Reducing systemic absorption and macrophages clearance of genistein by lipid-coated nanocrystals for pulmonary delivery
Yun He,Chng Liu,Run Hn,Yingmin Ling,Juith Choi Wo Mk,Yingho Zhu,Hifeng Li,Ying Zheng,*
a State Key Laboratory of Quality Research in Chinese Medicine,Institute of Chinese Medical Sciences,University of Macau,Macau,China
b Department of Pharmacy,Xuzhou Medical University,Xuzhou 221004,China
c LKS Faculty of Medicine,Department of Medicine,The University of Hong Kong,Hong Kong,China
d Institute of Applied Physics and Materials Engineering,University of Macau,Macau,China
Keywords:Pulmonary delivery Lipid-coated nanocrystals Cell uptake Transport Systemic absorption Macrophage clearances
ABSTRACT Pulmonary delivery is an effective drug delivery strategy for the treatment of local respiratory diseases.However,the rapid systemic absorption through the lung due to the thin barrier and persistent lung clearances influence the drug retention in the lung.In this study,we designed a lipid-coated genistein nanocrystals (Lipo-NCs) formulation to achieve enhanced efficiency of local pulmonary delivery.The Lipo-NCs were fabricated by modifying genistein nanocrystals (NCs) with phospholipid membrane through thin film hydration following the homogenization method.The prepared Lipo-NCs exhibited a decreased drug release rate compared with the naked NCs.Our results demonstrated that intracellular uptake and transcellular transport of NCs by the Calu-3 epithelial layer were reduced after lipid coating.Furthermore,the macrophages clearance was also impeded by this Lipo-NCs formulation. In vivo lung retention and distribution revealed that more genistein was retained in the lung after intratracheal administration of Lipo-NCs.The pharmacokinetic study displayed that the AUC(0-t) values of Lipo-NCs were 1.59-fold lesser than those of the NCs group,indicating a reduced systemic absorption.In conclusion,this research indicated that Lipo-NCs could be a suitable formulation for reducing systemic absorption and macrophages clearance,and thus enhancing drug concentration in lung by pulmonary delivery.
In recent years,lung diseases including pneumonia,asthma,chronic obstructive pulmonary disease (COPD) and cystic fibrosis have become a big threat to human health.According to the statistics,over 300 million adults and children suffered from asthma worldwide [1],and COPD was evaluated to be the third leading cause of death in 2030 [2].Pulmonary delivery of therapeutic drugs for these lung diseases is a promising strategy since it allows targeted drug to be delivered to the lung with a relatively high local concentration and low systemic exposure compared with the oral or parenteral routes [3–5].
Currently,the commercial inhalable drug products for the treatment of lung diseases are mainly fast-release formulations.Despite great success in commercial products,these formulations have faced the dilemma of reaching the peak concentration of the drug immediately after administration and the subsequent rapid decline in drug concentrations [5],leading to undesirable systemic side effects and increased administration frequency.This phenomenon was mainly attributed to the systemic absorption of drugs.Because the lung has a large alveolar surface area (about 100 m2),and the lung epithelial barrier is quite thin,the free drug molecules released from deposited particles are easily transported across the lung epithelium and to be absorbed into the bloodstream [6].While this property facilitates systemic delivery,it is not beneficial for local delivery of drugs that need to be retained in the lung.Besides,the lung clearances,including mucociliary clearance and macrophages clearance,could also reduce the available drugs [2,7].Many microparticles designed with suitable particle size for deposition were easily cleared by macrophage phagocytosis [7].These clearance and systemic absorption processes ultimately result in decreased retention of drugs in the lung.In this context,drugloaded particles with sustained-release property and prolonged retention ability are desirable for the treatment of local lung diseases by pulmonary delivery.
