Effect of maternal pregestational diabetes mellitus on congenital heart diseases
Zhi-Yan Chen · Shuang-Fa Mao · Ling-Hong Guo · Jian Qin · Li-Xin Yang · Yin Liu,,3,4
Abstract Background The increasing population of diabetes mellitus in adolescent girls and women of childbearing age contributes to a large number of pregnancies with maternal pregestational diabetes mellitus.Congenital heart diseases are a common adverse outcome in mothers with pregestational diabetes mellitus.However,there is little systematic information between maternal pregestational diabetes mellitus and congenital heart diseases in the off spring.Data sources Literature selection was performed in PubMed.One hundred and seven papers were cited in our review,including 36 clinical studies,26 experimental studies,31 reviews,eight meta-analysis articles,and six of other types.Results Maternal pregestational diabetes mellitus poses a high risk of congenital heart diseases in the off spring and causes variety of phenotypes of congenital heart diseases.Factors such as persistent maternal hyperglycemia,oxidative stress,polymorphism of uncoupling protein 2,polymorphism of adiponectin gene,Notch 1 pathway,Nkx2.5 disorders,dysregulation of the hypoxia-inducible factor 1,and viral etiologies are associated with the occurrence of congenital heart diseases in the off spring of mothers with pregestational diabetes mellitus.Treatment options including blood sugar-reducing,antioxidative stress drug supplements and exercise can help to prevent maternal pregestational diabetes mellitus from inducing congenital heart diseases.Conclusions Our review contributes to a better understanding of the association between maternal pregestational diabetes mellitus and congenital heart diseases in the off spring and to a profound thought of the mechanism,preventive and therapeutic measurements of congenital heart diseases caused by maternal pregestational diabetes mellitus.
Keywords Congenital heart disease · Maternal pregestational diabetes mellitus · Mechanism · Prevention
Introduction
Diabetes mellitus (DM) refers to a group of metabolic diseases characterized by hyperglycemia resulting from insulin secretion defect,insulin action disorder,or both.Population of global diabetes was estimated to be 463 million in 2019(corresponding to a prevalence of 9.3%) and to increase to 578 million (10.2%) by 2030 and 700 million (10.9%) by 2045 [1],among which diabetes in children,adolescents,and young adults keeps increasing at an alarming rate [2,3].Studies revealed a higher prevalence of diabetes in adults aged 20-22 years old (4.5%) [4],with a further 18%-29.3%of the young people having detectable pre-diabetes [4-6],not to mention those unaware cases.Among young people with diabetes,female adolescents and women of childbearing age make up a large portion [7,8],where 40% of females with diabetes were of reproductive age [9].This means that at the moment or in the near future there are or will be a large increasing number of females with diabetes facing childbearing need,which increases the potential risk of congenital diseases of the cardiovascular system,limbs,urogenital part,central nervous system,respiratory system,oral cleft,and other organ systems.Among these,the most common congenital disease is congenital heart disease(CHD) [10,11].
CHDs refer to the developmental disorders of the heart and great vessels.CHDs account for 0.9%-1% of all live births [12,13] and one-third of all major congenital malformations;therefore,CHDs are the most common birth defects worldwide [12,14].In addition,1% to 2% of the total population have bicuspid aortic valves [15].Extensive care and surgical interventions are required for CHD patients in the first year of life [16].The in-hospital costs for children with CHDs exceeded six billion dollars,making up 23% of the total hospital costs in 44 USA states [17].The survival rate of the patients with severe CHDs was about only 56%,and for those middle-aged and elder CHD survivors,it can become more complicated to care with the residual hemodynamic abnormalities despite the success of their earlier cardiac operative intervention [18].CHDs were commonly considered as the leading non-infectious factor to cause infant morbidity and mortality,representing 6% of neonatal death and 46% of death due to congenital anomalies,respectively[19,20].Thus,CHDs pose a significant burden to the world,both in the economy and in health care costs.
CHDs can result from genetics,epigenetic etiologies and environment factors,or the interaction of them.This has been corroborated by the confirmed association between CHDs and maternal factors of chemical exposure (alcohol,cigarette,etc.),nutrition deficiency,illness,and drugs during gestation [21,22].Maternal DM is a common risk factor for CHDs and is mainly composed of maternal pregestational diabetes mellitus (PDM) and maternal gestational diabetes mellitus (GDM).Pregnancy with DM diagnosed before pregnancy is called PDM;DM diagnosed during pregnancy is called GDM,which usually develops in the late second trimester of gestation [23,24].Heart is the first organ to develop in fetus,and the critical period of heart development is the 3rd-7th weeks of gestation [25].Thus,we can reasonably assume the effects of diabetic intrauterine environment on the fetus of mothers with GDM and PDM might be different.Various literatures have elucidated close association between maternal DM and CHDs in the off spring or between maternal PDM and congenial diseases in the fetus [26-28],but there is scant systematic information between maternal PDM and CHDs in the off spring.
This paper reviews cardiac development,risk of maternal PDM for CHDs in the off spring,research method of CHDs induced by PDM,the specific CHD phenotype,mechanisms of CHDs,and prevention of CHDs in the off spring of mothers with PDM.The content of the review contributes to a better understanding of the association between maternal PDM and CHDs in the off spring and to a profound thought of the preventive and therapeutic measurements of CHDs caused by maternal PDM.
Cardiac development
The heart is the first organ to function during human embryonic development [29].The population of cardiogenic mesoderm cells,proepicardium,and cardiac neural crest cells(CNCCs) have been widely identified as primarily distinct precursors in embryonic cardio-progenesis [30].Cardiac development is initiated at gastrulation on embryonic day 7.5 (E7.5) in mice and the corresponding day 14 in human gestation [Carnegie stage (CS) 7],followed by three-dimensional shape of the heart (an inverted Y) as a consequence of folding at the end of the 3rd-week CS9,to the well-septated heart chambers connected to the pulmonary trunk and aorta on E14.5 in mice and day 49 in human embryos [30-32].With the help of high-resolution magnetic resonance imaging and episcopic fluorescence image capture atlas,cardiac loop or looped heart tube could be observed at an estimated gestational age (EGA) of 6 4/7 weeks in human embryos,which is equivalent to CS13.The heart matures when the atrial and ventricular chambers expand into distinct subdivisions,recognized as the primitive left and right atria and presumptive left and right ventricles,respectively,at EGA7 5/7 weeks (CS17) in humans,with fully septated heart and maturing valves,observed at approximately EGA9 3/7 weeks [33].
