Identification of a three-gene prognostic signature for radioresistant esophageal squamous cell carcinoma
Xiao-Yan Wang,Narasimha M Beeraka,Nan-Nan Xue,Hui-Ming Yu,Ya Yang,Mao-Xing Liu,Vladimir N Nikolenko,Jun-Qi Liu,Di Zhao
Xiao-Yan Wang,Di Zhao,Department of Endocrinology,The First Affiliated Hospital of Zhengzhou University,Zhengzhou 450052,Henan Province,China
Narasimha M Beeraka,Nan-Nan Xue,Ya Yang,Jun-Qi Liu,Department of Radiation Oncology,The First Affiliated Hospital of Zhengzhou University,Zhengzhou 450052,Henan Province,China
Narasimha M Beeraka,Vladimir N Nikolenko,Department of Human Anatomy,I.M.Sechenov First Moscow State Medical University,Moscow 119991,Russia
Narasimha M Beeraka,Department of Pharmaceutical Chemistry,JSS College of Pharmacy,Mysuru 570015,India
Hui-Ming Yu,Department of Radiation Oncology,Peking University Cancer Hospital &Institute,Beijing 065005,China
Mao-Xing Liu,Key Laboratory of Carcinogenesis and Translational Research(Ministry of Education),Department of Gastrointestinal Surgery IV,Peking University Cancer Hospital &Institute,Beijing,China
Vladimir N Nikolenko,M.V.Lomonosov Moscow State University,Moscow 119991,Russia
Abstract BACKGROUND Esophageal squamous cell carcinoma(ESCC)is causing a high mortality rate due to the lack of efficient early prognosis markers and suitable therapeutic regimens.The prognostic role of genes responsible for the acquisition of radioresistance in ESCC has not been fully elucidated.AIM To establish a prognostic model by studying gene expression patterns pertinent to radioresistance in ESCC patients.METHODS Datasets were obtained from the Gene Expression Omnibus and The Cancer Genome Atlas databases.The edgeR,a Bioconductor package,was used to analyze mRNA expression between different groups.We screened genes specifically responsible for radioresistance to estimate overall survival.Pearson correlation analysis was performed to confirm whether the expression of those genes correlated with each other.Genes contributing to radioresistance and overall survival were assessed by the multivariate Cox regression model through the calculation of βi and risk score using the following formula:.RESULTS We identified three prognostic mRNAs(cathepsin S[CTSS],cluster of differentiation 180[CD180],and SLP adapter and CSK-interacting membrane protein[SCIMP])indicative of radioresistance.The expression of the three identified mRNAs was related to each other(r > 0.70 and P < 0.05).As to 1-year and 3-year overall survival prediction,the area under the time-dependent receiver operating characteristic curve of the signature consisting of the three mRNAs was 0.716 and 0.841,respectively.When stratifying patients based on the risk score derived from the signature,the high-risk group exhibited a higher death risk and shorter survival time than the low-risk group(P< 0.0001).Overall survival of the low-risk patients was significantly better than that of the highrisk patients(P = 0.018).CONCLUSION We have developed a novel three-gene prognostic signature consisting of CTSS,CD180,and SCIMO for ESCC,which may facilitate the prediction of early prognosis of this malignancy.
Key Words:Esophageal squamous cell carcinoma;CTSS;CD180;SCIMP;Radioresistance;TNM stage;Prognosis
INTRODUCTION
Esophageal cancer is one of the most commonly occurring gastrointestinal tumors and ranks 7thin incidence and 6thin death among all malignancies worldwide.The highest incidence rate was reported in China[1].Esophageal cancer includes two main pathological types,namely,esophageal adenocarcinoma and esophageal squamous cell carcinoma(ESCC),and 88% of ESCC cases originate in central and southern Asia[2].Surgery is the conventional method of treatment for early-stage esophageal cancer patients.Neoadjuvant radiotherapy is also reported to be a crucial therapeutic modality for treating advanced stage ESCC patients[3].However,the differences in sensitivity of each patient to radiation therapy result in variable prognoses among ESCC patients.ESCC is an aggressive malignancy with a poor overall survival[4].The available staging system is not very satisfactory in predicting the treatment outcome in ESCC patients,and the application of cancer genomics to predict clinical outcomes may improve the treatment of ESCC[5,6].
