敷料类水凝胶治疗糖尿病足溃疡的新型载体策略
潘敬科 邬浩明 王瑶 胡旭麟 唐璐 李开南 董志红
收稿日期:2023-03-20
基金项目:成都市科技局技术创新研发项目(2021-YF0501871-SN);成都市卫健委医学科研项目(2021059);
四川省科技厅成果转化示范项目(23ZHSF0321);成都大学国家级大学生创新创业计划(202211079001S)。
作者简介:潘敬科,硕士,主要从事生物医用材料的制备研究工作。
*通讯作者:董志红,博士,教授,硕士生导师,主要从事生物医用材料及表面处理。
摘要:糖尿病足溃疡(Diabetic foot ulcer,DFU)是糖尿病的一种慢性非愈合性并发症,主要影响因素有血管生长受限、神经再生困难、容易合并感染以及创伤面难以清理,会导致足部功能性障碍,严重者会导致截肢。开发高效新型敷料水凝胶是解决DFU有效的途径之一,它不仅可以提供伤口恢复所需的湿润环境、清除伤口渗出物、防止继发感染、还可促进组织再生,加速伤口愈合。水凝胶治疗DFU要求较高,本文通过敷料水凝胶的特点及类型,从生物材料的天然,合成及接枝改性角度,搭载纳米粒子、药物、及生长因子等探讨各种不同类型的敷料水凝胶,用于DFU伤口治疗,阐述敷料水凝胶治疗效果,为新型敷料的制备及研究提供指导。
关键词:糖尿病足溃疡;水凝胶;伤口敷料;天然和合成聚合物;活性物质;纳米颗粒
中图分类号:R318.08 文献标志码:A 文章编号:1001-8751(2023)06-0390-07
A Novel Carrier Strategy for Treating Diabetic Foot Ulcer with Dressing Hydrogels
Pan Jing-Ke1, Wu Hao-Ming2, Wang Yao2, Hu Xun-Lin2, Tang Lu2, Li kai-nan2, Dong Zhi-Hong1
(1 School of Mechanical Engineering,Chengdu University, Chengdu 610106;
2 Clinical Medical College and Affiliated Hospital of Chengdu University, Chengdu 610081)
Abstract: Diabetic foot ulcer (DFU) is a chronic non-healing complication of diabetes. The main influencing factors include vascular growth restriction, nerve regeneration, easy co-infection and cleaning of wound surface, which lead to foot functional disorders, even, amputation. The development of efficient new dressing hydrogels is one of the most effective ways to solve DFU, which can not only provide moist environment for wound recovery, remove wound exudation, preventing secondary infection, but also promote tissue regeneration