Risk of maternal PDM for CHDs in the off spring in clinical studies
Off spring of mothers with both type 1 diabetes (T1D) and type 2 diabetes (T2D) had higher risk of CHD than of those without diabetes [10,34],with prevalences [95% confidence interval (CI)] of 5% (4.0-6.0),3% (1.5-4.5),and 1%(0.9-1.0),respectively [10].Maternal PDM are strongly associated with CHD in fetuses especially before the 7th week of gestation in human,and the high prevalence is about 5%-7% [27,35].In addition,off spring of mothers with pregestational T1D and T2D shared a similar risk of CHD at birth [36].The association between maternal PDM and rate of CHDs in fetuses is significantly stronger than that between maternal GDM and prevalence of CHDs [27].
As for maternal PDM,the risk of CHDs in the off spring differs with different maternal conditions.Mean glycated A1c hemoglobin (HbA1c) level in the first trimester was significantly associated with major congenital anomaly [37].The prevalence of major CHD phenotype progressively increased in the off spring of PDM mothers with poorer glycemic control (measured by HbA1c) during pregnancy,and the highest risk occurred with HbA1c ≥ 9.5% [10].A Danish nationwide cohort study comprehensively elucidated the critical role of maternal PDM complications in the risk of CHDs in the off spring [36].It showed that maternal PDM with complications poses a higher CHD risk in the off spring than that without complications.In addition,maternal PDM with one acute complication (coma or ketoacidosis) was more likely to cause CHDs than that with one chronic complication (retinopathy,nephropathy,vasculopathy,etc.),with the prevalences of 6.2% and 3.1%,respectively.Children of mothers with diabetic complications have increased a prevalence of early onset cardiovascular disease from childhood to early adulthood [38].Furthermore,no significant association was detected between the risk of CHD in the off spring and maternal time period at diagnosis,maternal age at disease onset,duration of diabetes,diabetes type before giving birth and year of delivery [11,36,37].There was no obvious association between risk of CHD in the off spring and the initial maternal PDM treatment (insulin or oral anti-diabetic medicine) [36].
Research methods used in studying maternal PDM-induced CHDs
Growing prevalence of CHDs induced by maternal PDM poses a heavy burden on society,and professional profound researches have been carried out.Clinical research is an efficient way to gather direct and primary data on human CHDs and maternal PDM.Various types of clinical studies,such as population-based cohort study [35,36],case-control study[39],cross-sectional study [40],and meta-analysis [27],have been reported on the association (especially in prevalence and phenotype,and maternal conditions) between maternal PDM and CHDs.
Experimental research is of great importance for maternal PDM-induced CHDs and mainly includes the abnormal developmental observation,the pathological mechanism,prevention,and intervention.Whole embryo culture of rodents allows direct observation of the growth of embryos outside the maternal uterus with tightly controlled culture conditions [16].Whole embryo culture also makes neural tube defects easily detectable [41].Thus,culture of CNCCs in high-glucose environment is appropriate to study the mechanism and intervention of CHDs caused by maternal PDM [42,43].In addition,a variety of animal model experiments have been implemented on CHDs caused by maternal PDM [44-46].Instead of embryo culture,animal models,particularly female mice induced by single or multiple intraperitoneal injections of streptozotocin with or without highfat diet,followed by mating with healthy male,stimulated CHDs more commonly in the off spring of mothers with PDM [41,44,46-49] (Table 1).