Tumor radiotherapy can induce either direct damage to DNA by inducing DNA double-strand breaks,or indirectly modulate cell signaling cascades to foster tumor cell death[7].However,the clinical outcomes of radiotherapy in most esophageal tumor patients predominantly depend on the inherent sensitivity of tumor cells to radioactive rays.Furthermore,tumor cell insensitivity can lead to the occurrence of radioresistance,which involves several cellular mechanisms including cell cycle checkpoint regulation[8],stemness acquisition[9,10],epithelial mesenchymal transformation(EMT)[11],and activation of multiple pro-survival and pro-proliferation signaling pathways[12,13].Furthermore,radioresistance is also mediated by tumor-associated microenvironment factors,such as hypoxiainduced HIF-1 signaling factors[14,15],tumor-associated fibroblasts(CAFs)[16],and tumor-associated macrophages[17,18].Hence,radioresistance is one of the significant reasons for the failure of radiotherapy in ESCC patients.High-throughput sequencing technology is a promising novel approach to identify genes that are related to tumor radioresistance in ESCC.Maheret al[19]identified a set of five genes includingEPB41L3,RTKN,STAT5B,NMES1,andRNPC1as biomarkers for response to neoadjuvant radiotherapy in esophageal cancer.Overexpression of PTK7 can activate NF-kB to enhance raidoresistance in radiosensitive ESCC cells[20].Transcriptome analysis delineated that theMALAT1-ATG9BandDDIT4-MB-PLATgenes could regulate radioresistance inin vitromodels of ESCC cells by modulation of autophagy and hypoxia pathways[21].The prognostic role and underlying genomic pathways pertinent to the acquisition of radioresistance in ESCC patients have not yet been fully unraveled.Therefore,it is crucial to identify biomarkers and genes pertaining to radioresistance in ESCC for selecting novel therapeutic modalities to mitigate radioresistance in this malignancy.
The current study identified mRNAs as potential radioresistance markers in ESCC cells with the aid of merged mRNA data collected from the Gene Expression Omnibus(GEO)and Cancer Genome Atlas(TCGA)databases.The study identified a three-gene signature,includingCTSS,CD180,andSCIMP,that may predict the development of radioresistance in ESCC cells.Furthermore,we constructed a prognostic model for radioresistant ESCC based on the risk scores derived from clinical features and the three-gene signature.
MATERIALS AND METHODS
GEO database search:Identifying ‘radioresistance-promoting mRNAs’
Primarily,the microarray profiles in GSE81812 dataset pertaining to ‘non-radiated KYSE-180 cells’ and “12 and 30 Gy radiated KYSE-180 cells” were downloaded from the GEO database(http://www.ncbi.nlm.nih.gov/geo/)to identify mRNAs contributing to radioresistance in ESCC cells.The edgeR package(www.bioconductor.org/packages/release/bioc/html/edgeR.html)was used to analyze the differential expression of mRNAs between different groups(‘0 Gy groupvs12 Gy group’ and ‘0 Gy groupvs30 Gy group’)to identify genes related to radioresistance.The cutoff parameters were false discovery rate < 0.05 and|Log2fold change|>2.
TCGA database search:Identification of ‘radioresistance-promoting mRNAs’ associated with overall survival
Gene expression profile and clinical information of ESCC patients in the TCGA database were downloaded(https://gdc-portal.nci.nih.gov/).Overall survival rates were determined to ascertain the prognostic significance of the identified radioresistance promoting mRNAs in the TCGA database;the overall survival rates were analyzed by using survival package in R through Kaplan-Meier analysis and finally compared using the Log-rank test and Cox proportional hazards regression analysis.Then,radioresistance-promoting mRNAs associated with overall survival were screened.
Multivariate Cox regression analysis:Construction of prognostic model based on ‘radioresistancepromoting mRNAs’ associated with overall survival
The association of radioresistance-promoting mRNAs with overall survival was estimated using the multivariate Cox regression model,adjusted for age,gender,grade,and stage,to calculate βi.The forest plot was plotted to exhibit the hazards regression(HR)of the multivariate Cox regression model results.Later,risk score was estimated by using the following formula:.By using the maximally selected rank statistics from the ‘survminer’ package in R,all samples were divided into a low-risk group and a high-risk group subsequently,and survival analysis was conducted to assess prognosis differences between the two groups.
Confirmation of relationship of ‘radioresistance-promoting mRNAs’ with overall survival,tumor stage,and tumor grade
Pearson correlation coefficients(P< 0.05)were calculated using r.test()in R to confirm whether the identified radioresistance-associated mRNAs were typically related to the stage and grade of ESCC.The results are shown in violin plots.
Kyoto Encyclopedia of Genes and Genomes pathway analysis
Kyoto Encyclopedia of Genes and Genomes(KEGG)pathway enrichment analysis of mRNAs associated with radioresistance in ESCC was performed using the ClusterProfile package(http://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html)for a more comprehensive understanding of biological features.APvalue < 0.05 was set as the cut-off criterion in the KEGG pathway.Data pertinent to KEGG pathway analysis is attached as a supporting file.
Statistical analysis
Statistical analyses were executed with the aid of SPSS 22.0 software(IBM,Chicago,IL,United States)and R version 3.6.0.Overall survival rate was estimated using the Kaplan-Meier method.Multivariate Cox proportional HR analysis was executed to identify prognostic factors(the three-gene signature,age,gender,tumor stage,and tumor grade).Differences between groups were compared using the Student’st-test or paired samplest-test.P< 0.05 was considered to have statistical significance.