and accelerate wound healing. Hydrogel treatment of DFU requires higher requirements. Based on the characteristics and types of dressing hydrogels, this paper discussed different types of dressing hydrogels for DFU wound treatment by using nanoparticles, drugs and growth factors from the natural, synthetic and grafting modification of biomaterials, and expounds the therapeutic effect of dressing hydrogels. It is a good guidance for the preparation and research of new dressing.
Key word: diabetic foot ulcer; hydrogel; wound dressing; natural and synthetic polymers; active substance; nanoparticle
1 介绍
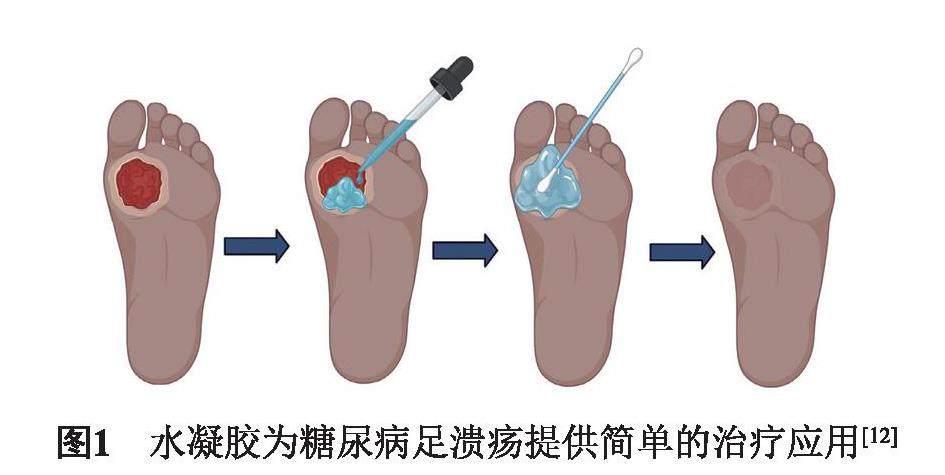
糖尿病足溃疡(Diabetic foot ulcer ,DFU)是一种严重的糖尿病并发症[1]。它是由胰岛细胞减少引起的碳水化合物、脂质和蛋白质代谢紊乱疾病[2],其特征是血清中葡萄糖水平升高,导致感知能力下降,进而导致血管并发症、血管失神经和伤口区域的低氧供应[3]。及时地注射长效胰岛素可补充治疗,但糖尿病是不可治愈的一种疾病,随着病情进展,高血糖会影响全身的血管,导致组织坏死,发炎,甚至溃烂。而伤口的愈合是一个复杂过程,包括止血、炎症、增殖(肉芽组织形成)、再上皮化和重塑几个阶段,需要细胞的成熟和分化,以及生长因子的搭载配合,才能达到细胞增殖并构建细胞外基质目的,从而增强伤口闭合[4-6]。临床上治疗DFU常用方法为手术清创[7],采用纱布换药、脉冲灌洗、水疗和低频超声,减少水肿以增加动脉氧压[8],但不能从根本上根治DFU。而采用敷料类水凝胶,不仅可保持伤口的润湿性,吸收伤口的渗出物;同时,还可促进角质形成细胞的迁移和分化[9],胶原蛋白形成及血管生成,移除时不会受损伤口,加速慢性伤口的愈合(图1)[10-11]。
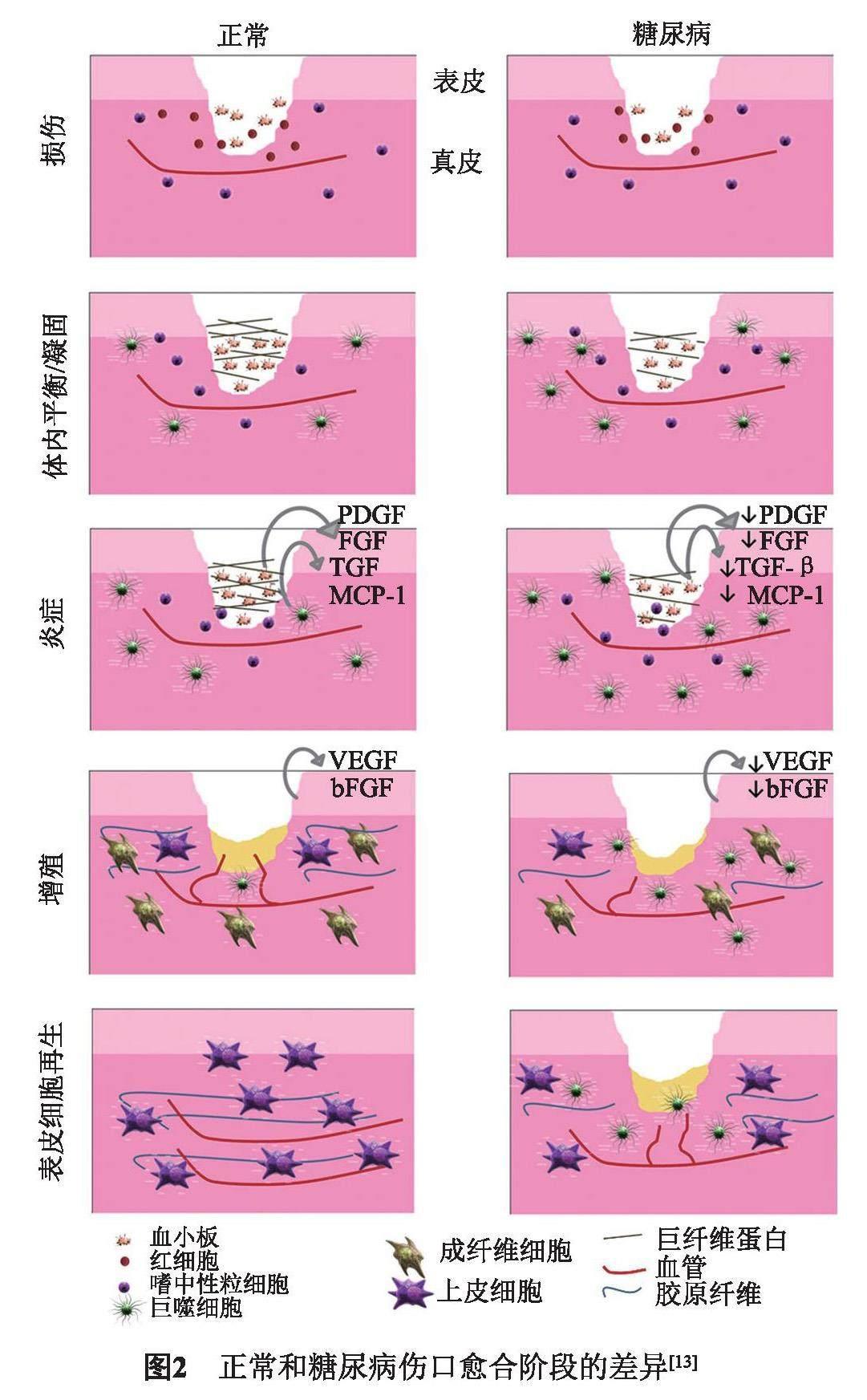
近几十年来,敷料水凝胶研究证明不仅可以促进和加速伤口愈合的过程,并且潮湿的微环境可诱导成纤维细胞增殖和角质形成分化,同时也可以增强胶原合成,从而减少疤痕(图2)[12-13]。优势就在于:(1)允许气体交换、隔热,并有助于清除排泄物和碎屑,以促进组织重建过程;(2)具有生物相容性,无毒,不易引起任何免疫或过敏反应;(3)防止生物膜形成和继发感染,易于移除而不会造成二次创伤[14]。因此,开发多元化的水凝胶敷料是DFU当前亟须解决的问题[15]。从DFU病理生理学和分子生物学角度考虑,最好的治疗策略是,选择正确的生物材料凝胶搭载纳米粒子、生物因子等,来积极促进伤口愈合。
因此,水凝胶类的敷料在治疗DFU中,发挥着较好的优势,本研究介绍不同类型的水凝胶敷料及搭载,为研究人员提供治疗糖足溃疡最新策略和方法。