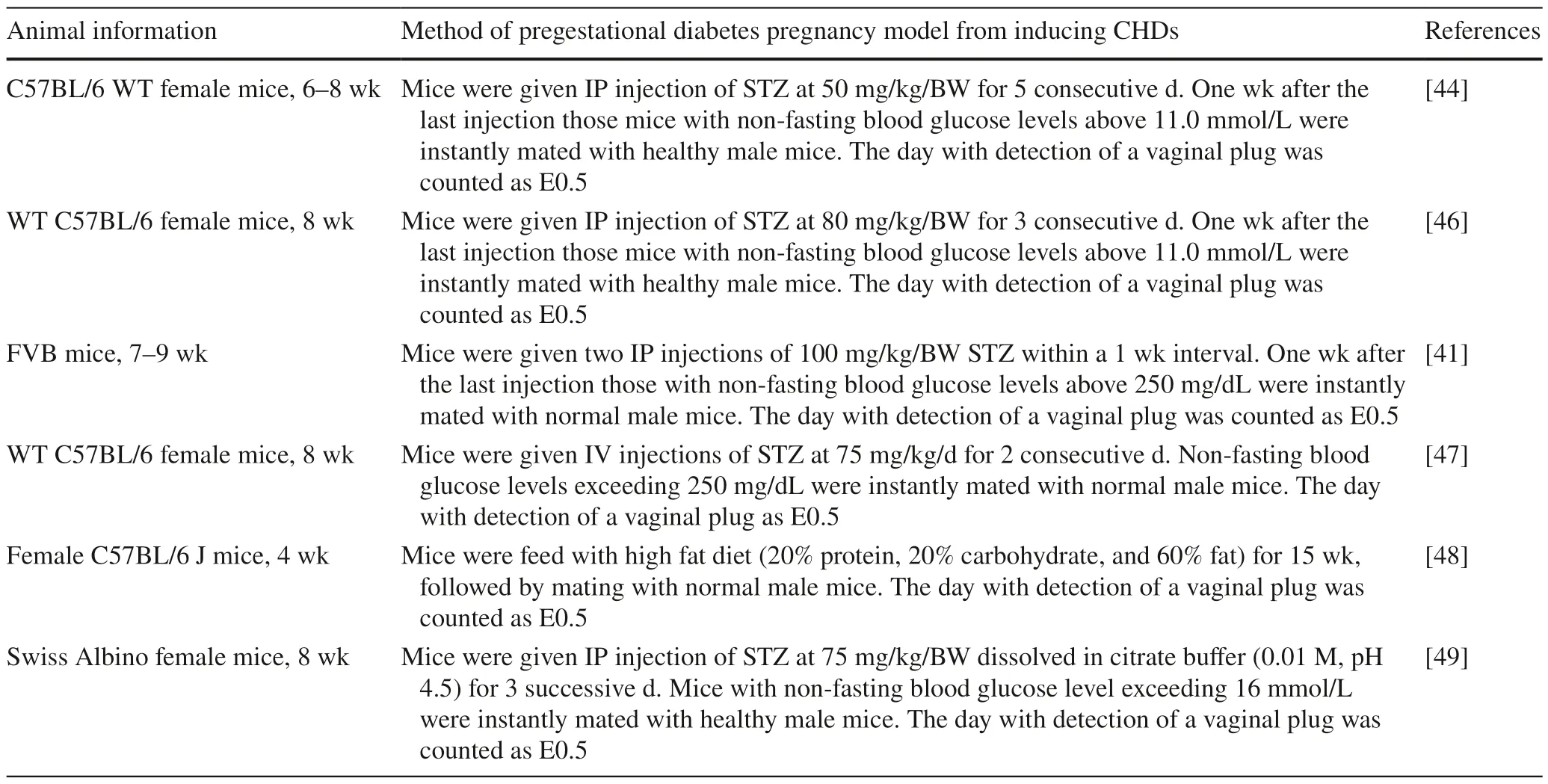
Table 1 Animal experimental research method of congenital heart diseases induced by maternal pregestational diabetes mellitus
CHD phenotypes and functional disorders in the off spring of maternal PDM
CHDs are featured mainly by developmental malformations of the cardiac chambers and abnormalities of the large blood vessels directly connected to the chambers [14,50].Common CHD phenotype mainly includes septal defects[ventricular septal defect (VSD),atrial septal defect (ASD)and atrioventricular septal defect (AVSD),etc.],conotruncal defects [d-transposition of the great arteries (d-TGA),tetralogy of Fallot (TOF),persistent truncus arteriosus (PTA),double-outlet right ventricle (DORV),etc.],chamber defects(cardiac hypertrophy,heterotaxia,single ventricle,etc.),left ventricular outflow tract obstruction (LVOTO: coarctation of the aorta,valvular aortic stenosis,etc.),right ventricular outflow tract obstruction (RVOTO: valvular pulmonary stenosis),valve defect,PDA,etc.
Most of the CHD phenotype occurred in the off spring of mothers with PDM in clinical studies.A population-based cohort study shows a high prevalence of CHD phenotype on VSD (1%),conotruncal defects (0.4%),ASD (0.3%),LVOTO (0.2%),RVOTO (0.2%),TGA (0.1%),AVSD(0.1%),TOF (0.1%),and others in the off spring of mothers with PDM [36].A meta-analysis showed prevalence of almost all CHD phenotype,with higher prevalence of VSD(2.1%) and ASD (1.8%) in the off spring of mothers with PDM [27].A case-control study showed that among 464 CHD off spring with maternal diabetic history,61.6% were diagnosed with VSD,28.0% with PDA,16.8% with ASD,10.8% with AVSD,5.6% with TOF,1.3% with aortopulmonary septal defect,and 0.4% with complete TGA (of note,some cases have been diagnosed with multiple subtypes of CHD) [39].The high risk of CHD phenotype in babies of maternal PDM above,particularly on VSD,PDA and ASD,was similar to another preceding comparable populationbased cohort study [11].Cardiac hypertrophy is a common CHD phenotype in off spring of PDM mothers,particularly when prenatal glycemia is poorly controlled [51].Interventricular septal hypertrophy is a frequent CHD phenotype in the off spring of mothers with maternal PDM,even though this malformation does not pose a risk to the fetuses or off spring unless it causes functional disorder [40].A retrospective cohort study revealed a high prevalence of cardiac hypertrophy (0.6%) among infants of diabetic mothers and a greater in-hospital mortality in patients with cardiac hypertrophy than in those without cardiac hypertrophy (4.9 vs.1.3%,P< 0.001) [52].
Most of the CHD phenotype occurred in the off spring of mothers with PDM in animal experimental studies.Pregestational maternal T1D significantly induced VSD(13%) and PTA (3.7%) in the developing heart of C57BL/6 J mice at E17.5 [48].Thickened inter-ventricular septum(IVS) and left ventricular free wall at E18.5 in the diabetic embryos in C57BL/6 J mice were reported compared to control,while these differences reduced at week 1 of age after birth [53].Another research reported that maternal PDM caused cardiac hypertrophy of the developing fetus in Kunming mice,where the thickness of the right ventricular anterior wall,the IVS,and the left ventricular posterior wall was significantly greater than that in the control group (P< 0.05,P< 0.001,P< 0.001);in addition,both the right ventricular internal diameter and the left ventricular internal diameter in the DM group were less than that in the control group(P< 0.001,P< 0.001) [54].Moazzen et al.reported a prevalence of 58% in CHDs,including VSD,ASD,TGA,DORV,and TOF,in the off spring of mice mothers with PDM [46].It was shown that CHDs (especially DORV and VSD) occurred in about 82% of the chick embryos with the CNCC exposed to elevated D-glucose [43] (Table 2).
