Wet-adhesive materials of oral and maxillofacial region: From design to application
Yilin Mao,Zhengyi Xu,Zihan He,Jian Wang,*,Zhou Zhu,*
a State Key Laboratory of Oral Diseases,National Clinical Research Center for Oral Diseases,West China Hospital of Stomatology,Sichuan University,Chengdu 610041,China
b West China School of Stomatology,Sichuan University,Chengdu 610041,China
Keywords:Oral cavity Oral and maxillofacial region Wet adhesion Stomatology Adhesive materials Mucoadhesion Tissue engineering
ABSTRACT Oral and maxillofacial diseases are a group of high-incidence disorders that affect people’s life quality to a great extent,while the wet and highly movable environment of the related regions brings challenges to traditional therapies.Faced with the obstacles of insufficient adhesive strength and ensuing short drug retention time,conventional oral therapeutic agents often have difficulty in achieving their desired efficacy.Oral and maxillofacial wet-adhesive materials have the advantages of excellent wet environment retention,internal stability,plasticity,and clinical potential,thus have become a significant research direction in the field of oral related disorders healing.In the past decade,the development of oral adhesive materials with good wet adhesion has accelerated based on the chemical molecular interaction,physical interlocking,and biological adhesion mechanisms,including biomimetic-inspired materials,naturally derived polymer–based materials and adhesive electrospun fiber films.These fancy wet-adhesive materials can be used for oral mucosal drug delivery,oral vaccination,wound healing,and bone defects treatments.Despite their numerous novel applications,wet-adhesive materials in stomatology still face unresolved challenges from material and biological aspects.Here,advances in designs of oral and maxillofacial wetadhesive materials are reviewed in terms of design backgrounds,attachment mechanisms,and common classifications.Recent demonstrations of wet-adhesive materials for oral and maxillofacial region medical applications from drug delivery to multifunctional tissue treatments are presented.To conclude,current challenges and prospects on potential applications of oral and maxillofacial wet-adhesive materials are also briefly discussed.
1.Introduction
Oral diseases are among the most prevalent diseases globally [1].At present,millions of people worldwide are afflicted with oral-related disorders such as oral mucositis,periodontitis,and maxillofacial bone defects.Currently,a variety of conventional treatments have been implemented in clinical practice to cure oral disorders,including ointments,powders,solutions,and wound suturesetc.[2–4].However,the oral cavity is characterized by its wet environment with continuously endogenous saliva secretion as well as the exogenous water flushing from food or drink.Besides,some movements caused by the oral cavity and its adjacent maxillofacial regions,such as masticating,speaking,swallowing also endows the oral tissue with the characteristic of high dynamic.Thus,based on the anatomical traits and physiological functions of the oral cavity and maxillofacial regions above,therapeutic obstacles such as loss of adhesion,blood contamination,weak resistance towards dynamic movement make the existing treatments less effective.
Adhesives are promising alternatives in clinical application as tissue adhesives or tissue sealants [5].While the majority of adhesives are applied externally to the skin,the necessity for adhesives to exert their functions under wet or submerged oral conditions is now being recognized.Synthetic polymers such as hydroxypropylmethylcellulose,synthetic polyacrylic acid and fibrin glues were used extensively in stomatology [6–8].However,these materials either have decreased interpenetration at the initial stages of adhesion or exhibit weak bonding strength [9,10].These limitations make the bio-adhesives listed above largely inappropriate for routine clinical applications.Therefore,achieving adequate adhesion of materials against oral and maxillofacial region remain a challenge to establish in the real market.
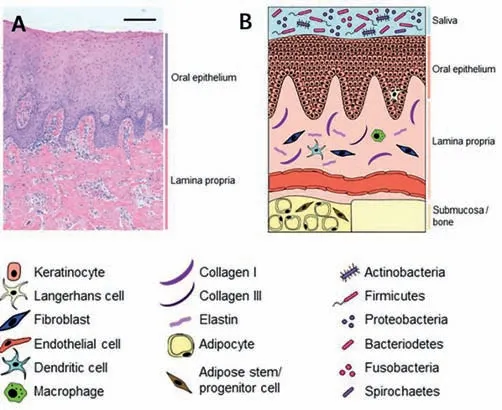
Fig.1.Healthy oral mucosa.(A) Histological healthy gingiva tissue.Hematoxylin and eosin staining of 5 μm paraffin-embedded tissue sections.Scale bar: 100 μm;(B) graphical illustration of oral mucosa.Copied with permission [24].Copyright 2021,MDPI.
New strategies in biomaterials engineering have led to the advancements of more oral-related wet adhesives.Recently,some materials with superior adhesive properties have demonstrated their potential in the wet environment and thus emerged as innovative solutions to address the obstacles of current therapeutics in stomatology.According to the various types of design mechanisms,oral and maxillofacial materials with wet-adhesive capacity can be classified into three categories: biomimetic-inspired materials,chemically modified materials,and physically interlocked materials.The most widely used forms of oral and maxillofacial wet-adhesive materials are hydrogels [11–13],biomimetic materials [14–16] and nanofibers [17–19].Additionally,composite materials are also gaining increased attention due to their ability to integrate the beneficial qualities of multiple components while avoiding the obvious drawbacks of a single material [20,21].
To date,there are few reviews available that summarized the full-scale demonstration and discussion of the wet-adhesive materials in oral mucosa and adjacent maxillofacial regions.Herein,we wrote this systematic overview for the purpose of providing a basic understanding of wet-adhesive materials used in the field of stomatology.To begin with,we proposed the ideal properties of wet-adhesive materials based on the difficulties of conventional therapeutics caused by special physiological characteristics of oral cavity and maxillofacial regions.For attachment onto the wet surface of oral-related regions,critical design principles and common classifications were investigated.Moreover,the applications of various wet adhesives in stomatology were described in detail.The final section discusses the future developments of wet adhesives in stomatology in light of the constraints that these materials currently face.
We hope that this review will offer a comprehensive understanding of oral and maxillofacial wet-adhesive materials to researchers and then inspire them to construct a somewhat mature oral wet adhesive system for the treatment of oral disease.