2 天然水凝胶敷料
天然水凝胶敷料能够模拟天然组织结构和功能,细胞更好地附着和生长,具有较好的生物相容性且无毒[16]。常见的天然敷料材料主要包括壳聚糖(Chitosan, CS)、海藻酸盐(Alginate, GLA)、透明质酸(Hyaluronic Acid, HA)、胶原蛋白(Collagen, Col)及细胞外基质(ECM)等。CS是甲壳素的N-脱乙酰基衍生物,是一种天然阳离子多糖,不但具有生物学特性,还具有抗菌、止血功效[17]。Escarcega-Galaz等[18]对8名DFU患者进行人体临床试验,在伤口区用2%壳聚糖凝胶和壳聚糖膜后,所有患者在伤口区均形成肉芽和新生组织。GLA是一种天然多糖,较好的生物相容性和黏附性,是细胞搭载的一种较好生物材料,通过添加二价阳离子(Ca2+、Mg2+及Mn2+等),在生理条件下更易于凝胶化[19]。Rezvanian等[20]利用链脲佐菌素(Streptozotocin, STZ)诱导糖尿病大鼠伤口愈合模型中,通过负载辛伐他汀的藻酸盐—果胶水凝胶膜,发现血管生成增加,胶原合成加速,可改善伤口愈合。HA是某些哺乳动物结缔组织(如软骨、脐带和滑液)细胞外基质(Extracellular matrix, ECM)的主要成分,在组织形成、修复和重塑中具有关键作用[21]。Zhang等[22]设计了一种接枝甲基丙烯酸酐的透明质酸基水凝胶,这种水凝胶可以通过光引发甲基丙烯酸酯部分之间的自由基交联,在3 s内原位光聚合,可延长培养基的释放。Col是人体组织(如皮肤、骨骼、软骨、肌腱和韧带)中天然存在的ECM最丰富的蛋白质,是用于伤口愈合应用的最常见材料之一。III型胶原(Col III)[23]已被鉴定为在无瘢痕伤口愈合过程中促进细胞迁移和增殖的重要结构蛋白。Chen等[24]对小鼠和猪的研究表明,胶原水凝胶在治疗急性伤口和其他遗传性皮肤病方面潜力巨大。然而,由于天然聚合物在体内降解速度快,机械性能弱,在临床应用中也受到限制,作为DFU敷料,研究人员进行了大量的改进。
3 合成水凝胶敷料
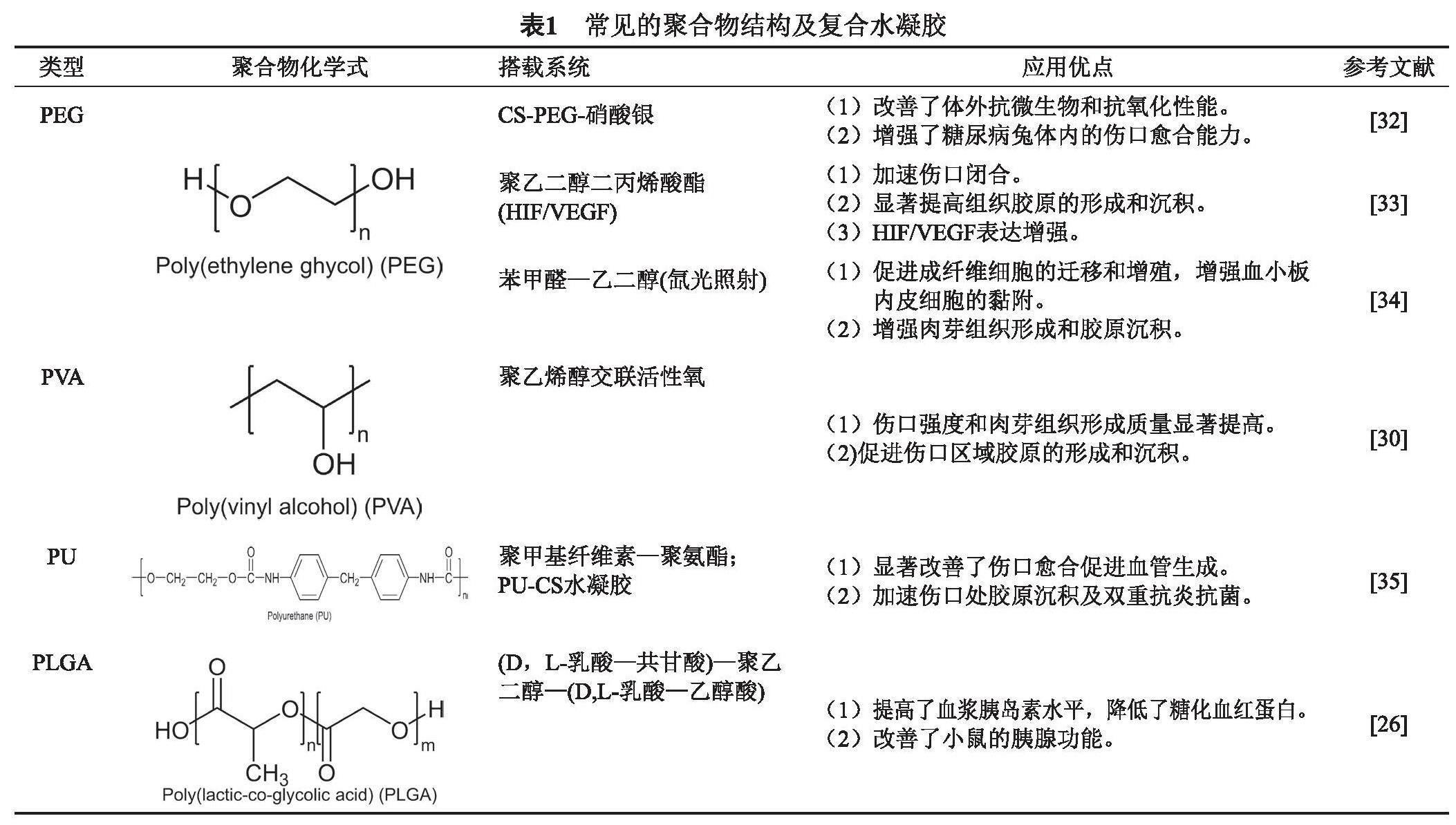
合成聚合物可改善机械性能、能够较容易地调节其降解速率、易于对其结构进行修饰和功能化,克服了天然聚合物的局限性,这有助于提高天然聚合物在复合DFU敷料中的性能,常用的聚合物在DFU敷料上的使用主要有聚丙交酯—共乙交酯(PLGA)、聚乙烯醇(PVA)聚乙二醇(PEG)及聚氨酯(PU)等[25],合成水凝胶大大改善了DFU的治疗,表1总结了常见的聚合物水凝胶特点。
PLGA是一种生物可降解聚合物,具有较好的生物相容性、可控的生物降解性和实现生物活性物质持续释放的潜力[26]。Zhuang等[27]制备了聚(D, L-乳酸-共甘酸)—聚乙二醇—(D,L-乳酸—乙醇酸)—水凝胶敷料,该种敷料不仅保持了温度诱导的凝胶化,而且在体内表现出更稳定的降解曲线,可有效的控制血糖浓度,提高血浆胰岛素水平,进而促进糖尿病伤口愈合。PVA[28]是一种可生物降解、生物相容、亲水、无毒和非致癌的聚合物,通过水解、醇解或氨解从乙酸乙烯酯中获得,已用于伤口敷料的开发,用于治疗急、慢性伤口愈合。Zhao等[29]通过使用聚乙烯醇(PVA)与活性氧(ROS)响应接头交联而开发的一种水凝胶,可以作为有效的ROS清除剂,通过降低ROS水平和调节伤口周围的M2表型巨噬细胞来促进伤口闭合。PEG是一种生物相容的亲水性聚合物,天然不降解,经过改性以获得可降解性,由海藻酸钠和PEG组成的复合支架在伤口愈合应用中表现出较好的效果[30]。通过丙烯酸酯基团来修饰PEG,获得PEGDA,为细胞黏附和支持提供介质;通过苯甲醛—乙二醇光交联改性,可以促进成纤维细胞的迁移和增殖,增强血小板内皮细胞的黏附,加速肉芽组织形成和胶原沉积[31],下表1总结常见的聚合物结构及复合水凝胶。