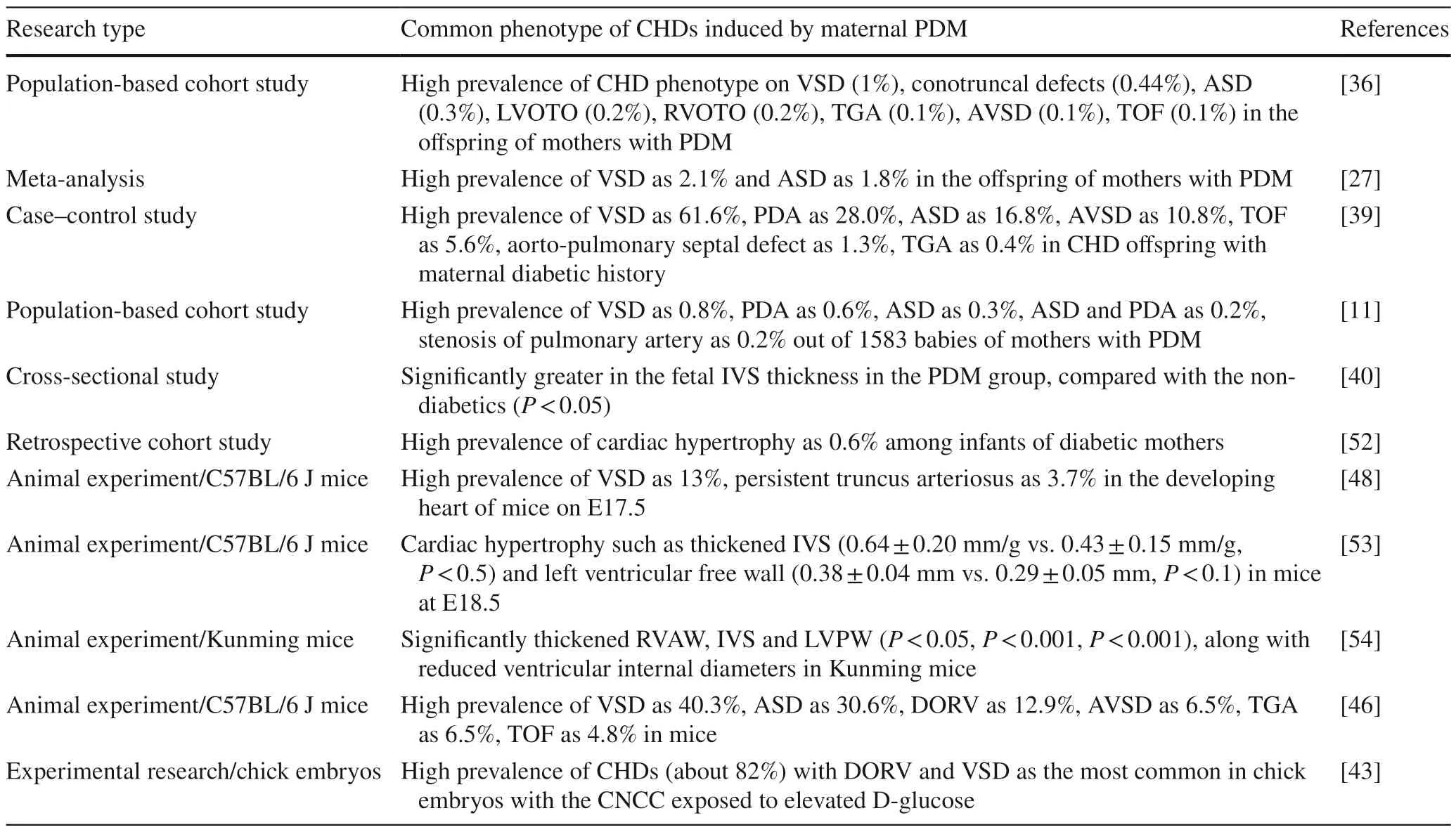
Table 2 Common phenotype of congenital heart diseases induced by maternal pregestational diabetes mellitus
The normal-structured heart and its adjacent blood vessels guarantee seamless physiologic function;thus,CHDs caused by maternal PDM might cause disordered cardiac function and systemic harm to the off spring.CHD is the most common cause of neonatal heart failure [55].Patients with VSD are at a risk of irreversible pulmonary hypertension [56] and may develop serious complications,such as aortic regurgitation and bacterial endocarditis to varying degrees [57].In addition,it was common for patients with ASD to suffer atrial arrhythmia,which might primarily cause recurrent stroke in these patients [58].Patients with PTA probably presented decreased myocardial contractility,resulting in decreased cardiac output[59].Reduced cardiac diastolic function [60],altered heart rate variability,and fetal academia [61] were observed in the neonates of mothers with PDM.Hypertrophy of the cardiomyocytes might gradually cause depressed left ventricular ejection fraction,atrial fibrillation,arrhythmic sudden death,and even heart failure [53,62,63].Furthermore,cardiac hypertrophy of the PDM fetal mice demonstrated diastolic dysfunction during early to mid-gestation [53] and presented lower stroke volume (1.64 ± 0.13 μL vs.2.22 ± 0.24 μL) and ejection fraction(78.42% ± 6.79% vs.86.65% ± 5.39%) than those of the control group [54].Taken together,these CHDs compromise the function of the heart (or cardiovascular system) to adequately nourish the body with oxygenated blood,and they lead to an elevated risk of cardiac complications.
Mechanisms of CHDs in the off spring of mothers with PDM
Genetic as well as in utero environmental factors could contribute to increased CHD.A cohort study based on data from Pediatric Cardiac Genomics Consortium concluded that the number of de novo variants per CHD patient born to DM mothers was higher (76.5 vs.72.1,Student'sttestP=3.03 × 10-11) compared with other children with CHD,but after accounting for parental age,the significant difference disappeared.These results strongly suggested the role of other mechanisms in the cause of CHD associated with maternal PDM [64].