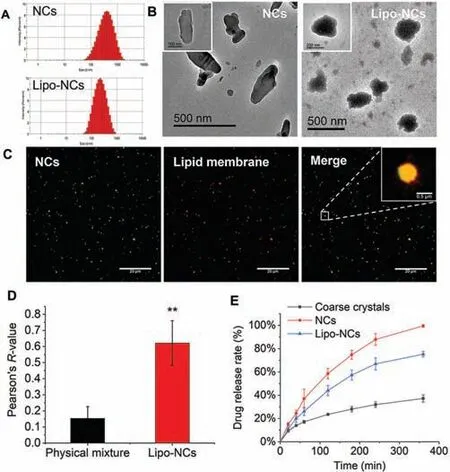
Fig.1.(A) Size distribution of GES NCs and Lipo-NCs; (B) TEM images of NCs and Lipo-NCs; (C) representative CLSM images of Lipo-NCs; the NCs was stained with coumarin 6 (green),the lipid membrane was stained with DiD (red),and the fluorescent points showed good colocalization in the merged Lipo-NCs group (the scale bar is 20 μm,the scale bar of the enlarged image on the right corner is 0.5 μm); (D)Pearson’s colocalization value of the physical mixture of NCs and lipid membrane and Lipo-NCs calculated by ImageJ software (n=6,**P<0.01); (E) in vitro drug release profile of GES formulations (n=3).
In recent years,the utilization of nano-drug systems for pulmonary delivery has attracted increasing attention because their small size could help avoid macrophages phagocytosis [8,9].Nanocrystals (NCs),the submicron drug particle dispersions system [10],are considered as a promising drug platform for pulmonary delivery due to their high drug loading capacity and low excipient addition property [11,12].For pulmonary delivery,NCs could improve the homogeneity of hydrophobic drugs in nebulized aerosols and exhibited well aerodynamic performance for deposition [13–15].However,the NCs dissolved very rapidly,which resulted in a fast systemic absorption in the lung [16].Amikacin liposome inhalation (ALIS; Arikayce®) with well biocompatibility and biosafety has been approved for use as part of a combination antibacterial drug regimen in the USA.More importantly,the phospholipid membrane enfolded vesicle displayed a declined drug release rate [17].Inspired by these properties,we considered combining the two nano-drug systems to obtain a novel lipid-coated nanocrystal drug delivery system with sustained-release property and high drug loading capacity.Furthermore,we would like to investigate how the release property influences the lung epithelial uptake and transport,and find out whether this formulation reduces lung clearance.
In this study,we fabricated a lipid-coated nanocrystal formulation to reduce lung clearance and prolong local retention by pulmonary delivery.The hydrophobic drug genistein (GES),which was an isoflavonoid compound derived from soy,was used as the model drug for nanocrystal preparation [18,19].It exhibited anti-inflammatory and antioxidant activities for the treatment of pulmonary diseases such as acute lung injury [20] and COPD [21].The lipid-coated NCs were prepared by coating the phospholipids on the surface of genistein NCs cores by the thin film hydration following the homogenization method.The systemic absorption and clearance of this nanocarrier were then assessed by thein vitroCalu-3 cell uptake,transport and macrophages phagocytosis studies.After that,the drug retention and biodistributionin vivowere also studied.The systemic absorption of GES was assessed by measuring the drug concentration in plasma after intratracheal administration.
The GES NCs were prepared by ball wet milling method.The parameters of the mill machine including rotation speed and milling time were adjusted to obtain GES NCs.After the preparation of NCs,the lipid membrane was formed and coated on the surface of NCs.In this lipid membrane,lecithin and cholesterol were used as the main lipid materials.TPGS was added to improve the stability of lipid coated NCs.Stearamine were incorporated to obtain a positive charged lipid membrane,so that the lipid membrane could successfully coat on the surface of NCs which possessed a negative charge.Size distributions of GES NCs and Lipo-NCs were shown in Fig.1A.The prepared GES NCs was at the size of 289.7±21.3 nm with the zeta potential of -30.4±8.1 mV.After coating with the lipid membrane,Lipo-NCs were 206.2±15.3 nm with the zeta potential of 21.1±4.1 mV.The size,polydispersity index (PDI) and zeta potential values were listed in Table S1 (Supporting information).The size reduction of Lipo-NCs was probably due to the high-pressure homogenization process.The stability of NCs and Lipo-NCs in cell culture mediums were shown in Fig.S1(Supporting information).
