprolol in ACS patients has been controversial in different reports.In several prior classical randomized trials performed decades ago, most patients did achieve the target dose (200 mg metoprolol tartrate equivalent to 190 mg metoprolol succinate),[34-37]and only a small excess in symptomatic fatigue and sexual dysfunction was reported.[38]Nevertheless, in routinely clinical practice, the beta-blocker doses used were significantly lower than those used in these pivotal trials, with most doses lower than 50%of 200 mg metoprolol tartrate.[30,39-42]Besides, the dose of beta-blockers in CHD patients in Chinese clinical practice is unsatisfactory in a recent study, which was 46 ±23 mg at discharge, lower than that recommended in the guidelines, brought about a relatively low standard-reaching rate of HR (48%), not conducive to the longterm prognosis.[33]Multiple reasons for underdosing of beta-blocker therapy in the clinical setting existed, including the clinician’s personal clinical experience, the doctor’s concern about the possible adverse effects of the target dose and the patient’s adherence to the clinician’s advice.[30,40]On the other side, several studies do not support the advantage of higher doses over lower doses.A previous trial showed that higher dose of beta-blocker therapy did not improve two-year survival compared with lower doses in myocardial infarction patients.[43]Further, a recent study showed that the incidence of major adverse cardiac events was similar in patients with ACS using high-dose and low-dose beta-blockers.[44]In present study, given the usage status of metoprolol in China, as well as the controversy over the optimal dose of metoprolol in previous studies, it can also be explained why the target dose of metoprolol was defined as medium level (50% of the recommended dose) in the present study, which was 95 mg of metoprolol succinate.
The titration of metoprolol in patients with ACS is more standardized by using the MSDP.For the PEACE (China Patient-centered Evaluative Assessment of Cardiac Events) study in China, among acute myocardial infarction patients treated with metoprolol, 73.6% received a cumulative dosage of no more than 25 mg in the first 24 h of admission, and only 4.0% received > 50 mg.[32]With the use of the MSDP, metoprolol tartrate (25 mg)and metoprolol succinate (47.5 mg) were prescribed to all ACS patients every 6-12 h within the first 48 h following admission.On the other hand, excessively rapid dose titration of metoprolol should also be avoided.The COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) trial demonstrated that in post-myocardial infarction patients whose dose was escalated rapidly during the in-hospital phase following myocardial infarction.The specific fashion of administration was early intravenous administration of metoprolol, and sustainedrelease tablets 200 mg/d would be given from the next day.However, there was no net benefit in this way.[35]Similarly, in the POISE (PeriOperative ISchemic Evaluation) trial, patients undergoing non-cardiac surgery started taking oral extended-release metoprolol 200 mg,12 h after the first postoperative dose.The results showed too rapid titration to target dose therapy could bring both beneficial (less myocardial infarctions) and harmful (more deaths and stroke) effects.[45]Therefore, it may be more beneficial to follow a step-by-step titration pathway.
In earlier studies, HR recommended by the guidelines was reported difficult to achieve.[9]In the present study, in hospitalized ACS patients, the standard-reaching rate of HR was 6.48%, which was distinctly lower than that in the earlier study (48.3%) and the average use dose of beta-blockers used was 63.22 ± 29.71 mg/d at discharge, which was much higher than that in the earlier study (46 ± 23 mg/d) in CHD patients.[33]Possible explanations might be related to different diseases of patients in each study, medical advice from clinicians, and differences in patients’adherence.Similar to another previous study in ACS patients, only 5.3% of patients achieved an average HR of 50-60 beats/min throughouta the hospital stay, with metoprolol averaged 58 mg at admission and 88 mg at discharge.[9]In present study,the HR of all ACS patients decreased significantly from baseline to discharge after applying the MSDP (Table 2).Moreover, the decrease of HR was more obvious in target group than in non-target group (Figure 5), which means that completing the MSDP may bring better clinical benefits.Although only about a third of patients(29.46%) achieved the target dose at V2, it still reveals that this MSDP can be completed in Chinese ACS patients and can effectively control HR.