RESULTS
GEO database search of mRNA expression profiles to identify radioresistance-promoting mRNAs
The gene count data of expression profiles of 22456 mRNAs in 41 samples of 0 Gy,92 samples of 12 Gy,and 89 samples of 30 Gy were obtained from the GSE81812 dataset downloaded from GEO.We identified upregulation of 1168 mRNAs in the ‘0 Gy groupvs12 Gy group’ comparison and 497 mRNAs in the ‘0 Gy groupvs30 Gy group’ comparison by using the edgeR package.To distinguish the differentially expressed mRNAs at different X-ray levels,the top 50 mRNAs are shown in a heatmap and principal component analysis(PCA)was performed(Figure 1A-D).A total of 379 intersection mRNAs were identified from the 0-12 Gy and 0-30 Gy comparisons as radioresistance associated genes.
Prognostic significance of radioresistance-promoting mRNAs from TCGA database
Log-rank test and Cox proportional hazards regression were adjusted for other confounding factors such as gender,age,stage,and grade.These statistical analyses were used to screen for prognostic genes,and a total of 5293 mRNAs were selected.Among them,44 mRNAs were significantly associated with radioresistance.We selected 23 mRNAs that were negatively correlated with prognosis for further analysis.The intersection of radioresistant prognostic mRNAs is visualized in a Venn diagram(Figure 1E).
Determination of correlations among radioresistance-promoting mRNAs
For the 23 mRNAs mentioned above,we primarily investigated whether their expression correlated with each other based on the data in the TCGA database.Although they were expressed at different levels in ESCC patients,the results showed strong correlations among three mRNAs,namely,CTSS,CD180,andSCIMP(r> 0.70 andP< 0.05).The correlations of 23 mRNAs are shown in a heatmap(Figure 2A).Hence,we selected these three mRNAs as radioresistance-promoting mRNAs of interest.Correlations of these three mRNAs are shown in a scattergram(Figure 2B-D).
Establishment of a gene signature as prognostic model for radioresistance
To explore the potential prognostic value of the above three mRNAs pertinent to radioresistance,we evaluated the overall survival rates of ESCC patients based on the expression patterns of these three mRNAs based on the data in the TCGA database by using Kaplan-Meier curves.As shown in Figure 3A,their low expression was associated with a good overall survival(TCGA database),and the median survival time was statistically significant(P< 0.05)for all the three mRNAs.
Subsequently,the connection between the three-gene signature and overall survival was explored through multivariate Cox regression model adjusted for patient age,gender,tumor grade,and tumor stage,for which,the HR with 95% confidence interval was depicted through the forest plot(Figure 3B).ROC analysis for the model is shown in Figure 3C(area under the curve:0.716 and 0.841 for 1- and 3-year survival,respectively).Accordingly,the risk score of each patient was calculated,and all the patients were divided into either a high risk group or a low risk group based on the risk score.
The patients of the high-risk group exhibited a ‘higher death risk and shorter survival time’ than the patients in the low-risk group;the heatmap of the three genes(CTSS,CD180,andSCIMP)showed that the high-risk patients typically had higher expression of these genes than the low-risk patients(Figure 3D-F).The Kaplan-Meier curves revealed that the low-risk patients typically with low expression of these three genes exhibited a good overall survival(Figure 3G).
External validation based on GEO dataset
To further validate the prognostic value of the three mRNAs,GSE53625 dataset was downloaded from the GEO database.As shown in Figure 4A,downregulation ofSCIMPexpression was associated with a good survival outcome.When patients were divided into two groups based onCTSSexpression,there was no statistically significant difference in their survival.CD180 expression also showed no significant correlation with survival.In the same manner,the risk score of GEO samples was calculated,and the overall survival of patient samples in the low-risk group was also higher than that of patient samples in the high-risk group(Figure 4B).The risk curve,scatter plot,and heatmap results were also similar to those obtained based on TCGA dataset(Figure 4C-E).

Figure 1 Comparative principal component analysis and heatmap analysis of up-regulated mRNAs between non-radiated and radiated KYSE-180 cell samples.A and B:Principal component analysis.Samples were clustered into two groups:0 Gy group vs 12 Gy group(A)and 0 Gy group vs 30 Gy group(B);C and D:Heatmap analysis.Upregulated genes are indicated in red whereas downregulated ones are indicated in green.The expression of mRNAs in radiated samples was comparatively higher than that in non-radiated samples:0 Gy group vs 12 Gy group(C)and 0 Gy group vs 30 Gy group(D);E:Venn diagram of radioresistance-promoting mRNAs associated with prognosis in esophageal squamous cell carcinoma.