4 新型搭载水凝胶敷料
无论是天然还是合成生物材料,在DFU治疗中,都需要复合或者搭载药物、生长因子等,才能达到治愈的效果,促进本身组织生长以及预防外源性感染,促进溃疡修复[36]。
4.1 搭载纳米颗粒
纳米颗粒不仅可以促进皮肤类伤口的愈合,还具有独特的抗炎特性、抗氧化和多药耐药(MDR)微生物的抗菌性。其机制是由于它们可能产生活性氧,从而杀死细菌且具有较好的附着性,附着在DNA或RNA上,从而进一步阻碍微生物的复制和繁殖[37]。常用于制作抗菌涂层、抗菌剂和抗生素输送系统[38]。常见的抗菌金属纳米颗粒包括银[39]、金[40]、铜[41]、铈等纳米颗粒[42]。Masood等[32]通过制备CS-PEG敷料水凝胶用来搭载纳米颗粒Ag促进糖尿病大兔伤口愈合修复,结果该类水凝胶可以持续释放Ag离子颗粒,表现出较好的抗菌性和抗氧化性能,加速伤口愈合。Choudhary等[43]制备的CS/Ca-AlgNps/AgNPs水凝胶对革兰阴性(大肠埃希菌、铜绿假单胞菌)和革兰阳性(枯草芽孢杆菌、金黄色葡萄球菌)均具有广谱抗菌性能。Zhao等[44]开发了一种搭载聚多巴胺修饰的纳米Ag颗粒的聚苯乙烯—聚乙烯醇导电混合水凝胶(PDA@Ag NPs),不仅可调节水凝胶的机械和电化学性能、增强其可加工性,以及提升自愈能力和可重复黏附性,还可以促进血管生成、加速胶原沉积、抑制细菌生长、控制伤口感染,对糖尿病足部伤口有显著的治疗作用。壳聚糖—明胶—聚乙烯吡咯烷酮搭载纳米Ag粒子具有高的热稳定性和水解稳定性、较强的机械性能、最佳的亲水性和孔隙率。同时,能显著减少受伤区域,表现出强大的抗菌活性。
纳米Zn颗粒在DFU治疗中也发挥着重要的作用,纳米ZnO颗粒由于其抗炎和防腐剂的特性,可加入皮肤软膏中治疗皮炎及皮肤感染[45-46]。Tavakoli等[47]将甲基丙烯酸基卡拉胶作为水凝胶基质用来搭载聚多巴胺修饰氧化锌(ZnO/PD)纳米颗粒来提升水凝胶的力学、抗菌和细胞性能。体内外研究发现复合物纳米ZnO水凝胶具有显著的力学性能和促进伤口恢复能力,与人体正常皮肤的弹性和黏附性相当,水凝胶的拉伸强度从64.1 ± 10kPa增强到80.3 ± 8 kPa,伸长率从20 ± 4%上升到61 ± 5%。纳米ZnO颗粒能明显改善水凝胶的特性,使细胞活力增强,更好地促进溃疡修复。Yin等[48]用敷料水凝胶搭载EGCG修饰纳米ZnO颗粒治疗糖尿病伤口,可有效清除95.6%的金黄色葡萄球菌和97%的大肠杆菌,杀菌活性大大提高。伤口治疗15 d后,大鼠皮肤病变闭合率为96.3%,疗效远胜于对照组的65.4%。纳米ZnO不仅促进了角质形成细胞的迁移,而且可穿透细菌细胞膜,导致氧化损伤,从而更好地促进伤口愈合,防止感染。Sun等[49]在体内大鼠模型中,用SiO2纳米颗粒修饰的GelMA/明胶/PEG生物打印支架促进了巨噬细胞向糖尿病骨修复中的抗炎M2表型极化,达到糖尿病伤口愈合目的。
4.2 搭载活性物质
水凝胶搭载活性物质,在急性和慢性伤口愈合中已被广泛研究,如生长因子、蛋白质,可促进组织修复和伤口愈合。常见的生长因子和/或细胞因子如表皮生长因子(EGF)、血管内皮生长因子(VEGF)、转化生长因子(TGF-β),成纤维细胞生长因子(FGF)、血小板源性生长因子(PDGF)、胰岛素样生长因子(IGF)、肿瘤坏死因子-α(TNF-α)等,可提高伤口愈合过程中细胞的生物活力[50]。