Transmission electron microscope (TEM) images revealed that NC had a rod-like shape.Whereas,the Lipo-NC had a more spherical shape with a core-shell structure (Fig.1B).As shown in the Lipo-NCs group,the black core possibly referred to the NC,the grey shell referred to the phospholipids on the surface of NC.The core-shell structure of Lipo-NCs was further validated by the double fluorescence labelling method by using confocal laser scanning microscopy (CLSM,Leica TCS SP8,German).According to Fig.1C,the green and red fluorescence showed well colocalization in the Lipo-NCs group.Pearson’sR-value of the Lipo-NCs group was 0.62,which was significantly higher than that of the physical mixture group 0.15 (P<0.01) (Fig.1D).This indicated that the phospholipids were successfully coated on the NCs.
Thein vitrorelease study of GES NCs,Lipo-NCs and coarse crystals was carried out in a dialysis bag in PBS 7.4 medium,and the release profile was shown in Fig.1E.According to the result,the GES coarse crystals displayed the slowest drug release rate.There was only about 37% of drugs released within 6 h.For the NCs group,the drug release rate was significantly increased with nearly 100% GES dissolving within 6 h,compared with the group of the coarse crystal.This enhancement was attributed to the reduced particle size of crystals and increased total surface area [11].After lipid coating on NCs in the Lipo-NCs group,the drug release rate decreased.About 78% of the drug was released from Lipo-NCs after 6 h.This indicated that coating phospholipid membrane on the surface of NCs could impede the release of drug.
Since systemic absorption of the drug from the lung to blood circulation is a major lung clearance pathway that influences local drug concentration,reducing systemic absorption is a useful strategy to increase drug retention in the lung.In this study,the systemic absorption process was elucidated by lung epithelial cells uptake and transport studies,and Calu-3 cells were used as the cell model because the characteristics of these cells were similar with bronchiolar epithelium [22].The viability of cells treated with GES formulations with different concentrations was assessed by MTT method (Fig.2A).The NCs group showed no obvious cytotoxicity.However,for the Lipo-NCs group,the cell viability decreased at the concentration of 200 μg/mL.It has been reported that the integrity and viability of Calu-3 cells decreased when particles with positive charge were used in the cell layers without mucus protection.However,when there was a mucus blanket lying on the Calu-3 cells,the integrity and viability remained [23].Therefore,the decreased cell viability in this study was possible due to the positive charge of Lipo-NCs.In contrast,under thein vivocondition,the integrity and viability of lung epithelial cells would not be influenced due to the presence of the mucus on the airways of the lung.In further cell studies,a concentration of GES at 50 μg/mL was chosen to ensure the cell viability.
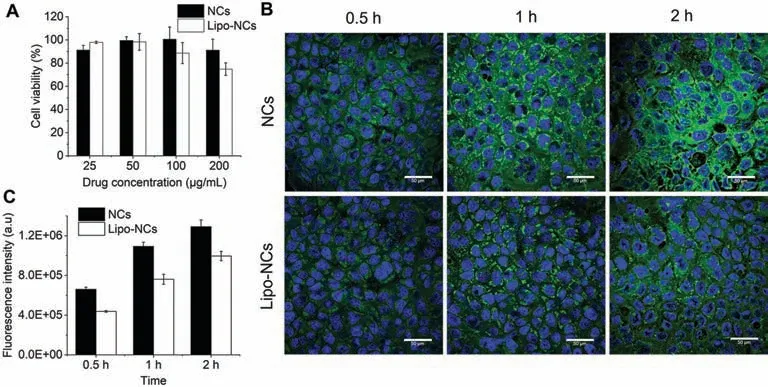
Fig.2.(A) Cell viability of Calu-3 upon exposure to GES formulations at different drug concentrations; (B) CLSM images of Calu-3 cells after incubation with GES NCs and Lipo-NCs at different time points (the blue fluorescence represented nucleus; the green fluorescence was C6 labelled GES formulations; the scale bar is 50 μm); (C) mean fluorescence intensity of cells determined by flow cytometry after incubation (n=3).