Association of the three mRNAs with pathological grade and tumor-node-metastasis stage
We next explored the association between the three radioresistance-promoting mRNAs and pathological grade(Figure 5A-C)and tumor-node-metastasis(TNM)stage(Figure 5D-F).CTSS,CD180,andSCIMPexhibited significantly higher expression in advanced pathological grades(2-3vs1)and tumor stages(II-IVvsI).
Functional characteristics of CTSS,CD180,and SCIMP mRNAs
To further explore the underlying biological features of the three mRNAs in ESCC,we performed Pearson correlation between the three mRNAs,namely,CTSS,CD180,andSCIMP,and the other mRNAs to identify co-expressed mRNAs.A total of 539 mRNAs were selected for KEGG pathway enrichment analysis(P< 0.01,r> 0.4).Our results showed that the co-expressed mRNAs were mainly enriched in 50 pathways,including NF-kB,JAK-STAT,cell adhesion molecules signaling,and PD-L1 expression &PD-1 checkpoint pathways(Figure 6).

Figure 2 Heatmap and scattergram depicting the correlations of mRNAs.A:Heatmap showing correlations of 23 mRNAs contributing to radioresistance;B-D:Scattergram showing correlations between two of the three mRNAs:CTSS vs SCIMP(B),CTSS vs CD180(C),and CD180 vs SCIMP(D).
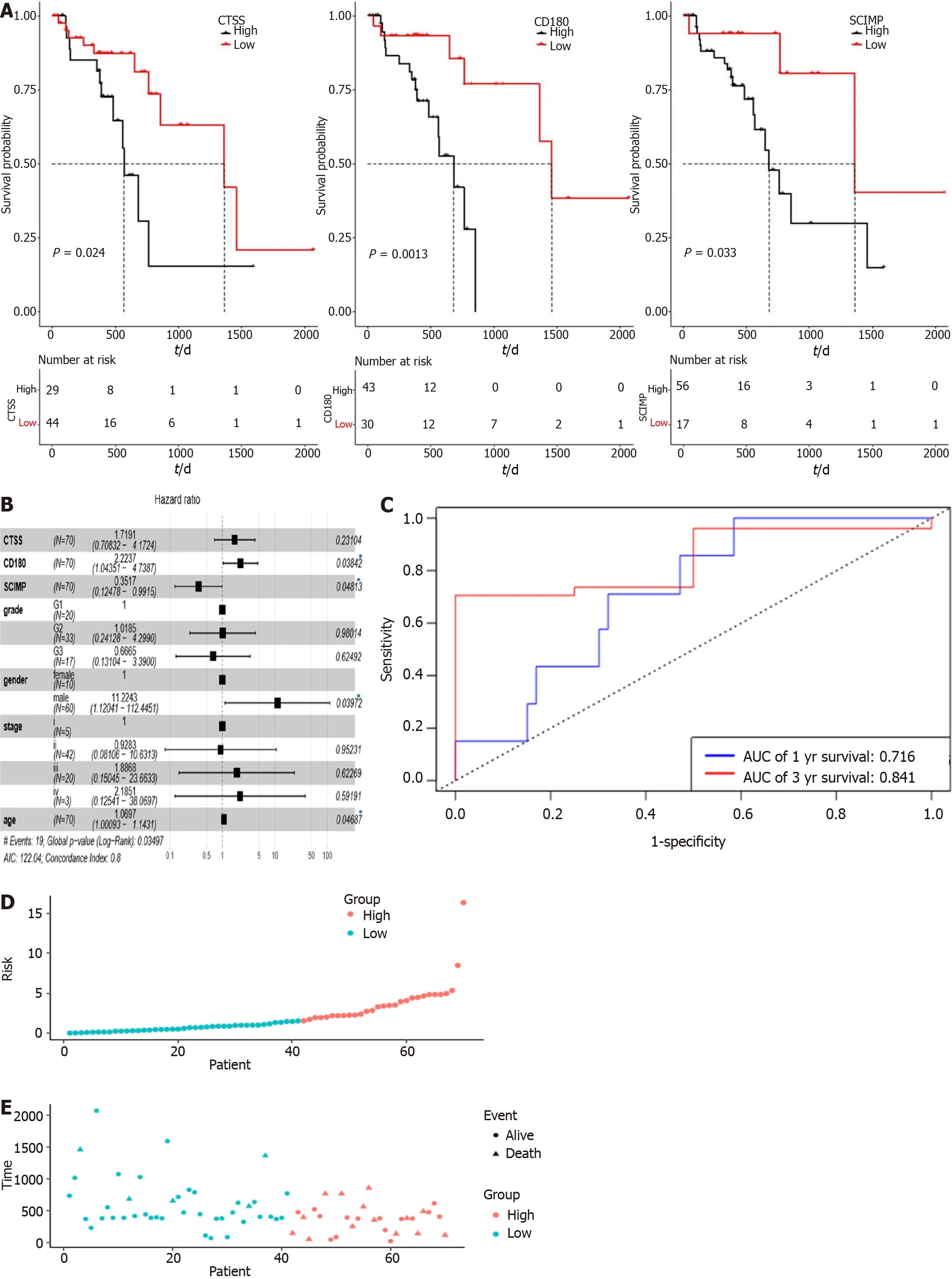
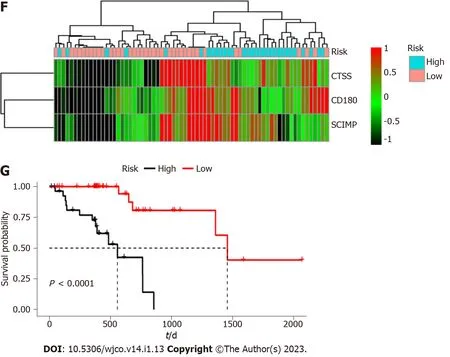
Figure 3 Kaplan-Meier curves.A:Kaplan-Meier survival curves by expression of CTSS,SCIMP,and CD180 based on data from TCGA database;B:Forest plot established with a hazard ratio calculated through multivariate Cox regression model adjusting for age,gender,grade,and stage(P < 0.05);C:Receiver operating characteristic analysis for 1- and 3-year overall survival prediction;D-F:Risk score distribution for patients in high risk group and low risk group.SCIMP,CD180,and CTSS had higher expression in high risk group than in low risk group;G:Kaplan-Meier survival curves for the high risk group and low risk group.