2.Obstacles of wet-adhesive materials in stomatology due to characteristics of oral and maxillofacial region
2.1.Histophysiological factors of oral cavity
Oral mucosa consists of the epithelium and lamina propria lining all the surfaces of the oral cavity (Fig.1) [22].Generally,oral mucosa can be classified into three types based on its location and function: masticatory,lining,specialized mucosa [23].The physiological structure of oral mucosa is one of the main reasons for the difficulties of wet adhesive materials adherence.
The masticatory mucosa is a keratinized epithelium found on the covering hard palate and gingival,which are subjected to pressure and friction during mastication [24].Therefore,the wetadhesive materials are prone to lose their retention and peel off the mucosa under the high strength of friction,finally resulting in adhesion failure.The lining mucosa has a loose submucosa,which leads the lining mucosa to be elastic and mobile and thus has a negative effect on the retention of the wet adhesive material.Besides,the buccal mucosa is attached to the buccal muscleviathe submucosa with great tension.While the ventral tongue mucosa is characterized by the smooth and thin features and close; to the connective tissue around the lingual muscle bundle.Therefore,the high-frequency mechanical movements of the buccinator muscle and tongue shorten the retention time for materials to perform their intended function.Additionally,in order to meet the aesthetic requirements,oral adhesives are often designed to be small and concealed as much as possible,which also affect retention ability to a certain extent.On the other hand,the oral cavity is a closed environment with continuous saliva secretion.Moreover,exogenous water consumption also contributes to oral moisture and hence hamper the materials to perform effectively.
Oral microorganisms also cause adverse influences on materials’adhesion.The oral environment is colonized by over 700 species of microorganisms under healthy physiological conditions [25].Along with the favorable reproductive conditions created by the consistently wet oral environment,the relatively stable oral temperature further contributes to the microbial community’s thriving.As a result,a significant number of microbes can multiply at a fast pace within this habitable temperature,resulting in the destruction of the adhesive between the materials and the mucosal surface.
2.2.Histophysiological factors of maxillofacial bone
From an anatomical perspective,the oral cavity is closely connected to the maxillofacial bone nearby.For instance,lamina propria of the hard palate is attached directly to the base of the maxilla through the mucoperiosteum and thus can be regarded as an entirety [23].Once maxillofacial tissue defect occurs,it comes to a challenge to maintain a relatively stable state.Furthermore,if the maxillofacial bone defects are resulted from chronic bacterial infections for a long period such as periodontitis [26] and periimplantitis [27],the wet-adhesive capacity of oral and maxillofacial materials can be further limited owing to the continuous release of inflammatory products.
3.The ideal properties of wet-adhesion materials in stomatology
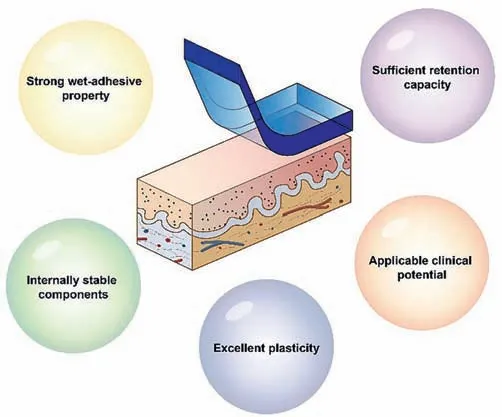
Fig.2.Schematic diagram of the desired properties of oral and maxillofacial wetmaterials.
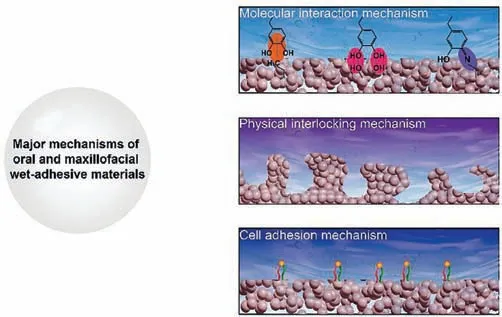
Fig.3.Major mechanisms of oral and maxillofacial wet-adhesive materials,including chemical,physical and cellular mechanisms.
Adhesive is a general term for a class of substances that can bind two materials to be an entirety and resist their separation.In recent years,adhesives tailored for biomedical purposes have undergone a rapid development,with numerous encouraging applications including skin wound healing,hemostatic sealing,and surgical suture replacementetc.[28–30].Thus,it can be regarded as an excellent alternative to traditional oral treatments.In order to adapt to the features of oral and maxillofacial tissues,as well as the needs of novel oral-related disease treatment,the adhesive materials used in the stomatology field are supposed to exhibit the following characteristics (Fig.2): Strong wet-adhesive property,sufficient retention capacity,internally stable components,excellent plasticity,and applicable clinical potential.Once materials are exposed to a wet/underwater environment,they should form a robust and rapid adhesion with sufficient bonding force and will not be easily peeled away from the mucosa surface,even if oral specific substances such as blood,saliva or food residues exist.Moreover,during the process of masticating,swallowing,or speaking,the materials should have sufficient retention stay at the treatment site to withstand destabilizing forces.Additionally,the stability of the material should not be compromised by its size.Then,a stable molecular structure within wet-adhesive material should be maintained to prevent contamination from external liquids or endogenous inflammatory processes.Due to the complicated anatomical structure of oral and maxillofacial region,great plasticity is also required to adapt to different shapes of defects.Besides,it is critical to highlight that this plasticity trait should work prior to the adhesive effect.In addition to the desired properties introduced above,it is essential for the materials to possess detailed therapeutic functions such as superior osteogenesis/angiogenesis abilities or high drug delivery efficiency to cure oral disorders.
4.Major design mechanism of oral and maxillofacial wet-adhesive materials
In this section,the main mechanisms involved in oral and maxillofacial wet-adhesive materials are introduced,including chemical,physical and cell-related mechanisms (Fig.3).