The cell uptake studies were conducted by adding C6 labelled GES formulations in Calu-3 cells and incubating them for different time lengths (the concentration of C6 in GES formulations was about 2.5 μg/mL).The images acquired by CLSM were shown in Fig.2B.Both GES formulations exhibited a time-dependant uptake manner that the fluorescence increased with incubation time.The Calu-3 cells treated with the NCs group showed a brighter green fluorescence at every time point when compared with the Lipo-NCs.This indicated that coating phospholipid on NCs could decrease the cellular uptake amount.Quantitative analysis of the intracellular fluorescence intensity by flow cytometry also showed that the uptake of Lipo-NCs by Calu-3 was lower than NCs at all tested time points which was consistent with the CLSM result(Fig.2C).Previously,researchers found that nanoparticles with positive surface charge were more easily internalized by Calu-3 cells compared with neutral and negative charged nanoparticles[23].However,in this study,the cell uptake of positive charged Lipo-NCs was less than the negative charged NCs.To further explore the underlying mechanism,we calculated the ratio of drug release rate in NCs and Lipo-NCs at 40 min (0.5 h points were lacked in release profile),1 h and 2 h points.The result showed that the release rate of NCs were 1.23-fold,1.41-fold and 1.33-fold greater than Lipo-NCs,respectively.The intracellular fluorescence intensity determined at 0.5 h,1 h,and 2 h showed that the uptake of NCs were 1.51-fold,1.44-fold and 1.30-fold higher than Lipo-NCs,respectively.This result showed that the drug release rate and cellular uptake had a good correlation.The decreased cellular uptake of Lipo-NCs group was possibly due to the decreased drug release rate.
The penetration of GES formulations across lung epithelium was then investigated by using the Calu-3 cell layer by the air-liquid interface culturing (ALC) method.This culturing method formed a pseudostratified Calu-3 columnar epithelium which was similar to thein vivobronchiolar epithelium because tight junctions were formed,and mucus was secreted on the cell surface [24,25].The schema of NC formulations transport across the Calu-3 cell layer was depicted in Fig.3A.After 2 h exposure,results showed that a lower amount of GES transported across the Calu-3 layer at every time point in the Lipo-NCs group compared with that of the NCs group (Fig.3B).And the calculatedPappvalue of Lipo-NCs ((2.32±0.31)×10-6cm/s) was also significantly smaller than that of NCs ((3.46±0.48)×10-6cm/s) (P<0.05) (Fig.3C).The GES coarse crystals group had the lowest drug transport amount and thePappvalue ((1.67±0.16)×10-6cm/s) because of its slow dissolution rate.Then,the fluorescence of the Calu-3 layer after transport of GES formulations was observed by 3D andz-stack confocal models (Figs.3D and E).It is worth mentioning that the Calu-3 cells cultured by the ALC method formed a pseudostratified layer(Fig.3E).The cells incubated with Lipo-NCs had a weaker fluorescence than naked NCs.Images taken by using thez-stack scanning model (Fig.3E) also exhibited that the green fluorescence of Lipo-NCs in the basolateral side of the Calu-3 cell layer was weaker than that of the NCs group.These results indicated that after lipid coating,the transport of GES had been impeded,fewer drugs were transported across the lung epithelium.According to the result of Calu-3 uptake study (Fig.2),the uptake amount of Lipo-NCs was lower than NCs because of the slower drug release rate.The impeded transport of Lipo-NCs could attribute to less drug accumulation within the cells.Another reason that was responsible for the transport was the mucus secreted on the surface of Calu-3 layer.It was reported that the respiratory mucus had a mesh-like structure with negative charge.The positive-charged Lipo-NCs could interact with the mucus layer resulting in reduced contact with cells and decreased transport.This feature of Lipo-NCs could help increase the local retention of the drug in the lung.