Figure 4 Validation of prognostic value of the three mRNAs based on dataset downloaded from the Gene Expression Omnibus database.A:Kaplan-Meier survival curves for SCIMP,CD180,and CTSS based on GSE53625 dataset;B:Kaplan-Meier survival curves for the high risk group and low risk group;C-E:Risk score distribution for patients in high risk group and low risk group.SCIMP,CD180,and CTSS had higher expression in high risk group than in low risk group.
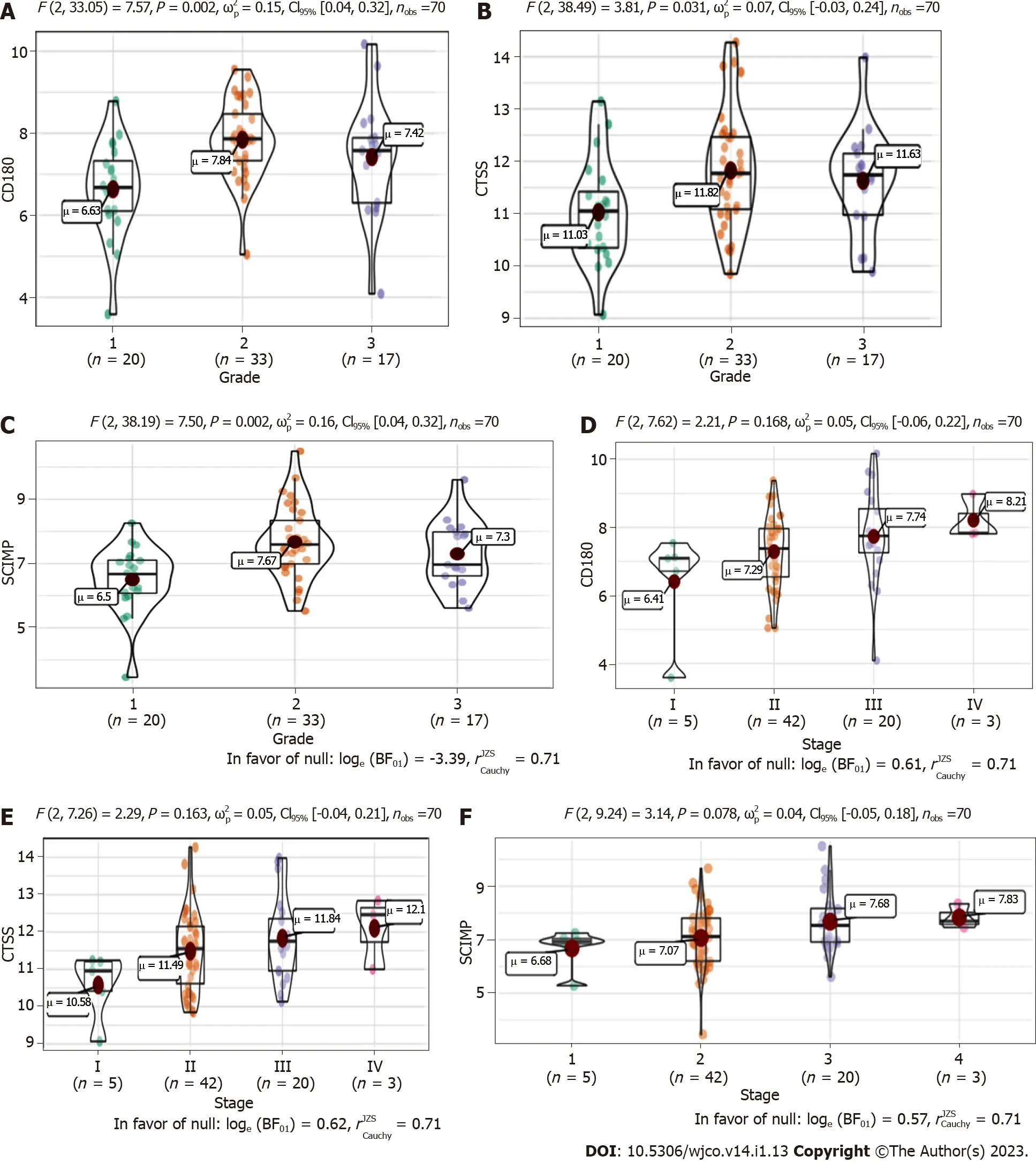
Figure 5 Association of the three radioresistance-promoting mRNAs(CTSS,CD180,and SCIMP)with tumor characteristics.A-C:Box plots showing a positive correlation of SCIMP,CD180,and CTSS with pathological grade;D-F:Box plots showing a positive correlation of SCIMP,CD180,and CTSS with tumor-node-metastasis stage.