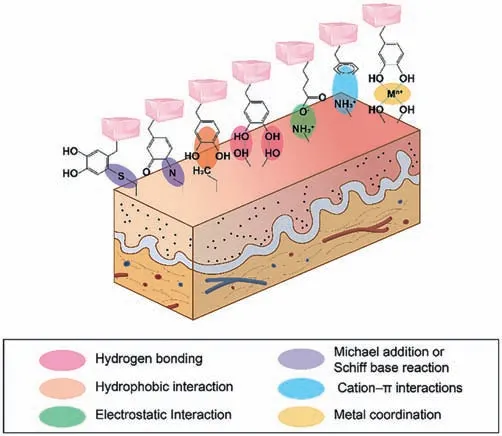
Fig.4.Schematic diagram of the molecular interaction mechanisms involved in the oral and maxillofacial wet-adhesive materials.
4.1.Functional groups on tissue surfaces
From a structural classification perspective,tissues of the oral and maxillofacial region consist of cells and extracellular matrix(ECM).And proteins,the material basis of biological life,provide mechanical supports for cells and ECM [31].Amino acids make up the proteins,and some of them carry functional groups that help with tissue adhesion.Primary amines,carboxylic acids,hydroxyls,and thiols are some of the functional groups found on tissue surfaces [32].They are widely used for reacting with adhesions because they are freely available.Among them,primary amines are the most commonly used in adhesion design because of their excellent chemical reactivity.Through supplying an active electron pair conjugated to the reactive group of the tissue binder,nucleophilic primary amines could then activate numerous chemical reactions to have adhesiveness [33].
4.2.Molecular interaction mechanism
Molecular interaction comprises noncovalent interactions and covalent bonding interactions.Their more detailed classification and bonding strength are shown in Table 1.
4.2.1.Noncovalent interactions
Noncovalent interactions (NCIs) are ubiquitous in nature.Though the intra- and intermolecular noncovalent interactions are relatively weak,they are intimately connected with the cohesion within chemical substances (Fig.4) [34].
(a) Hydrogen bonds: Hydrogen bonds are formed between an electronegative atom and a hydrogen atom bonded to a second electronegative atom and are extensively involved in chemical,physical,and biological processes [35].Various chemical groups,such as primary amines,hydroxyl,and carboxylic acid,can form hydrogen bonds with wet adhesives on the mucosa surface.For instance,one study proposed that the bidentate hydrogen bond formed by two Dopa molecules is closely related to the adhesion of mussel protein Mfp-3F on foreign substrates,while another demonstrated that the number of hydroxyl groups within the catechol derivatives also exert significant impacts on the strength of hydrogen bonding [36,37].Some thermosensitive mucoadhesive hydrogels canform hydrogen bonds with mucins,thus dramatically prolonging the duration of contact between the formulation and the mucosa [38].
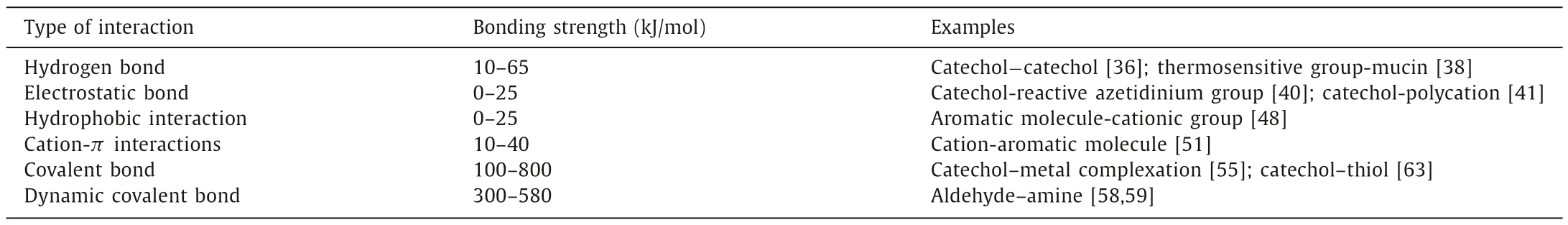
Table 1 Various types of bonds utilized to form adhesive matrix,and their molecular strength.
(b) Electrostatic attraction: Electrostatic attraction is an electrostatic force existing in the aqueous solution with different particles.It always occurs between the attractive electrostatic adsorption [39].With respect to the effect of the electrostatic attraction on wet adhesion,several types of groups have been already proved their related function.One representative group is primary amine which can be found in chitosan extensively.Primary amine groups enable chitosan to form an electrostatic bond with negatively charged mucin at physiological pH,allowing it to adhere to tissues firmly.While catechol-based ions,for example,could produce robust adhesiveness with reactive azetidinium groups or polycations of quaternized chitosan through electrostatic absorption,respectively [40,41].Besides,other groups like sulfonic acid groups or arginine units contained in the cell-penetrating peptide (CPP)-conjugated bacterial cellulose nanofibrils could interact with anions of skin or cell membranes,thus contributing to the adhesive performance due to this mechanism [42,43].
(c) Hydrophobic interaction: Hydrophobic interaction refers to the attractive interaction between the hydrophobic parts of a system [44].In this procession,hydrophobic molecules tend to aggregate with the exclusion of water molecules [45].In some reports,aromatic monomers can offer the hydrophobic function and are often used to play a cooperative role with adhesive cationic groups [46,47].For instance,one barnacle CPsinspired hydrogel consists of aromatic 2-phenoxyethyl acrylate (PEA) monomers and a cross-linked copolymer of cationic 2-(acryloyloxy)ethyl trimethylammonium chloride (ATAC) was produced,which imitate hydrophobic amino acids and the cationic in CPs,respectively.Aromatic groups can disrupt the hydrated layer on the surface,thus providing a low dielectric constant for the inner region to enhance electrostatic interactions of adjacent cationic groups and form interfacial hydrophobic bonds [48].Hydrophobic groups can be alternatively used as water repellants on the tissue surfaces,preventing the adhesive-reactive molecules from coming into contact with water and boosting their responses’effects.Such an enhancement could also be found in mussel-inspired underwater adhesives.The positively charged amine group mimics the structure of the mussel foot protein,which displaces the water layer from the tissue and allows the catechol group to bind tightly to the functional groups on the tissue surface [49].
(d) Cation-πinteractions: In the presence of electron-rich orbitals and cations,a cation–πinteraction is essentially an electrostatic force that plays a vital role on tissues [50].Mytilus californianus foot protein-1 (mcfp-1),for example,shows unique strong adhesion because of this interaction [51].Studies have shown that as the K+concentration increases,this adhesive ability decreases,which represents an ionic strength-dependent property.This phenomenon can be contributed to the ability that K+can replace the cationic lysine and form a new cationπinteraction with the aromatic molecule.