Persistent maternal hyperglycemia during pregnancy can cause an adverse outcome for fetus [9] and has been recognized as a teratogen leading to CHDs [6 5].Hyperglycemia in PDM may cause cardiac hypertrophy owing to increased size of individual cardiomyocytes and to inhibition of cell proliferation and promoted cell apoptosis in mice.In these mice the PH3+cell (a marker of cell proliferation) numbers in the ventricular septum,left ventricular wall (LVW) and right ventricular wall (RVW) at E18.5,E15.5,and E13.5 in PDM groups were lower than their corresponding controls [54].Cellular oxidative stress could affect cell survival,proliferation,differentiation,and ultimately could lead to myocardial apoptosis and fibrosis [66].T2DM induced CHDs in mice embryos,along with increased oxidative stress,endoplasmic reticulum stress,and apoptosis [48].Exposure of CNCCs of chicken embryos to high-leveled D-glucose induced CHDs prevalence as 82%,which was obviously reduced to 27% with the intervention of N-acetylcysteine,along with the decreased level of oxidative stress[43].Hyperglycemia-induced excess reactive oxygen species (ROS) oxidizes cardiogenic and angiogenic molecules;thus,ROS is not conducive to heart and coronary artery development [44].Hyperglycemia can induce reduced glycogen content and thicker myocardium in the right ventricle and left atrium,can retard the growth,extension and density of the embryonic vascular plexus,and can reduce cardiomyocyte viability in the fetus by inducing ROS [67].Taken together,hyperglycemia can lead to abnormal angiogenesis and cardiogenesis in the off spring of mothers with PDM by inducing excess ROS or oxidative stress.It is important to note that both ROS-dependent and independent mechanisms(such as viral etiologies) increase the risk of CHD in PDM mothers;however,in this review we will mostly focus on ROS-dependent mechanisms.
Endothelial nitric oxide synthase (eNOS) is critical to embryonic heart development and coronary arteries because nitric oxide (NO) regulates transcription factor expression,progenitor cell growth,and epithelial-to-mesenchymal transition [68].Deficiency in eNOS caused most of the CHD phenotype in mice [68,69].Tetrahydrobiopterin (BH4),an antioxidant,is a co-factor of eNOS that can improve the stability and activity of eNOS [70] and vascular endothelial function in diabetes [71,72].Oxidative stress produces excessive peroxide with BH4 heavily consumed,and the decreased number or activity of BH4 triggers eNOS uncoupling,leading to excessive peroxide instead of NO,thus forming a vicious cycle (exacerbated oxidative stress)[72-74].Furthermore,uncoupled eNOS along with oxidative stress were closely associated with CHDs in the off -spring of mothers with PDM [44].
Hyperglycemia during early embryogenesis would cause CHD in the off spring of mothers with PDM through Notch signal path.Endothelial Notch1 was essential in the proper development of the semilunar valves and cardiac outflow tract [75].Cardiovascular malformation was found in 75% ofNotch1mutation carriers,and this strongly demonstrated the close link betweenNotch1mutation and CHDs [76].There was genetic interaction between eNOS andNotch1: deficient eNOS could lead to further reduction in Notch1 signaling through loss of NO [75].Furthermore,it was revealed that in circumstance of hyperglycemia,oxidative stress (induced by excessive ROS and peroxide) resulted in uncoupled eNOS and decreased NO bioavailability,which was associated with enrichment of JARID2,a transcriptional repressor on theNotch1locus,leading to further reduction in Notch1 levels below a critical threshold level required for normal heart development [77].Thus,we conclude that gene-environment interaction between hyperglycemia and Notch1 signaling is closely linked with CHD in off spring with maternal PDM history.
Suppression,ablation,and genetic variants ofNkx2.5in maternal PDM are associated with CHDs in the off spring.Nkx2.5 is an essential transcription factor for the differentiation of cardiomyocytes and can regulate the expression of several other important transcription factors during cardiac development.Cardiac malformations,arrythmia,and contraction defects occurred whenNkx2.5gene was ablated at mid-embryonic stage in mice [78].High glucose suppressed Nkx2.5 expression in the ventricular septum,RVW,and LVW of the PDM mouse fetus hearts (at E15.5 and E18.5),and the down-regulation of Nkx2.5 further inhibited the expressions of its downstream target genes of potassium voltage-gated channel subfamily E member 1 and connexin 43 [54].In addition,mutation inNkx2.5is associated with cardiac malformations and atrioventricular conduction disorders [79].More than 40 mutations ofNkx2.5are involved in a three-generation American family affected with CHD with Nkx2.5 activity reduced [80].Another case-control study showed thatNkx2.5genetic variants at rs11802669 and rs2277923 are most probably involved in heart malformation in the off spring of mothers with PDM history [81].Furthermore,dysregulation of the hypoxia-inducible factor 1 (HIF-1) pathway altered cardiovascular development-related-gene expression (includingNkx2.5,T-box transcription factor 5,and myocyte-specific enhancer factor 2C) in the developing heart and increased susceptibility to CHDs in a mouse model of diabetic pregnancy [82].In addition,hyperglycemia during early embryogenesis would alter gene expression of paired like homeodomain-2,Wnt,and GATA binding protein 5,which were essential cellular components of the developing heart [41].Besides,reduction in bone morphogenetic protein 4,Msh homeobox-1,and paired box 3,which were involved in the development of CNCC,was confirmed to be responsible for defective hearts in fetuses with maternal PDM [49].
Uncoupling protein (UCP),especiallyUCP2polymorphism in maternal PDM,may be associated with CHDs in the off spring.UCP2,distributed across a wide range of tissue and cell types of the body,can regulate free fatty acid metabolism and transport,attenuate the production of ROS,and regulate insulin secretion [83,84].Clinical studies showed that polymorphism ofUCP2-866G/A (rs659366)may relate to pre-diabetes and T2DM by affecting insulin secretion [85,86].Another case-control study showed that the interaction of maternal PDM andUCP2gene polymorphism at rs659366 [T/C vs.C/C: odds ratio (OR)=1.49,95% CI=1.02-2.16;T/T vs.C/C: OR=2.77,95% CI=1.67-4.62] and rs660339 (A/A vs.G/G: OR=2.19,95%CI=1.34-3.58) are associated with the formation of CHD in off spring [87].However,it was controversial in the relationship betweenUCP2-Ala55Val (rs660339) and DM that a meta-analysis claimed no evidence between theUCP2rs660339 and the susceptibility to T2D [84,86].