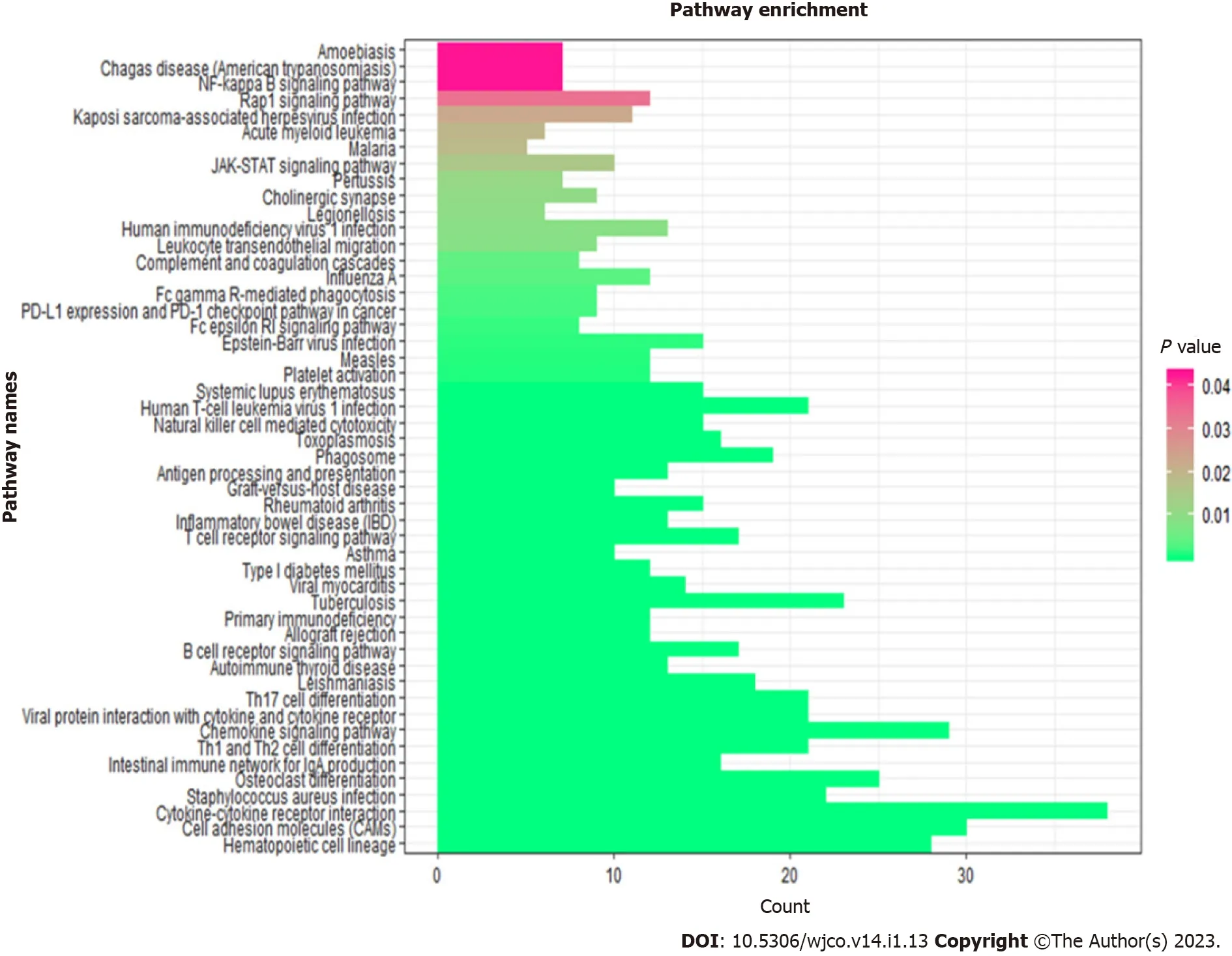
Figure 6 Kyoto Encyclopedia of Genes and Genomes enrichment analysis of co-expressed mRNAs.CTSS,CD180,and SCIMP genes can interact with other co-expressed mRNAs,which were mainly enriched in 50 pathways,such as NF-kB,JAK-STAT,PD-L1 expression &PD-1 checkpoint pathway,and cell adhesion molecules signaling pathways.