4.2.2.Covalent bonding interactions
Covalent bonds are atom-to-atom interactions that share electron pairs and have more energy and directionality than noncovalent bonds (Fig.4) [52].
(a) Metal coordination bonds: Metal coordination bonds are recognized as one significant covalent bonding that contribute to the enhancement of the wet adhesive properties [53,54].For instance,the interaction of catechols with metallic materials occurs mainly through coordination bonds formed between the oxygen atoms in the hydroxy group and the metal atoms of the surface,with a strength that is highly dependent on the metal.Dopa-metal complexes (e.g.,the Dopa-Fe3+coordination) contribute to the mechanical integrity of mussel adhesive plaques[55].
(b) Schiff base reaction or Michael addition: Schiff base is a nucleophilic addition process that is synthesized from amine and carbonyl groups to generate an imine group [56].Michael additions are C–C bond formation reactions in which the nucleophile or carbanion can have a nucleophilic addition with anα,β-unsaturated carbonyl compound [57].Under an oxidative or alkaline environment,it is easy for the catechol groups to oxidize as a form ofo-quinone.The latter production then can form covalent bonds with the nucleophilesviathose two reactions.Besides,adhesives ontaining aldehyde groups can generate dynamic Schiff base interactions with the amino groups of the proteins on the tissue surface due to the presence of amino groups in the proteins,which promotes the adhesion ability of biomaterials on the tissue surface [58,59].
(c) Surface modification based on covalent bond: Adhesive materials,such as hydrogels,are frequently modified on the surface to increase their adhesive capabilities.Hydrogels functionalized with phenolic moieties (e.g.,catechol and gallol)have demonstrated outstanding tissue-adhesion capacity,making them one of the best-modified groups for improving adhesion [60,61].As for the immobilized sulfhydryl group,the tremendous advantages such as mucoadhesion,permeation enhancement and stability improvement make it have an extensive application [62].One study modified arabinoxylan (ARX) with the thiol and developed a mucoadhesive oral film for better tizanidine hydrochloride administration(TZN HCl) [63].
(d) Cross-linking reaction: Covalently cross-linked approaches are a strategy to improve wet-adhesive materials’properties.When chitosan comes into contact with saliva,for example,it can quickly convert into an insoluble,adhesive hydrogel-like substance due to the coordinated actions of covalent crosslinking [64].The photoinitiated polymerization of multifunctional monomers or UV-radiation curing arouse attentions in many chemical applications.Under the irradiation of a standard clinical blue light (the range is generally 395–500 nm) [65,66],some active molecular groups (e.g.,aldehyde group) are born,and then crosslinks with the molecular groups at the foreign tissue interface to strengthen the adhesive ability [67].
4.3.Physical interlocking mechanism
Physical interlocking can anchor the adhesive onto tissuesviasteric hindrance.The adhesive effect produced by physical interlocking usually occurs on materials like nano-sized electrospun fiber films,which could provide a good contact chance for adhesion to mucosal surfaces due to its large specific surface area[19].Besides,materials with the characteristic of hygroscopic nature also can improve adhesion ability.For example,hygroscopic polymers with gel-forming abilities,such as chitosan,are capable of creating a partially dehydrating phenomenon at the contact area of the mucosal surface,resulting in robust adhesiveness when the mucosa reaches the equilibrium in water absorption.Finally,the processes described above cause a swelling reaction,which allows the chitosan in the nanofibers to entangle with molecules such as mucins located on the mucosal surface [68].
4.4.Cell adhesion mechanism
In addition to the physical or chemical adhesive mechanism of the material itself,cells adhesion mechanism cannot be ignored either.The cell-substrate affinity is a crucial aspect in biomaterial design and development [69].Through integrin and other transmembrane proteins,cells can adhere to the ECM (collagen,fibronectin,and laminin) and other substrates [70].Additionally,they stick to other cellsviamolecules of cellular adhesion such as cadherin and desmosome.Since many wet-adhesion materials used in stomatology are fiber membranes with spatial structure,it is an opportunity for osteoblast or angiogenesis-related cells to distribute inside and then exhibits obvious pseudopodia shapeviavinculin (an intracellular protein in the cell adhesion plaque complex),which indicate the successful cell adhesion on the surface of materials [6,17].
5.Common classification of oral and maxillofacial wet-adhesive materials
5.1.Naturally derived polymer-based adhesives
5.1.1.Polysaccharide-based hydrogels
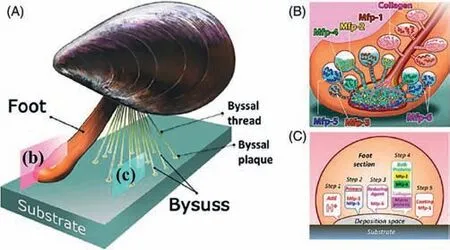
Fig.5.Illustration of the mussel byssus and proposed model of the mussel’s adhesion mechanism.(A) Schematic overview of a mussel.(B) Schematic of mfp delivery from various precursors.(C) Corresponding functions under the mussel’s foot.Copied with permission [94].Copyright 2021,John Wiley and Sons.