Polymorphism of adiponectin gene in maternal PDM is significantly associated with CHDs in the offspring.Adiponectin gene was responsible for encoding protein adiponectin [88] and can increase insulin sensitivity and can improve islet β-cell dysfunction and fatty acid betaoxidation.Several adiponectin variants at rs266729 [89],rs1501299 [90],rs2241766 [91],and rs12495941 were reported to associate with T2DM.Furthermore,in a case-control study Luo et al.[3 9] reported that polymorphism of maternal adiponectin gene may probably lead to CHDs in the off spring.More specifically,the maternal PDM,adiponectin genetic variants [especially the polymorphism of maternal adiponectin gene at rs1501299 (T/T vs.G/G: adjusted OR,aOR=3.45;T/G vs.G/G: aOR=1.73)and rs2241766 (G/G vs.T/T,aOR=3.36;G/T vs.T/T,aOR=1.93)],and the interaction of maternal PDM and adiponectin gene polymorphism were significantly associated with high risk of CHD in off spring.
Viral etiologies play a potential role in certain diseases including CHDs,among which coxsackievirus(CV) B infection has long been associated with T1DM and myocarditis [92].Sharma et al.revealed that CVB infection (particularly during the critical window before fetal heart development) in pregnant mice induced CHD(VSD).CVB3 infection during early gestation in C57Bl/6 pregnant mice induces CHDs through suppression of fetal cardiomyocyte proliferation [93].Wook Kim et al.reported a different gut virome profile between pregnant women with T1DM and healthy controls in the Environmental Determinants of Islet Autoimmunity study.In this study CVA2,CVB4,CVB5,rhinovirus B,and ECHO viruses were detected exclusively in women with T1D,and the two viruses of picobirnaviruses (OR=4.2,95%CI=1.0-17.1;P=0.046) and tobamoviruses (OR=3.2,95% CI=1.1-9.3;P=0.037) were more prevalent in T1D pregnant women [94].From this result,we can speculate that it is more susceptible to CV infection (particularly CVB) in maternal PDM,which probably contributes to the strong association between maternal PDM and CHDs.In addition,Lin et al.reported that hyperglycemia may impede pathogen clearance,increasing the duration of gestational infections in pregnant women with T1D [95].These studies above suggest that hyperglycemia in pregnant women with DM is an essential factor to induce the altered composition of the gut virome and distinct enterovirus infection,which together lead to the occurrence of CHDs in the off spring (Fig. 1).
Prevention and treatment of CHDs induced by maternal PDM
Preconception care focusing on glycemic control can reduce adverse pregnancy outcome and frequency of congenital anomalies in the off spring of mothers with PDM [96].Insulin is the preferred pharmacological treatment for diabetes during pregnancy because insulin does not cross the placenta owing to its molecular size [97,98].Insulin analogues,specifically lispro,aspart,and detemir,are viable therapeutic options for diabetes in pregnancy,with comparable safety and efficacy of human insulin [99].This finding was generally consistent with another metaanalysis,which again reported the safety and efficiency(without causing complications for the mothers or fetuses)of aspart and detemir in women with diabetes during pregnancy along with glargine,but with a relatively negative view of lispro [100].Furthermore,a retrospective multinational study of 533 pregnancies with PDM demonstrated no increased risk of congenital anomalies overall with insulin lispro treatment compared with the use of human insulin [101],which was later confirmed again by another retrospective population-based cohort study [37]: insulin analogues treatment in the first trimester reduced the risk of CHDs to a significantly lower rate of 7.6‰,compared with 40.2‰ of human insulin use only and 35.5‰of treatment with both in the off spring of mothers with PDM.What cannot be ignored was that the use of insulin analogues led to a higher rate of spontaneous abortion(1.5% compared with 1.0% of human insulin treatment).In addition,other hypoglycemic drugs have been tried to treat diabetes in pregnancy with consistent view of metformin as an treatment option with its efficiency and safety almost comparable to insulin [97,102,103].