DISCUSSION
Prolonged and fractionated irradiation during radiotherapy in ESCC patients could confer radioresistance and result in distant metastasis,which may lead to treatment failure[22,23].CAFs can foster radioresistance in ESCC tumor cells through the long noncoding RNA DNM3OS by modulating the PDGFβ/PDGFRβ/FOXO1 signaling pathway,suggesting that CAFs-promoted DNM3OS could be a crucial target to reverse radioresistance in ESCC tumor cells.A study by Zhaoet al[24]in 2020,showed that three genes(FOXL2,TCF4,andNR2F2)exhibited a significant correlation with the prognosis of endometrial carcinoma;biological pathways associated with the low expression of these three genes were significantly enriched in cell cycle and fatty acid metabolism of cancer cells.However,there is limited evidence to validate the gene signatures involved in conferring radioresistance in ESCC patients to delineate accurate and efficient disease prognosis[25].Maet al[26]demonstrated that HMGB1 promotes radioresistance through the activation of autophagy.Furthermore,differentially expressed genes(DEGs)including ‘CFLAR,LAMA5,ITGA6,ITGB4,andSDC4’ in five signaling cascades(PI3KAKT pathway,CYCS gene-based apoptosis pathway,S100AX-AKT3-related pathway,SDC4 and HSPG2 pathway,and mTOR signaling pathway)were reported to be associated with radioresistance inin vitroESCC models,and tissue biopsies of ESCC patients[27].In the present study,we,for the first time,constructed a risk score model based on three radioresistance-associated mRNAs(CTSS,CD180,andSCIMP)and clinical features of ESCC patients;this model could facilitate oncologists to predict overall survival of ESCC patients with acquired radioresistance in radiotherapy.
A research study showed that the insulin-like growth factor 2 mRNA-binding protein 3 can contribute to the development of radioresistance in ESCC[28].miR-205 promotes radioresistance in ESCC typically through enhancing DNA repair,impairing apoptosis,and stimulating EMT[29].Another factori.e.,eEF2K,could foster the progression of radioresistance in ESCC[30].In our study,the involvement of three mRNAs(CTSS,CD180,andSCIMP)in radioresistance was analyzed through the transcriptome profiling of ESCC samples between non-radiated KYSE-180 cells and 12 or 30 Gy far infrared radiation-treated KYSE-180 cells and by constructing a risk score model.However,the overall survival information in GSE81812 dataset is unavailable,so we conducted univariate and multivariate Cox regression analysis based on the TCGA database,and identified 49 radioresistance-associated mRNAs associated with survival,of which 23 were inversely correlated with survival.After comprehensive correlation analysis,we selected three radioresistance-associated mRNAs(CTSS,CD180,andSCIMP)that were strongly correlated with each other based on the data in the TCGA database.Subramanianet al[31]deciphered that the well-developed genomic signatures are significantly beneficial for improving clinical outcomes in ESCC patients.Results of the overall survival of patients in this study suggested that patients with a higher risk score exhibited a poorer prognosis.Moreover,we downloaded the GSE53625 dataset as independent validation data to validate the prognostic role of the three-mRNA signature.Our result confirmed that the risk score model could also predict the survival outcome based on the external validation datasets.
Among the three mRNAs investigated,CTSSencodes a cysteine protease.Seoet al[32]showed radiation-inducedCTSSoverexpression,which can consequently promote radioresistance,and knockdown ofCTSScould induce impairment of radioresistance by modulating the ROS-IFN-γ pathway[32].Additionally,a plethora of research studies have found that CTSS is particularly involved in modulating autophagy pathways[33],PI3K/Akt and Ras/Raf/MAPK signaling pathways[34],and EGFR-ERK signaling pathway[35]as these signaling cascades are more or less involved in conferring radioresistance.However,there are no reports available in the literature to delineate that CD180 and SCIMP are involved in causing radioresistance in ESCC patients.CD180 belongs to the family of Tolllike receptors.Its expression has been reported to be associated with acute or chronic leukemia[36].SCIMPencodes a transmembrane adaptor protein that shapes host defense and inflammationviadirect modulation of TLR4[37].