Hydrogels,which are made up of 3D crosslinked networks containing huge volumes of water,have a significant biological promise in the field of wet-adhesive materials [71,72].For example,chitosan (CS) is a biopolymer derived from the deacetylation of chitin and it mainly comprises D-glucosamine andN-acetyl-Dglucosamine [73,74].The–OH and–NH2groups contained in chitosan allow it to create hydrogen and covalent bonds.As a result of this property,several chitosan chemical derivatizations can be created [75].Under an acidic condition,the amino groups of chitosan can be protonated and subsequently interact intensely with the negatively charged proteins,such as mucins,existing both on epithelial mucosal interfaces and in saliva [76].Furthermore,due to its hygroscopic properties with strong swelling abilities,chitosan has also been extensively investigated as a mucoadhesive polymer [68].Therefore,researchers are currently focusing their efforts on modifying chitosan to improve its adhesive qualities,including generating thiolated chitosan,carboxymethyl chitosan 3-(3,4-dihydroxyphenyl)propionic acid-modified chitosan and catechol modified quaternized chitosanetc.[77–80].Alginate,which could also be used to build wet-adhesive materials,is a natural linear anionic polysaccharide polymer comprised ofβ-(1-4)-D-mannuronic (M-blocks) andα-L-guluronic acid (G-blocks) [81].Amongst a plethora of favorable biomedical properties,its excellent wet adhesive capacity distinguishes it from other materials and makes it an effective natural viscosity modifier.In fact,apart from its bio-adhesive properties,alginate is also regarded as a natural source of a high concentration of carboxylic groups,which can be used to initiate the crosslinking reaction of carbodiimides and thus act as a crosslinking agent to enhance the adhesiveness [82].In addition,among the most common biological glues is bacterial cellulose (BC),which is an extracellular polymer released by bacteria such asKomagataeibacter(Gluconacetobacter) bacterium [83].BC is a homopmer composed ofβ-D-pyranose units,which has a representative chair conformation,with all -OH groups positioned in the equatorial region.This unique chemical construction plays a key role in the formation of the intermolecular H-bonding between two adjacent chains.Thus,the presence of H-bonding makes it possible for BC to form a fiber with the fine diameter with excellent mechanical properties and high tensile strength.Furthermore,enough surface area per unit mass of BC endows it with a special capacity to store a large volume of water [84].Above all,BC-containing polymers can be employed as a good wet-adhesive in wound healing areas.Besides,hyaluronic acid (HA) is a hydrophilic polysaccharide made up of repeating disaccharide units ofN-acetylglucosamine and D-glucuronic acid.Possesses carboxyl and hydroxyl groups on its backbone,HA is suitable for chemical modification then it can be cross-linked to form hydrogels [85,86].However,as a nonadhesive material,not only does HA limit surgical placement but also it offers no sites for integrin-mediated cell contacts,which could prevent endogenous cell invasion.Therefore,it has to combine with other adhesive molecular groups to acquire the adhesion [87–89].
5.1.2.Gelatin
As one of the most investigated materials for tissue adhesives,gelatin can be derived from collagen in nature.The mechanism of its natural adhesive behavior in solution lies in the ability to form physically crosslinked hydrogel structures [90].Nevertheless,the weak mechanical strength of pure gelatin makes it difficult to produce enough adhesion.Therefore,chemical additives with crosslink function are often used in conjunction with gelatin so as to improve adhesive capacity as well as appropriate mechanical properties [91,92].
5.2.Mussels inspired wet-adhesive materials
In nature,marine mussels are well known for their excellent adhesion as they can cling tenaciously to other surfaces beneath the water [93].Numerous researches have revealed the reason why mussels can produce such a robust adhesion even in a completely wet environment.As the Fig.5 shows,the excellent underwater adhesion of mussels can be contributed by the byssal thread protein,with which the tough adhesive plaques can be formed subsequently [94,95].More specifically,this forceful,wet adhesion capacity at the plaque–substrate interface mainly results from six important mussel foot proteins (mfps),which are all abundant with a main active composition: catecholic amino acid 3,4-dihydroxyphenylalanine (DOPA).Since the DOPA’s primary molecular group component is the catechol functional group,it can form strong covalent and noncovalent bonds with inorganic and organic materials (Fig.5) [96].As a result,a great deal of materials inspired by mussels is being developed,involving mucoadhesive film,bioactive hydrogels,and hybrid protein materials [20,97,98].
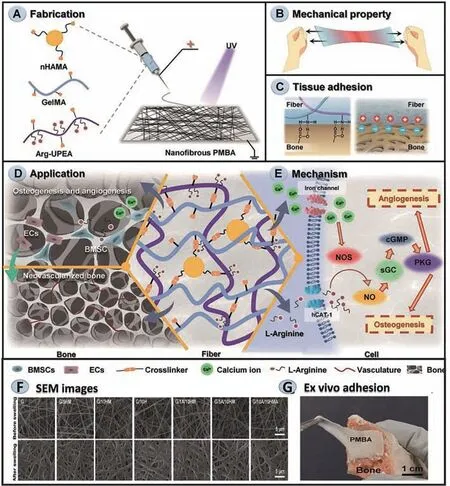
Fig.6.An example of the fabrication,characteristics,mechanism,and application of adhesive electrospun fiber films.(A) The fabrication process of nanofibrous PMBA.(B)PMBA mechanical properties.(C) PBMA’s superior adhesion performance.(D) A well-coordinated osteogenic–angiogenic coupling effect and accelerated bone regeneration caused by PMBA.(E) Activate/upregulate the NO–cGMP signaling pathway.(F) Representative SEM images of electrospun fiber films.(G) Illustrations of ex vivo adhesion of PMBA to the natural porcine bone.Reproduced with permission [98].Copyright 2021,John Wiley and Sons.
5.3.Adhesive electrospun fiber films
As a simple and efficient nano-fibers produce techniques from a fluidviautilizing a high electrostatic field,electrospinning has unique features such as high surface-area-to-volume ratio,tunable surface structures and facilitate controlled drug release,and thus has been extensively applied in diverse areas [99,100].Numerous studies have proved that it is the high surface-area-to-volume ratio that confers on it an excellent adhesive capacity; Additionally,this characteristic also prevents functional nanoparticles from being cleared immediately,thereby maintaining a long-term concentration gradient of nanoparticles at the oral mucosal site [101].For example,a synthetic periosteum with similar structure and function to natural periosteum was designed based on this technique,which could guarantee tissue adhesion and sustained activation of nitric oxide–cyclic guanosine monophosphate (NO–cGMP) signaling pathway,and ultimately achieve the purpose of accelerated bone regeneration (Fig.6) [102].
6.Applications of wet-adhesive materials in stomatology
With the increasing understanding of the wet-adhesive mechanism of oral and maxillofacial materials,a variety of promising adhesives based on the features of oral and maxillofacial region have been developed.Therefore,we summarize the current applications of oral and maxillofacial wet-adhesive materials below and in Table 2.