临床上,Park等[51]对167例患者进行局部EGF治疗慢性DFU的临床试验,73.2%的治疗伤口达到完全愈合,而对照组为50.6%。Lee等[52]利用CS复合水凝胶搭载EGF,表现出明显的水化能力,包括膨胀程度和平衡含水量,14 d后伤口闭合程度达到97%,比商业敷料HeraDerm和纱布组高7.4%和18.9%,同时可加速伤口上皮化以及胶原沉积。Lin等[53]开发了一种基于CS的非均质复合水凝胶,该水凝胶包封了全氟化碳乳液、表皮生长因子(EGF)和聚六亚甲基二胍(PHMB),使大鼠的糖尿病伤口愈合效果显著增强,15 d后,胶原成熟度达到95%,比用商业敷料治疗组高12.6%。Chu[54]等提出了一种改进的双乳化法,负载rhEGF和PLGA纳米颗粒,制备水凝胶敷料,用于治疗DFU,在糖尿病大鼠中,这些负载rhEGF纳米颗粒促进了成纤维细胞增殖分化,从而使得伤口更快地愈合。HA也可用于递送生物制剂以控制释放,提高治疗效果,HA上的EGF和bFGF在2型糖尿病小鼠模型上有助于增强血管生成,促进伤口愈合,是糖尿病伤口愈合的有效治疗剂[37]。
DFU的损伤还包括神经损伤,Li等[55]通过肝素—波洛沙默热敏水凝胶来搭载碱性成纤维细胞生长因子(bFGF)和神经生长因子(NGF)治疗患有坐骨神经挤压损伤的糖尿病大鼠。通过载体递送的方式不仅对大量生长因子(GFs)具有良好的亲和力,而且释放可控。在体内,与单独使用的水凝胶或直接给予GFs相比,GFs-HP水凝胶治疗能够促进细胞增殖,增加神经相关结构蛋白的表达,促进轴突再生和髓鞘再生,并改善恢复运动功能。利用敷料型水凝胶搭载活性物质可以从多个方面促进足底溃疡修复,包括神经损伤、血管损伤、抗感染以及隔离创伤面等,这为临床治疗提供了新的研究方向。
4.3 搭载药物
敷料型水凝胶的搭载作用不仅仅局限于纳米颗粒、活性物质等。同时还可以搭载促进伤口修复的药物。Wang等[56]制备了PVA/CS水凝胶,通过缓慢释放西藏十八味党参丸(TEP)的活性成分来治疗糖尿病伤口。体外研究表明,TEP负载的水凝胶可以有效和持续地释放TEP的活性成分,并具有抗菌和抗氧化性能。此外,该水凝胶系统对L929细胞没有细胞毒性,并能显著促进内皮细胞(HUVEC)的增殖。当TEP负载水凝胶用于大鼠糖尿病伤口时,它减少了炎症反应,改善了胶原沉积,进而促进了皮肤愈合。丝蛋白水凝胶金属螯合二肽—姜黄素,也具有加速糖尿病伤口愈合的潜质[57];另一项研究开发了硫酸软骨素接枝和热增强藻酸盐负载姜黄素的水凝胶,原位形成注射水凝胶,可调控药物释放,抑制炎症,加速糖尿病伤口模型中的组织再生。明胶/右旋糖酐搭载芍药苷,具有抗菌和促血管化,止血功效[58],也可以加入一些抗生素的药物,如莫匹罗星,来治疗原发性皮肤感染及溃疡合并感染等。
5 总结
加速敷料的开发治疗DFU,旨在提高DFU患者的生活质量,减轻疼痛,这对研究人员来说是一个挑战。理想的敷料应具有水分平衡、蛋白酶隔离、生长因子刺激、抗菌活性、透氧性和促进自溶清创的能力,从而促进肉芽组织的产生和上皮化过程。此外,在药物系统的情况下,它应具有延长作用时间、提高效率和改善持续递送药物等功效。