Macrophage phagocytosis is another important lung clearance pathway [26].To evaluate whether lipid coating could help avoid macrophages clearance,the phagocytosis of GES formulations by RAW 264.7 cells was conducted.The CLSM images at 0.5 h and 2 h were shown in Fig.4.The fluorescence intensity of RAW 264.7 cells in both NCs and Lipo-NCs groups increased with the incubation time.Whereas the macrophage phagocytosis of Lipo-NCs decreased when compared with naked NCs.According to the particle size of NCs and Lipo-NCs in DMEM (Fig.S1),the particle size kept stable within 2 h in both groups.But the size of Lipo-NCs was smaller than NCs.It was reported that the macrophages preferred to phagocytize particles with larger size [3].Therefore,the reduced phagocytosis effect of Lipo-NCs was possibly due to the smaller particle size in the culture medium.We further investigated the integrity of Lipo-NCs after phagocytosis by RAW 264.7 cells.The C6 labelled NCs and DiD labelled lipid showed well colocalization which demonstrated that the particles were phagocytized integrally.This study indicated that the Lipo-NCs formulation could decrease macrophages phagocytosis clearance in the lung and further contribute to prolong drug retention.
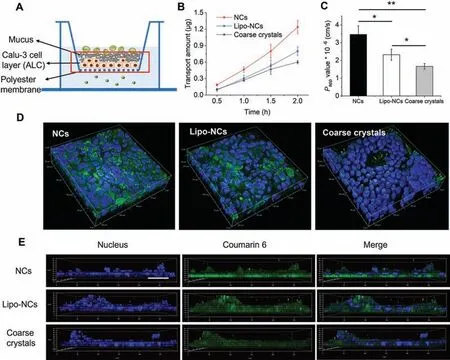
Fig.3.(A) A schema of NCs transport across Calu-3 cell layer; (B) transport amount of GES across Calu-3 cell layer at different time points; (C) Papp values of GES formulations after transport across Calu-3 cell layer (n=3,*P<0.05,**P<0.01); (D) 3D images of Calu-3 cell layer after transport of GES formulations; (E) z-stack confocal images of the Calu-3 cell layer with the polyester membrane after transport of GES formulations for 2 h (the scale bar in xy plane was 50 μm).
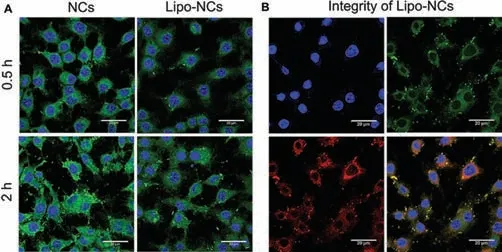
Fig.4.(A) CLSM images of RAW 264.7 cells after phagocytosis of NCs and Lipo-NCs at 0.5 h and 2 h (the blue fluorescence represented nucleus; the green fluorescence was C6 labelled GES formulations; the scale bar is 20 μm); (B) integrity of Lipo-NCs after phagocytosis by RAW 264.7 cells (the green fluorescence was C6 labelled GES NCs,and the red fluorescence was DiD labelled lipid membrane).
The retention of GES in the lung after intratracheal administration of C6 labelled GES formulations were observed byin vivoImaging System (IVIS,Lumina XR III) (Fig.5A).In this study,all relevant animal experiments were approved by the Animal Ethics Committee of the University of Macau.The result showed that the fluorescence in the lungs was brighter after 0.5 h administration than at 2 h.The decreased fluorescence was attributed to the lung clearance and systemic absorption.By comparing the fluorescence between the two GES formulations,the fluorescence of the Lipo-NCs group was brighter than the NCs group.It is important that these results were consistent with ourin vitrorelease and transport studies,indicating that Lipo-NCs would obtain a slower drug release rate,a decreased Calu-3 cell layer transport amount and a higher retention in the lung after lipid coating.All above results suggested that the less retention of NCs mainly attributed to their fast systemic absorption.Then,the distribution of GES formulations within the lung was observed by tissue sections using CLSM visually.The fluorescence intensity shared a similar trend with the IVIS images.In the airway of the lung,a lot of Lipo-NCs particle fluorescence could be found in the lumen after 0.5 h administration (Fig.5B),while only a few particles could be observed in NCs.In the parenchyma region,many bright spots could also be found in Lipo-NCs and NCs groups.After 2 h administration,the fluorescence of both GES groups became weaker.Some particles of Lipo-NCs still remained in the interval space of cilium and parenchyma of the lung.But the bright spots could hardly be observed in the NCs group at 2 h.