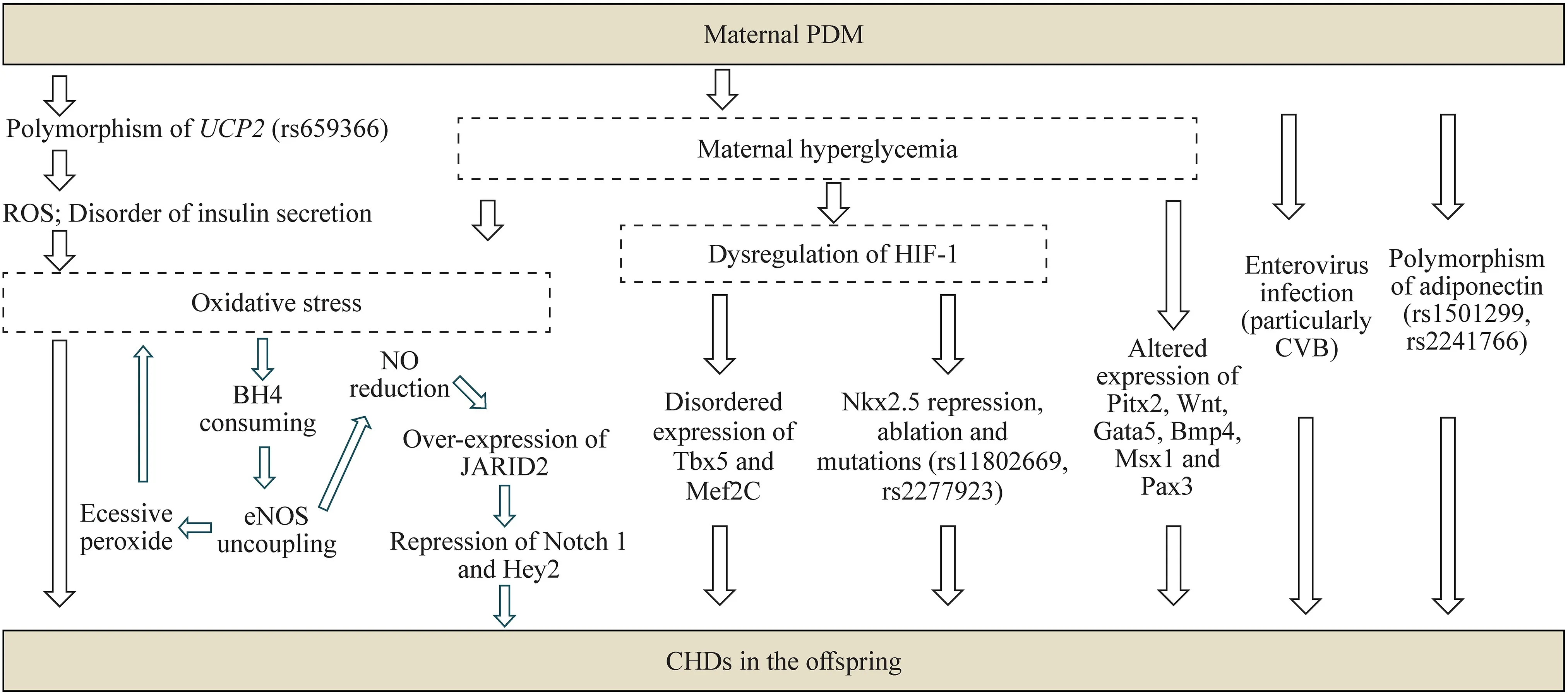
Fig.1 Mechanisms of CHDs in the off spring induced by maternal PDM.Main mechanisms: (1) hyperglycemia in maternal PDM and polymorphism of UCP2 rs659366/ROS and disorder of insulin secretion pathway can cause oxidative stress.Oxidative stress leads to BH4 consumed and eNOS uncoupling,resulting in excessive superoxide and consequently worse oxidative stress,which lead to high risk of CHDs in the off spring;in addition,oxidative stress-induced eNOS uncoupling will contribute to NO reduction,resulting in increased expression of JARID2,and the over-expression of JARID2 will subsequently lead to repression of Notch1 and Hey2 to cause CHDs in the off spring;(2) in circumstances of maternal PDM,hyperglycemia can lead to dysregulation of HIF-1,resulting in suppressed Nkx2.5 expression,Nkx2.5 ablation and mutations,and disordered expression of Tbx5 and Mef2C,which together result in the occurrence of CHDs in the off spring;(3) in circumstances of maternal PDM,hyperglycemia may alter the expression of Pitx2,Wnt,Gata5,Bmp4,Msx1 and Pax3,which finally cause CHDs in the off spring;(4) maternal PDM may cause CHDs in the off spring through enterovirus infection (particularly CVB);(5) maternal PDM may cause CHDs in the off spring through polymorphism of adiponectin (rs1501299/rs2241766). CHDs congenital heart diseases,PDM pregestational diabetes mellitus,UCP2 uncoupling protein 2,ROS reactive oxygen species,eNOS endothelial nitric oxide synthase,NO nitric oxide,BH4 tetrahydrobiopterin,HIF-1 hypoxia-inducible factor 1,Tbx5 T-box transcription factor 5,Mef2C myocyte-specific enhancer factor 2C,Pitx2 paired like homeodomain-2,Gata5 GATA binding protein 5,Bmp4 bone morphogenetic protein 4,Msx1 Msh homeobox-1,Pax3 paired box 3,CVB coxsackievirus B
Inhibition or improvement in oxidative stress has been demonstrated as an effective preventing or treating therapy for CHDs in the off spring of mothers with PDM.Injection of 2 mM N-acetylcysteine to the chick embryos CNCCs exposed to elevated D-glucose reduced the prevalence of CHDs from 82% to 27% [43].Oral administration of 4 mg/mL N-acetylcysteine (1 g/kg body weight/day) in drinking water from E0.5 to the end of gestation or harvesting of the embryos completely avoided the occurrence of AVSD,TGA,TOF and significantly reduced the prevalence of VSD and ASD in the off spring of mice with PDM [46].N-acetylcysteine is the precursor of glutathione,which is a common antioxidant.So,we can propose that the intervention of N-acetylcysteine inhibits oxidative stress to reduce the risk of CHDs.Furthermore,oral administration of sapropterin(a stable,orally active,synthetic form of BH4) at 10 mg/kg/day to pregnant C57BL/6 mice with PDM from E0.5 to E18.5 successfully reduced coronary artery malformation from 50.0% to 20.6% in the fetuses,probably through increasing eNOS activity and lowering oxidative stress [44].The administration of sapropterin probably may prevent or reduce the occurrence of CHDs in the off spring of mothers with PDM by inhibiting eNOS uncoupling and improving oxidative stress.
A wealth of research showed that exercise during pregnancy is safe and beneficial to both the mothers and fetuses[104-106].Maternal aerobic exercise during pregnancy contributes to improved off spring cardiac autonomic health[107].An animal experiment claimed that maternal voluntary exercise (running wheels),starting from 1st week before mating until E18.5 in gestation,mitigated ROS and oxidative stress in the fetal heart and resulted in a lower occurrence of CHDs from 59.5% to 25% in the off spring of maternal PDM in mice [45].However,it is hard to conclude which exercise type or intensity is the best during pregnancy,and further studies need to be conducted (Table 3).