6.1.Drug delivery through oral mucosa
In comparison to oral and parenteral formulations,medication deliveryviathe oral mucosa channel is widely known for its noninvasive,painless,and convenient advantages,which could save patients from injection dread or high-dose adverse effects [103].Within the oral cavity,the buccal and sublingual regions are two major mucosal delivery sites due to their non-keratinized and stratified epithelium,which can provide a more elastic and permeable tissue than other parts of the mouth and thus facilitating drug absorption [104].However,difficulties in the wet oral environment,such as continual saliva secretion,meal flushes,and even active hemorrhage owing to ulceration,have hindered its further application.Here,we introduce a variety of materials that have outstanding capabilities for strong wet adhesion and so considerably improve drug delivery effects.
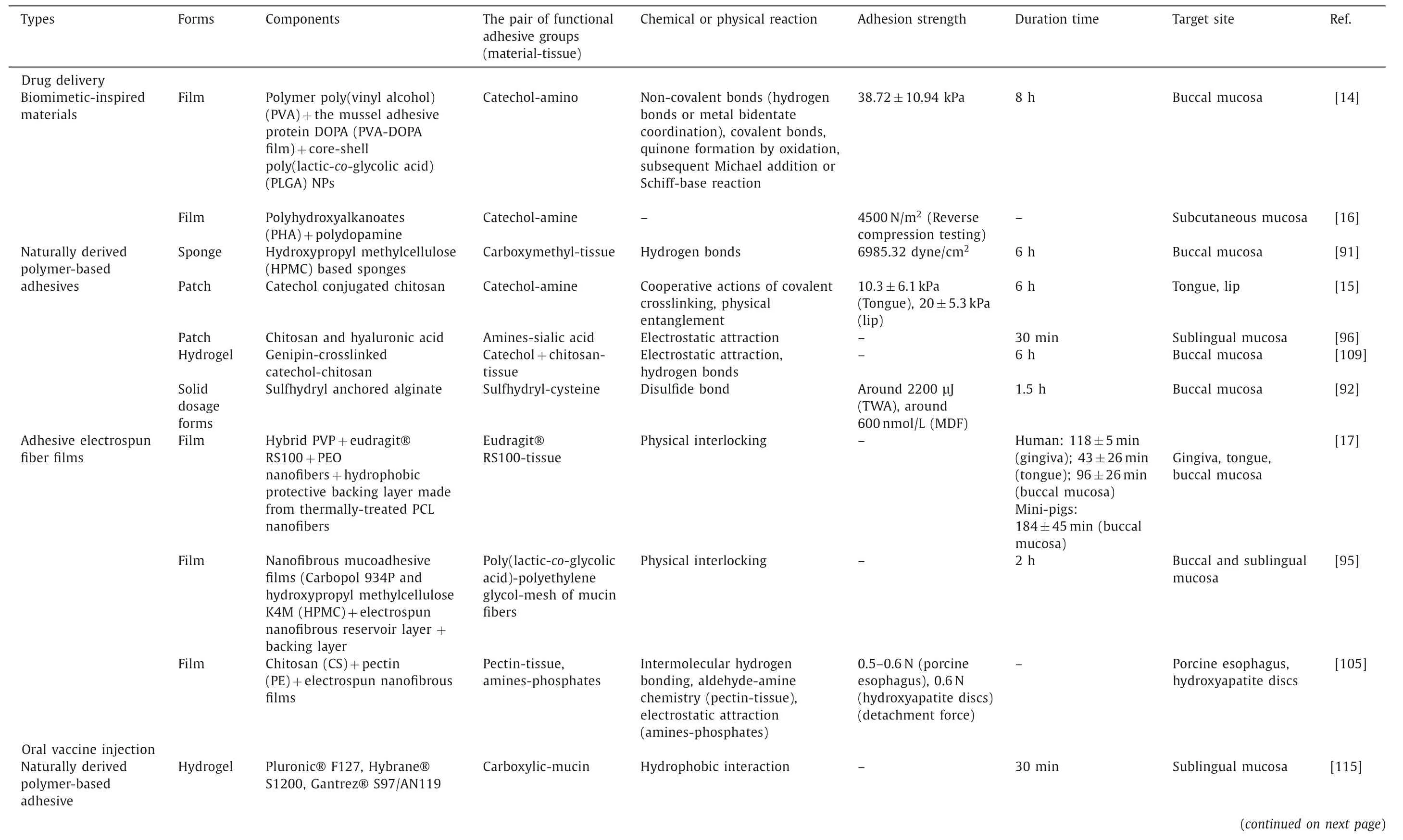
Table 2 Current applications of oral and maxillofacial wet-adhesive materials.