The GES retention in the lung after intratracheal administration was then quantitatively detected by LC-MS/MS.According to the results in Fig.6A,the GES concentration in lung tissue of the Lipo-NCs group was higher than the NCs group without lipid coating at 0.5 h point.This result coincided with the tendency of fluorescence observation in Fig.6 that the lipid coating on NCs could increase the retention of GES in the lung.After 2 h administration,the concentration of GES in the lung was further decreased in both groups.The decline of Lipo-NCs group was even faster than NCs from 0.5 h to 2 h.The reason was possibly that NCs dissolved quickly after deposition,the dissolved drug was then rapidly absorbed from the lung epithelium into the blood circulation leading to a fast decline of drug within 0.5 h.For the Lipo-NCs group,because of the slower drug release,the decline was gentler within 0.5 h.However,the unreleased Lipo-NCs particles in lung would undergo the lung clearance processes including mucociliary movement clearance,macrophages clearance and translocation from the lung to lymphatic system.These clearances preferred to clear particles rather than dissolved drug,therefore resulting in a faster decline of Lipo-NCs from 0.5 h to 2 h.
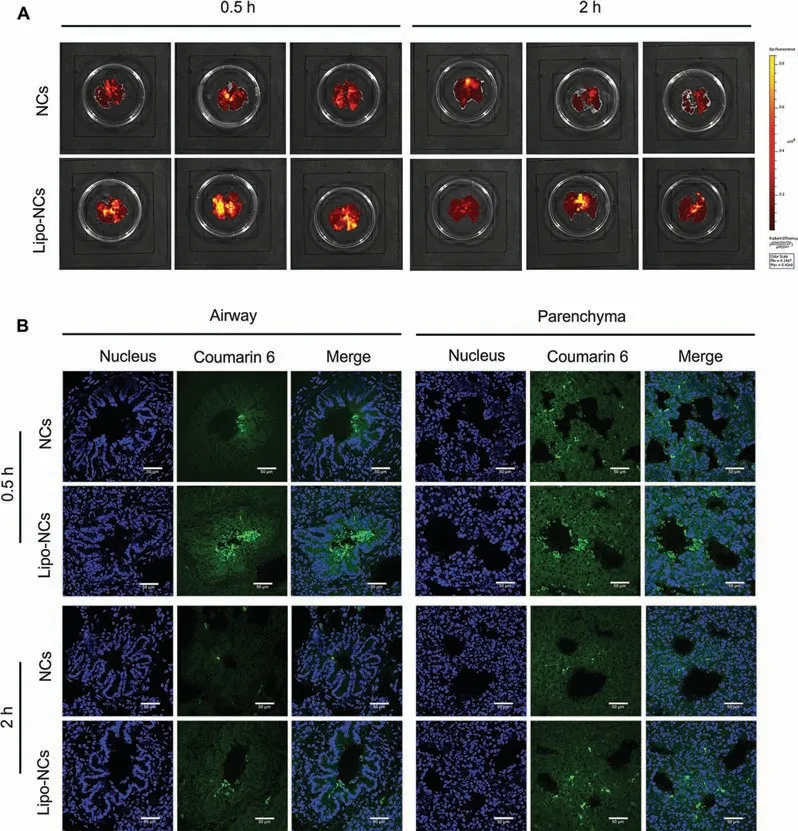
Fig.5.(A) Ex vivo imaging of the whole lungs after intratracheal administration of GES formulations at the different time points (0.5 and 2 h); (B) histological examination of GES formulations distribution in the airway and parenchyma regions of the lung after intratracheal 0.5 h (upper panel) and 2 h (lower panel) administration (the scale bar is 50 μm).