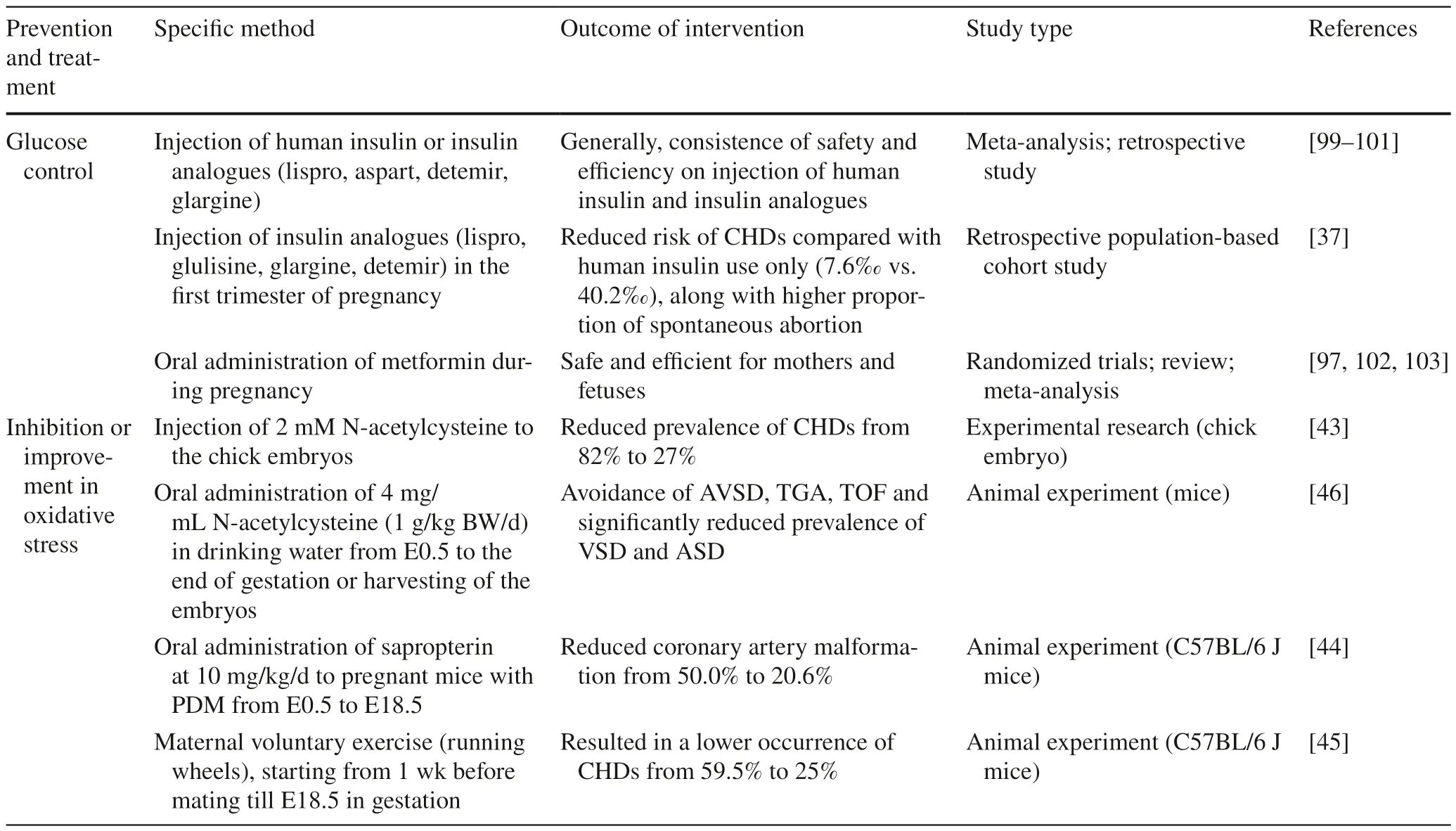
Table 3 Prevention and treatment of congenital heart diseases in the off spring induced by maternal pregestational diabetes mellitus
In addition to drug or non-drug intervention before and during pregnancy,postpartum management is necessary.It is important to continue a regular cardiac follow-up withelectrocardiography (ECG),Holter ECG,and echocardiography,particularly for those patients with cardiac hypertrophy,because cardiac hypertrophy in off spring of diabetic mothers is usually reversible and has a good prognosis [51].
Conclusions
DM population has significantly increased in the past few decades,along with adolescent female and women of childbearing age;consequently,an increasing number of pregnancies with PDM will cause a severe global burden to both health and economy.CHDs can be caused by multiple factors mainly composed of genetics,environmental factors,and gene-environment factors.Maternal PDM is considered a key risk factor for CHDs and its specific phenotype;especially,defects of VSD,ASD,PDA,DORV,and cardiac hypertrophy in the off spring have been demonstrated in both animal experiments and clinical studies.Maternal hyperglycemia,along with oxidative stress,eNOS uncoupling,polymorphism ofUCP2,polymorphism of adiponectin,Notch1mutation or reduction,decreased activity of Nkx2.5,dysregulation of HIF-1,and enterovirus infection,has been proposed to elucidate the mechanism of maternal PDM in inducing CHDs.Preventive or treatment options,such as glucose control with insulin and metformin,oxidative stress inhibition with oral and injection interventions,and exercise,have been explored.More studies are needed to provide systematic and accurate knowledge of the mechanisms and treatment of CHDs caused by maternal PDM.
AcknowledgementsWe thank Yan-Qiu Chen for her expert assistance with the image processing technology.
Author contributionsCZY contributed to conceptualization and writing of the original draft.MSF and GLH contributed to validation.QJ and YLX contributed to software.LY contributed to reviewing and editing,and funding acquisition.All authors have made substantial contributions to the conception and design of the study,drafting the article,or revising it critically for important intellectual content.All authors have provided approval for the final version to be submitted and published.Each author has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FundingThis work was supported by Sichuan Vocational College of Health and Rehabilitation (No.CWKY-2020Z-02),the Department of Science and Technology of Sichuan Province (No.2019YJ0079),and the National Natural Science Foundation of China (No.81900283).
Data availabilityThe authors confirm that the data supporting the findings of this study are available within the article.
Declarations
Ethical approvalNot applicable.
Conflict of interestNo financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.The authors have no conflict of interest to declare.
 World Journal of Pediatrics2023年4期
World Journal of Pediatrics2023年4期
- World Journal of Pediatrics的其它文章
- Psychiatric comorbidities in children and adolescents with chronic urticaria
- Effectiveness of resilience-promoting interventions in adolescents with diabetes mellitus: a systematic review and meta-analysis
- Addition of respiratory exercises to conventional rehabilitation for children and adolescents with cerebral palsy: a systematic review and meta-analysis
- Effects of synbiotic supplementation on anthropometric indices and body composition in overweight or obese children and adolescents: a randomized,double-blind,placebo-controlled clinical trial
- Estimated prevalence and trends in smoking among adolescents in South Korea,2005-2021: a nationwide serial study
- Factors of heavy social media use among 13-year-old adolescents on weekdays and weekends