已经开发的传统敷料最新替代品包括混合不同的聚合物,并使用更有效的交联方法来确保伤口最佳微环境的保障。如,天然(CS、HA、ALG、COL)或合成(PVA、PEG、PVP、PU)聚合物已被组合或交联;此外,装载纳米粒子及药物敷料已被认为可有效送递药物或其他生物活性因子,加速组织的再生和抗菌特性,被用于DFU的治疗。搭载抗生素、血小板衍生物、患者自身干细胞、生长因子等的水凝胶敷料可平衡慢性伤口炎症,促进愈合;掺入一些天然提取物,在治疗DFU方面也能发挥巨大潜力。随着新材料的开发和新技术的出现,在水凝胶敷料治疗DFU领域,会开发出更高效、更经济、具有更良好的生物相容性和生物降解性能的复合水凝胶敷料,加速患者伤口愈合及修复。
参 考 文 献
Ahmed I, Goldstein B. Diabetes mellitus [J]. Clin Dermatol, 2006, 24(4): 237-246.
Hussain Z, Thu H E, Katas H, et al. Hyaluronic acid-based biomaterials: A versatile and smart approach to tissue regeneration and treating traumatic, turgical, and chronic wounds [J]. Polym Rev, 2017, 57(4): 594-630.
Fadini G P, Albiero M, Bonora B M, et al. Angiogenic abnormalities in diabetes mellitus: mechanistic and clinical aspects [J]. J Clin Endocrinol Metab, 2019, 104(11): 5431-5444.
Li Z, Zhao Y, Liu H, et al. pH-responsive hydrogel loaded with insulin as a bioactive dressing for enhancing diabetic wound healing [J]. Mater Des, 2021, 210:2587-2604.
Li N, Zhan A, Jiang Y, et al. A novel matrix metalloproteinases-cleavable hydrogel loading deferoxamine accelerates diabetic wound healing [J]. Int J Biol Macromol, 2022, 222(Pt A): 1551-1559.
Ozdemir D, Feinberg M W. MicroRNAs in diabetic wound healing: Pathophysiology and therapeutic opportunities [J]. Trends Cardiovasc Med, 2019, 29(3): 131-137.
Skorkowska-Telichowska K, Czemplik M, Kulma A, et al. The local treatment and available dressings designed for chronic wounds [J]. J Am Acad Dermatol, 2013, 68(4): 117-126.
Crystal Holmes C J, Garneisha T, Sari P. Wound debridement for diabetic foot ulcers:A clinical practice review [J]. The Diabetic Foot Journal Vol 2019: 64.