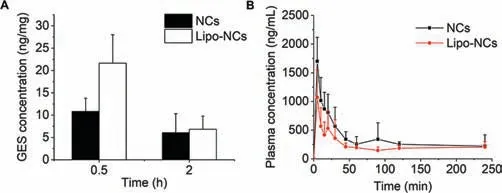
Fig.6.(A) Lung concentration of GES after intratracheal administration of NC formulations (n=3); (B) plasma concentration of GES after intratracheal administration of NC formulations at a dose of 5 mg/kg (n=4).
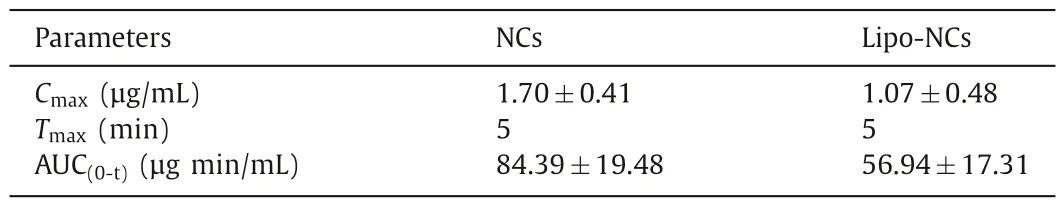
Table 1 Pharmacokinetic parameters of GES after intratracheal administration of formulations to rats (n=4).
The pharmacokinetic profiles of GES were indicative of the combined effect of several influence factors including dissolution,clearance and absorption in the lung.The plasma concentration of GES over time and pharmacokinetic parameters were shown in Fig.6B and Table 1,respectively.From the profiles,both NCs and Lipo-NCs groups reached the peak concentration at 5 min.Then the plasma concentration declined with time.The rapid pulmonary absorption could be attributed to the large surface area of lung alveoli and the thin anatomical barriers that limit the access to the blood [27].It was reported that the pulmonary absorption of lipophilic small molecules (logP >0) was in approximately 1 min [28].The GES peak concentration of the NCs group was 1.70±0.41 μg/mL which was 1.59-fold higher than that of the Lipo-NCs group.The calculated AUC(0-t)value of the NCs group was 1.48-fold greater than Lipo-NCs.This result indicated that the GES in the NCs group was more easily absorbed into the blood circulation.However,the statistical analysis of AUC(0-t)between NCs and Lipo-NCs groups was not significant (P >0.05) possibly because of the large difference between individual animals.Since the absorption of lipophilic GES free drugs happened within a minute,the rate-limiting step of absorption should be the drug release process.Therefore,the main reason leading to less absorption of GES from lung to blood in Lipo-NCs was attributed to the slower drug release rate.On the other hand,less systemic absorption facilitates more accumulation of drugs in the lung.
In this study,lipid coated GES NCs were fabricated to enhance the drug accumulation and retention in the lung after pulmonary delivery.These Lipo-NCs showed a slower drug release ratein vitrowhen compared with the naked NCs.The Calu-3 cell uptake and transport studies demonstrated that Lipo-NCs formulation could decrease the transport of GES from apical side to basolateral side of the lung epithelium.And this lipid-coated formulation could also reduce macrophage clearance.Thein vivoretention and distribution studies verified that Lipo-NCs enhanced the drug concentration and residence in the lung by reducing the systemic absorption and macrophages phagocytosis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Multi-Year Research Grants from the University of Macau (No.MYRG2019–00032-ICMS),Natural Science Foundation of Jiangsu Province (No.BK20210912),the startup grant of Xuzhou Medical University (D2021004),and Macau Science and Technology Development Fund (No.0017/2019/AKP).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.04.082.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