A report by Yanget al[27]described the activation of the PI3K-Akt signaling pathway(KEGG ID:hsa05200)with upregulation of DEGs such asLAMA5,LAMB2,LAMB3,ITGA6,andITGB4at 12-Gy and 30-Gy fractionated irradiation.Thus,PI3K-Akt is reported to be involved in protecting KYSE-180 cells from undergoing apoptosis after irradiation.CYCSgene-based apoptosis pathway(KEGG ID:hsa04210)is impaired after 12-Gy irradiation due to the induction ofCYCSdownregulation.KEGG pathway analysis of S100AX-AKT3 signaling depicted that the activation of this pathway could enhance the migration and metastasis of HSCC KYSE-180-12 Gy and KYE-180-30 Gy cells[27,38].SDC4 and HSPG2[KEGG ID:hsa05205]are two proteoglycans that were reported to be upregulated during the irradiation of KYSE-180 cells at doses of 12 Gy and 30 Gy.These genes are responsible for tumor cell invasion and metastasis[27].In the present study,KEGG pathway analysis was performed to clarify the underlying mechanisms of the three mRNAs contributing to the radioresistance of ESCC cells.Our results showed that these mRNAs were mainly enriched in pathways that are related to radioresistance,such as the JAK-STAT signaling pathway[39]and NF-kB signaling pathway[40].Our results also demonstrated the radioresistance-promoting ability of these three mRNAs.Besides,these mRNAs were enriched in immune-related pathways,such as antigen processing and presentation,cytokine-cytokine receptor interaction,and Th17 cell differentiation.Hence,these three radioresistance-associated mRNAs might be involved in the regulation of immune pathways contributing to ESCC cell radioresistance.
CONCLUSION
In summary,our study proved that CTSS,CD180 and SCIMP can promote the development of radioresistance in ESCC patients.The novel three-gene signature developed based on the three genes can be used as a prognostic model to predict the prognosis of patients with radioresistant ESCC.
ARTICLE HIGHLIGHTS
Research background
Esophageal squamous cell carcinoma(ESCC)is causing a high mortality rate due to the lack of efficient early prognosis markers and suitable therapeutic regimens.
Research motivation
The prognostic role of genes responsible for the acquisition of radioresistance in ESCC has not been fully elucidated.
Research objectives
To establish a prognostic model by studying gene expression patterns pertinent to radioresistance in ESCC patients.
Research methods
Datasets were obtained from the Gene Expression Omnibus and The Cancer Genome Atlas databases.The edgeR,a Bioconductor package,was used to analyze mRNA expression between different groups.We screened genes specifically responsible for radioresistance to estimate overall survival.Pearson correlation analysis was performed to confirm whether the expression of those genes correlated with each other.Genes contributing to radioresistance and overall survival were assessed by the multivariate Cox regression model through the calculation of βi and risk score using the following formula:.
Research results
We identified three prognostic mRNAs(cathepsin S[CTSS],cluster of differentiation 180[CD180],and SLP adapter and CSK-interacting membrane protein[SCIMP])indicative of radioresistance.The expression of the three identified mRNAs was related to each other(r> 0.70 andP< 0.05).As to 1-year and 3-year overall survival prediction,the area under the time-dependent receiver operating characteristic curve of the signature consisting of the three mRNAs was 0.716 and 0.841,respectively.When stratifying patients based on the risk score derived from the signature,the high-risk group exhibited a higher death risk and shorter survival time than the low-risk group(P< 0.0001).Overall survival of the low-risk patients was significantly better than that of the high-risk patients(P= 0.018).
Research conclusions
We have developed a novel three-gene prognostic signature consisting ofCTSS,CD180,andSCIMOfor ESCC.
Research perspectives
The three-gene signature developed in this study may facilitate the prediction of early prognosis of this malignancy.
FOOTNOTES
Author contributions:Wang XY,Beeraka NM,Xue NN,Yu HM,Yang Y,Liu MX,Nikolenko VN,Liu JQ,and Zhao D conceptualized and designed the study;Beeraka NM,Wang XY,Liu JQ,Zhao D,Xue NN,Yu HM,Nikolenko VN,and Yang Y performed the literature analysis and drafted the manuscript;Beeraka NM,Liu JQ,and Zhao D revised,edited,and extended the final draft;all authors have reviewed and approved the manuscript before submission;Wang XY and Beeraka NM contributed equally to this work.
Institutional review board statement:Since the data of this study were obtained from the TCGA and GEO public databases,in which no personal identification information was included,informed consent was waived by the First Affiliated Hospital of Zhengzhou University.
Conflict-of-interest statement:All the authors declare that they have no conflict of interest to disclose.
Data sharing statement:All the supplementary files can be provided upon request by the editorial office as the data was obtained from the TCGA and GEO databases.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution Non Commercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Jun-Qi Liu 0000-0002-6015-1033.
S-Editor:Liu JH
L-Editor:Wang TQ
P-Editor:Liu JH