In terms of evaluating patient adherence after discharge, 70.64% of the patients maintained the dose given at discharge, and about 22% of the patients reduced or stopped the drug, indicating that they did not continue to follow the MSDP of up-titration.This is similar to previous research results.In a previous study, between discharge and three weeks, 76.4% had no change in beta-blocker dose in myocardial infarction patients.[30]In another study, 80% of myocardial infarction patients continued to take beta-blocker within 30 days after discharge, which dropped sharply to about 60% around 30 days, and maintained for one year.[46]Nonetheless, in the present study, more patients’HR achieved the target at four weeks after discharge (17.99%) than that at discharge (6.48%), which may be attributed to 7.31% of patients increase the dose and 70% of patients maintained the dose.The above showed that this pathway was effective and well worth promoting, but the titration was often less than desirable in the outpatient setting simultaneously.Besides, both a larger population base and more limited medical resources in China lead to a shorter average hospital stay than in western countries.Previous studies have shown that the median hospital stay of ACS patients in China was nine days, and in the present study, it was seven days among all patients.[47,48]Therefore, it appeared to be increasingly imperative for ACS patients that beta-blocker therapy should be initiated early and titrated rapidly and stably to the target dose (95 mg) following the MSDP in a short hospital stay.
In view of the non-standard administered dose of metoprolol, clinicians’individualized difference of practice mode, the non-standard titration method, and the current situation of unsatisfactory HR control in ACS patients, we propose the MSDP.The MSDP helps clinicians to more standardize the use of metoprolol in these high-risk ACS patients, and has been proved to be effective, worth persisting and promoting after discharge.Based on the efficacy of completing the pathway and the patients’adherence to the pathway, it was concluded that the feasibility of the clinical pathway was acceptable.
On the aspect of tolerability profile, during the study period, the incidence of bradycardia, hypotension, and transient cardiac dysfunction was relatively low, and only a small proportion of adverse events (2.51%) and SAEs (0.10%) were related to MSDP in target group.This result indicated that the application of MSDP in Chinese ACS patients was relatively safe.These findings provided sufficient evidence to eliminate clinicians’concerns regarding the application of MSDP, patients’concerns about following clinicians’advice, and promote metoprolol up-titration in the clinical pathway.The MSDP provides Chinese clinicians with specific operation steps supported by evidence to standardize the management of ACS patients in the clinical setting, and enable them to titrate to the target dose of 95 mg as soon as possible during hospitalization.
LIMITATIONS
This study still had several limitations that must be noted.Firstly, the follow-up of the study lasted only four weeks after discharge; therefore, long-term benefits and adverse events could not be assessed after the completion of the MSDP.Therefore, long-term follow-up outside the hospital should be strengthened.Secondly, although the MSDP was proposed in present study, clinicians often have their thought in clinical practice, and we could not control for variations in practice patterns of individual clinicians.Besides, patients also have their considerations about following clinicians’advice.Therefore,whether a patient’s final dose achieves the target is affected by a variety of factors.Given this, a large-scale prospective randomized controlled trial is necessary to validate these results.Last but not least, the number of patients achieving 190 mg or more was too small to evaluate the efficacy and side effects of these high doses.In future research, strict training of clinicians on the implementation steps, methods and requirements of the MSDP needs to be provided.Interviews on the patient requirement and clinicians’reflections on the application of the clinical pathway need to be added.Sub-group analysis of patients with 190 mg or more should be conducted to evaluate the efficacy outcomes with high dose.
CONCLUSIONS
In Chinese ACS patients, the feasibility and tolerability of the MSDP have been proved to be acceptable, and this pathway can be popularized in clinical practice.This study confirmed that part of Chinese ACS patients can complete this clinical pathway to achieve the target dose,and achieve effective HR control with only a few SAEs.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (2016YFC1300 300).All authors had no conflicts of interest to disclose.
 Journal of Geriatric Cardiology2023年4期
Journal of Geriatric Cardiology2023年4期
- Journal of Geriatric Cardiology的其它文章
- Characteristics and in-hospital mortality of elderly patients with heart failure in Spanish hospitals
- Feasibility and clinical benefits of the double-ProGlide technique for hemostasis after cryoballoon atrial fibrillation ablation with uninterrupted oral anticoagulants
- Minimally invasive valve surgery: pushing boundaries over the eighty
- Prevalence and incidence of heart failure among community in China during a three-year follow-up
- Safety of butylphthalide and edaravone in patients with ischemic stroke: a multicenter real-world study
- How to effectively manage the refractory coronary thrombus? A systemic mini-review