Junker J P, Kamel R A, Caterson E J, et al. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments [J]. Adv Wound Care (New Rochelle), 2013, 2(7): 348-536.
Korting H C, Schollmann C, White R J. Management of minor acute cutaneous wounds: importance of wound healing in a moist environment [J]. J Eur Acad Dermatol, 2011, 25(2): 130-137.
Okan D, Woo K, Ayello E A, et al. The role of moisture balance in wound healing [J]. Adv Skin Wound Care, 2007, 20(1): 39-53.
Moura L I, Dias A M, Carvalho E, et al. Recent advances on the development of wound dressings for diabetic foot ulcer treatment-a review [J]. Acta Biomater, 2013, 9(7): 7093-7114.
Bardill J R, Laughter M R, Stager M, et al. Topical gel-based biomaterials for the treatment of diabetic foot ulcers [J]. Acta Biomater, 2022, 138: 73-91..
Fuchs S, Ernst A U, Wang L H, et al. Hydrogels in emerging technologies for type 1 diabetes [J]. Chem Rev, 2021, 121(18): 458-526.
Wang Y, Wu Y, Long L, et al. Inflammation-responsive drug-loaded hydrogels with sequential hemostasis, antibacterial, and anti-inflammatory behavior for chronically infected diabetic wound treatment [J]. ACS Appl Mater Interfaces, 2021, 13(28): 84-99.
Ullah S, Chen X. Fabrication, applications and challenges of natural biomaterials in tissue engineering [J]. Appl Mater, 2020, 20:7357-7373
Wang C H, Cherng J H, Liu C C, et al. Procoagulant and antimicrobial effects of chitosan in wound healing [J]. Int J Mol Sci, 2021, 22(13):88-98
Escarcega-Galaz A A, Cruz-Mercado J L, Lopez-Cervantes J, et al. Chitosan treatment for skin ulcers associated with diabetes [J]. Saudi J Biol Sci, 2018, 25(1): 130-135.
d'Ayala G G, Malinconico M, Laurienzo P. Marine derived polysaccharides for biomedical applications: chemical modification approaches [J]. Molecules, 2008, 13(9): 2069-2106.
Rezvanian M, Ng S F, Alavi T, et al. In vivo evaluation of Alginate-pectin hydrogel film loaded with simvastatin for diabetic wound healing in streptozotocin-induced diabetic rats [J]. Int J Biol Macromol, 2021, 171: 308-319.
Figueira T G, Dos Santos F V, Yoshioka S A. Development, characterization and in vivo evaluation of the ointment containing hyaluronic acid for potential wound healing applications[J]. J Biomat Sci-Polym E, 2022, 33(12): 1511-1530.
Zhang Y, Zheng Y, Shu F, et al. In situ-formed adhesive hyaluronic acid hydrogel with prolonged amnion-derived conditioned medium release for diabetic wound repair [J]. Carbohydr Polym, 2022, 276: 118-132.
Xu L, Liu Y, Tang L,et al. Preparation of recombinant human collagen III protein hydrogels with sustained release of extracellular vesicles for skin wound healing [J]. Int J Mol Sci, 2022, 23(11)3421-3426.
Chen M, Jin Y, Han X, et al. MSCs on an acellular dermal matrix (ADM) sourced from neonatal mouse skin regulate collagen reconstruction of granulation tissue during adult cutaneous wound healing [J]. RSC Advances, 2017, 7(37): 2998-3010.
Mir M, Ali M N, Barakullah A, et al. Synthetic polymeric biomaterials for wound healing: a review [J]. Prog Biomater, 2018, 7(1): 1-21.
Wu N, Yu J, Lang W, et al. Flame retardancy and toughness of poly(lactic acid)/GNR/SiAHP composites [J]. Polymers (Basel), 2019, 11(7):1129.
Zhuang Y, Yang X, Li Y, et al. Sustained release strategy designed for lixisenatide delivery to synchronously treat diabetes and associated complications [J]. ACS Appl Mater Interfaces, 2019, 11(33): 04-18.
Chen X, Lu B, Zhou D, et al. Photocrosslinking maleilated hyaluronate/methacrylated poly (vinyl alcohol) nanofibrous mats for hydrogel wound dressings [J]. Int J Biol Macromol, 2020, 155: 03-10.
Zhao H, Huang J, Li Y, et al. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds [J]. Biomaterials, 2020, 258: 120-126.
Ilhan E, Cesur S, Guler E, et al. Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material [J]. Int J Biol Macromol, 2020, 161: 40-54.
Asawa R R, Belkowski J C, Schmitt D A, et al. Transient cellular adhesion on poly(ethylene-glycol)-dimethacrylate hydrogels facilitates a novel stem cell bandage approach [J]. PLoS One, 2018, 13(8): 2300-2306
Masood N, Ahmed R, Tariq M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits [J]. Int J Pharm, 2019, 559: 23-36.
Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair [J]. Theranostics, 2020, 10(11): 29-43.