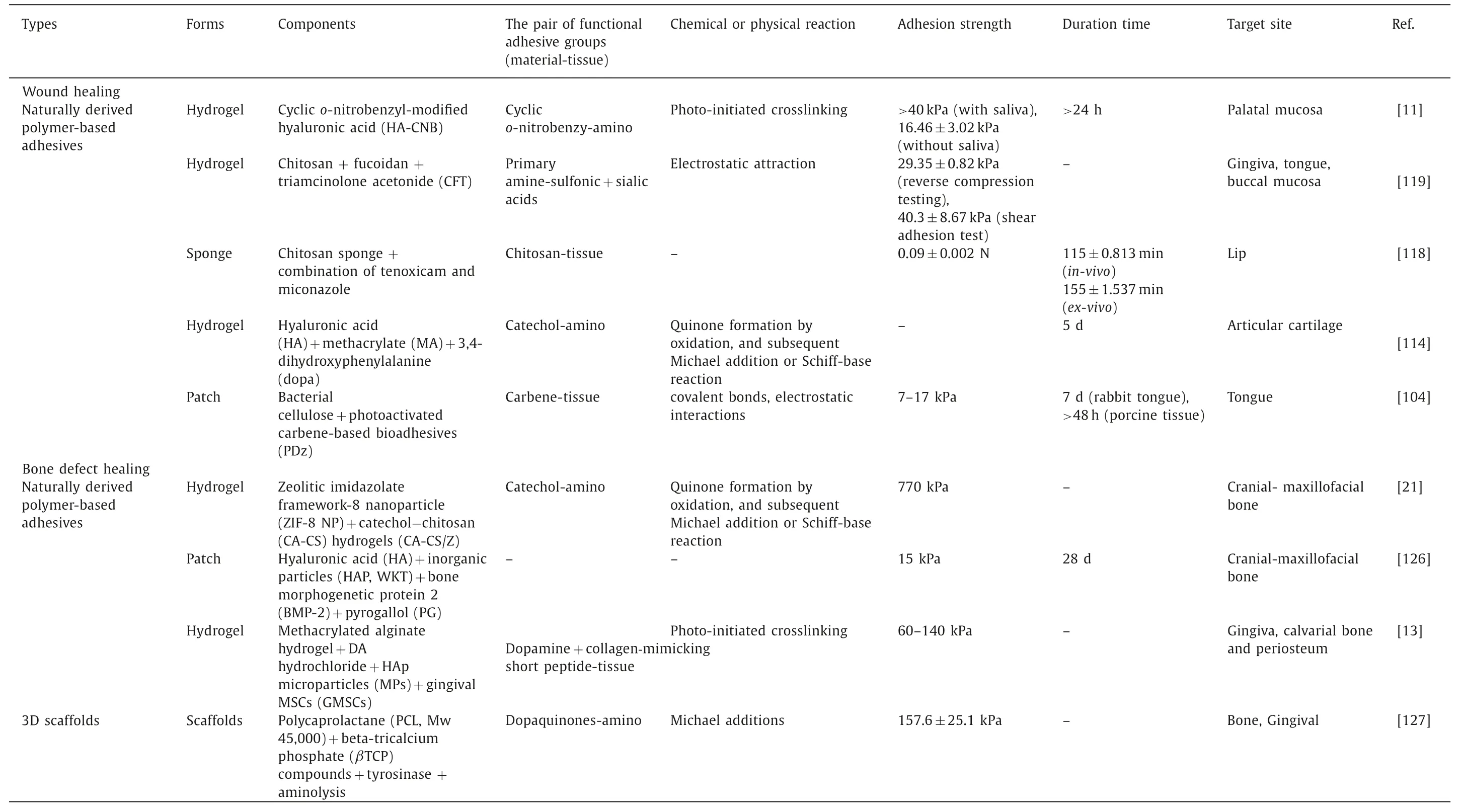
Table 2 (continued)
6.1.1.Buccal mucosa delivery route
In one study,a tunable thin film designedviacombining the mucoadhesive polymer poly(vinyl alcohol) (PVA),the mussel adhesive protein DOPA (PVA-DOPA film) and core-shell poly(lactic-coglycolic acid) (PLGA) NPs together exhibited better residence duration and adhesion strength than existing commercial products.The results showed that after only 10 s of contact,the PVA-DOPA film established strong adhesion with porcine buccal mucosa.This phenomenon can be partly attributed to the interpenetration and entanglement of polymer chains with mucus.The amount of DOPA also contributes to the film’s adhesive strength.Meanwhile,the incorporation of modified nanoparticles ensures the entire film with better mucus-penetrating and cellular uptake properties,resulting in comparatively 6 folds in healing oral mucositis than traditional oral medication (Figs.7A–E) [14].Another research coupled polyhydroxyalkanoates (PHAs) with polydopamine (PD) to form the drug delivery system.As a result,because of the enhanced adhesiveness provided by PD,this coated film can be acted as a promising longlasting potential oral patch with localized drug delivery [16].
Polysaccharide-based materials have also been extensively investigated to administer buccal doses [105].Freaget al.purposed a chitosan-based composite sponge through a polymer blending approach.When the ratio of CS to hydroxypropyl methylcellulose(HPMC) was around 1:1,the composite sponge could up to its best wet adhesive performance,which could be owing to the highest hydration capacity of the sponge that allows mucin entanglement to facilitate their mucoadhesion [106].Besides,some derivatives,such as chitosan-catechol (Chi-C) conjugate,also form a spongy network structure that could bring an appropriate porosity and hence greatly increase capillary force for salivary liquid adsorption and complexation.This type of oral patch could induce instant and robust mucoadhesion properties,which offer long-lasting therapeutic effects of medications and improved oral ulcer healing [15].There are also some novel adhesive polymeric excipients based on alginate that have been developed nowadays to cure oral mucosa diseases.In one study,sulfhydryl groups of amino acid cysteine(SH) were anchored on the polymeric backbone of alginate (AL)and then the ambroxol was loaded onto it for analgesia and aphthous stomatitis treatment [107].Thus,sulfhydryl anchored alginate showed 11.56 times increase in mucoadhesive performance.
Colleyet al.recently reported the successful fabrication of a novel electrospun dual-layer mucoadhesive system.The inclusion of polyoxyethylene (PEO) particles to the inner layer (electrospinning polyvinylpyrrolidone and Eudragit® RS100) boosts the overall adhesiveness of the system.The large surface area it offered allows drugs to persist for a long time.As a result,once loaded with clobetasol-17-propionate,the high-potency topical corticosteroids to cure oral mucosis,the system exhibited its potential to enhance therapeutic efficacy by highly targeted and controlled drug delivery to the mucosal surface [17].
6.1.2.Sublingual mucosa delivery route
The sublingual area has many advantages,including easy access for self-administration,appropriate immunological induction ability,high permeability,a lack of enzymatic barriers,mild pH,and opportunities to bypass first-pass metabolismetc.[108,109].Therefore,attempts to enhance the adhesion of a sublingual drug delivery system have become a hot topic.