Cheng C, Zhong H, Zhang Y, et al. Bacterial responsive hydrogels based on quaternized chitosan and GQDs-epsilon-PL for chemo-photothermal synergistic anti-infection in diabetic wounds [J]. Int J Biol Macromol, 2022, 210: 77-93.
Almasian A, Najafi F, Eftekhari M, et al. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies [J]. Mater Sci Eng C Mater Biol Appl, 2020, 114: 11-39.
Arvizo R R, Bhattacharyya S, Kudgus R A, et al. Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future [J]. Chem Soc Rev, 2012, 41(7): 43-70.
Hobizal K B, Wukich D K. Diabetic foot infections: current concept review [J]. Diabet Foot Ankle, 2012, 3:5150-5154.Tian J, Wong K K Y, Ho C-M, et al. Topical delivery of silver nanoparticles promotes wound healing [J]. Chem Rev, 2007, 2(1): 29-36.
Dunn K, Edwards-Jones V. The role of Acticoat? with nanocrystalline silver in the management of burns[J]. Burns, 2004, 30: S1-S9.
Chen S A, Chen H M, Yao Y D, et al. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products [J]. Eur J Pharm Sci, 2012, 47(5): 75-83.
Tiwari M, Narayanan K, Thakar M B, et al. Biosynthesis and wound healing activity of copper nanoparticles [J]. IET Nanobiotechnol, 2014, 8(4): 2-7.
Chigurupati S, Mughal M R, Okun E, et al. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing [J]. Biomaterials, 2013, 34(9): 194-201.
Choudhary M, Chhabra P, Tyagi A, et al. Scar free healing of full thickness diabetic wounds: A unique combination of silver nanoparticles as antimicrobial agent, calcium alginate nanoparticles as hemostatic agent, fresh blood as nutrient/growth factor supplier and chitosan as base matrix [J]. Int J Biol Macromol, 2021, 178: 41-52.
Zhao Y, Li Z, Song S, et al. Skin-Inspired Antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings [J]. Adv Funct Mater, 2019, 29(31):1901474.
Jaiswal M, Gupta A, Dinda A K, et al. An investigation study of gelatin release from semi-interpenetrating polymeric network hydrogel patch for excision wound healing on Wistar rat model [J] J Appl Polym Sci, 2015, 132(25): 1735-1746.
Stoica A E, Chircov C, Grumezescu A M. Nanomaterials for wound dressings: an up-to-date overview [J]. Molecules, 2020, 25(11):11-30
Tavakoli S, Mokhtari H, Kharaziha M, et al. A multifunctional nanocomposite spray dressing of kappa-carrageenan-polydopamine modified ZnO/L-glutamic acid for diabetic wounds[J]. Mater Sci Eng C, 2020, 111: 110837.
Yin X, Huang S, Xu S, et al. Preparation of pro-angiogenic, antibacterial and EGCG-modified ZnO quantum dots for treating bacterial infected wound of diabetic rats[J]. Biomaterials Advances, 2022, 133: 112638.
Sun X, Ma Z, Zhao X, et al. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus [J]. Bioact Mater, 2021, 6(3): 57-69.
Barrientos S, Stojadinovic O, Golinko M S, et al. Growth factors and cytokines in wound healing [J]. Wound Repair Regen, 2008, 16(5): 585-601.
Park K H, Han S H, Hong J P,et al. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: A phase III multicenter, double-blind, randomized, placebo-controlled trial [J]. Diabetes Res Clin Pract, 2018, 142: 35-44.
Lee Y H, Hong Y L, Wu T L. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing [J]. Mater Sci Eng C Mater Biol Appl, 2021, 118: 75-85.
Lin Y J, Chien B Y C, Lee Y H. Injectable and thermoresponsive hybrid hydrogel with antibacterial, anti-inflammatory, oxygen transport, and enhanced cell growth activities for improved diabetic wound healing[J]. Eur Polym, 2022, 175: 111364.
Chu Y, Yu D, Wang P, et al. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats[J]. Wound Repair Regen, 2010, 18(5): 499-505.
Li R, Li Y, Wu Y, et al. Heparin-poloxamer thermosensitive hydrogel loaded with bFGF and NGF enhances peripheral nerve regeneration in diabetic rats [J]. Biomaterials, 2018, 168: 24-37.
Wang Z, Gao S, Zhang W, et al. Polyvinyl alcohol/chitosan composite hydrogels with sustained release of traditional Tibetan medicine for promoting chronic diabetic wound healing [J]. Biomater Sci, 2021, 9(10): 1-9.
Sonamuthu J, Cai Y, Liu H, et al. MMP-9 responsive dipeptide-tempted natural protein hydrogel-based wound dressings for accelerated healing action of infected diabetic wound [J]. Int J Biol Macromol, 2020, 153: 58-69.
Shah S A, Sohail M, Khan S A, et al. Improved drug delivery and accelerated diabetic wound healing by chondroitin sulfate grafted alginate-based thermoreversible hydrogels[J]. Mater Sci.Eng C, 2021, 126: 112169.