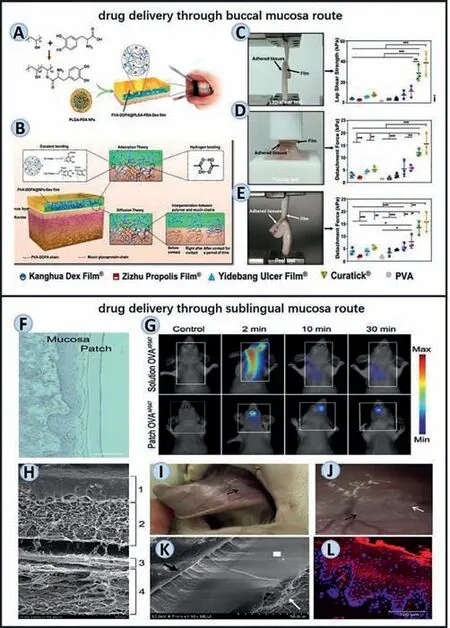
Fig.7.Representative oral and maxillofacial wet-adhesive materials of buccal and sublingual drug delivery.(A–E) The method and mechanisms used to assemble a mucoadhesive film and evaluations to test its adhesive ability.Reproduced with permission [14].Copyright 2021,Nature Publishing Group.(F,G) The duration time of the sublingual adhesive patch from assessments of hematoxylin staining (sublingual mucosa get touch with the patch 100,20 min after administration.Scale bar:100 μm.) and fluorescence molecular tomography (fluorescent signal was detected 2,10 or 30 min after administration) respectively.Reproduced with permission [111].Copyright 2021,Elsevier.(H–L) Another sublingual patch’s adhesive ability: (H,K)Scanning electron microscopy representation of tight adhesion between the nanofibrous mucoadhesive film and the sublingual mucosa; (I,J) Function of wet adhesive material in the vivo model.Black arrow indicates mucoadhesive layer 2 h after the administration; (L) determines the quantity of released nanoparticles from the reservoir nanofibrous layer via immunofluorescence.Reproduced with permission[110].Copyright 2017,Elsevier.
Mašeket al.designed nanofiber-based mucoadhesive films for oromucosal administration of nanocarriers for medication vaccine delivery (Figs.7H–L) [110].Among three parts consisted in this mucoadhesive film,the mucoadhesive layer made by Carbopol 934P and hydroxypropyl methylcellulose K4M (HPMC) provide firm adherence between the complete layers and wet sublingual mucosa.The potential adverse impact of mucosal self-cleaning effects generated by a continuous flow of saliva could also be avoided thoroughly.In another work,Pariset al.presented a simple protein delivery scaffold composed of chitosan and hyaluronic acid.Assembled by the layer-by-layer methodology,this natural polymer can produce a mucoadhesive and oro-dispersible freestanding membrane.The functionalized membrane was then used to create a bioactive patch with efficient protein loading and release as well as appropriate mechanical characteristics for manipulation (Figs.7F and G) [111].As a result,the model protein called ovalbumin (OVA)can be detected as a concentrated signal in the mouth during at least 30 min,which verified the function this scaffold exerted.
6.2.Wet-adhesive materials to vaccine injection in oral cavity
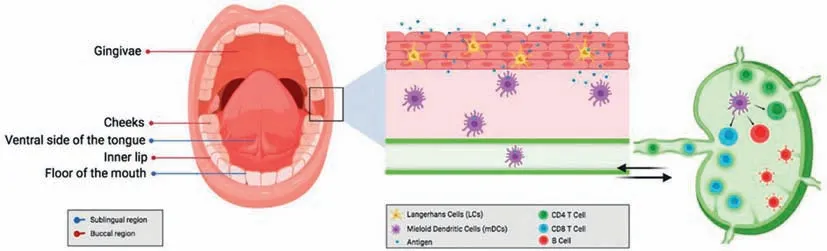
Fig.8.Antigen uptake and presentation by dendritic cells via the oral mucosal route for immunization.Copied with permission [104].Copyright 2021,MDPI.
The entire oral mucosa area can operate as a potentially favorable site for inducing a specific immune response or tolerance to specified antigens and allergens due to the densely-distributed dendritic cells (Fig.8) [112].And the sublingual mucosa,in particular,has long been thought to be one of the most suitable immuneinducing areas for mucosal vaccination and immunomodulation[113].
A sublingual vaccine is defined as a formulation deposited under the tongue [114].Recently,researchers purposed ternary thermosensitive and mucoadhesive hydrogels as one sublingual vaccine that could achieve sol-gel transition at body temperature[115].Specifically speaking,they used Pluronic,a self-assembly amphiphilic copolymer,in conjunction with two other mucoadhesive materials to deliver entire attenuated microbesviathe orotransmucosal route.The result indicated that the highest mucoadhesive hydrogels were obtained when Pluronic concentrations were between 18.5% and 21% while Gantrez (the adhesive substance) concentrations were below 1.5%.Such a strong adhesion ability successfully ensured hydrogels not to be swallowed before antigen was released into the oral cavity.
6.2.1.Wound healing in oral cavity
Wound healing necessitates a rather stable and dry environment.Despite the fact that recurrent oral ulcers are typically 2–5 mm defects that can self-heal within approximately 10 days,there are other oral mucosal disorders that cause larger defects,which may require a much longer time to cure [116].However,not only does the oral cavity secrete saliva,but the diet and drinking water from outside sources make it hard to keep the oral environment dry and immobile.As a result,keeping the treatment working until the incision heals is a challenge for researchers.Actually,the oral patches used in clinical trials could only function for one hour at best,which is significantly less than the recommended repair period for treating oral mucosal disorders [117].
To circumvent this crucial aspect affecting drug effectiveness,numerous adhesives has been designed [118].Zhanget al.created a photo-crosslinking hydrogel adhesive,which was inspired by the successful usage of light-curing procedures technique in dentistry.This hydrogel adhesive is made up of a single component of big hyaluronic acid that has been modified by a cycliconitrobenzyl compound,which can be photo-responsive under the 395 nm light irradiation.Then the cyclico-nitrobenzyl compounds release three active groups and protect mucosal wounds for at least 24 h from liquid rinsing,oral movement,and friction.Besides,considering that the defect of oral tissue may be large in certain circumstances,such as tumors or trauma,the capacity of the modified patch to heal huge defects was also examined.By day 6,the wound-healing rate in the cyclico-nitrobenzyl-modified hyaluronic acid (HA-CNB) group was 94.87%,much higher than that of the control group (77.19%),which also reflect its superior adhesive ability (Figs.9A–G) [11].Researchers also use chemical crosslinking to construct triamcinolone acetonide (TA)-loaded chitosan/fucoidan (CF) composite hydrogels.Based on the fact that hydrogel films could get the connection with oral mucosa in the presence of water,the films could partially swell and form a mucous layer.Therefore,the hydrogel patches can adhere to the gingiva,tongue,and buccal mucosa easily.The addition of TA and CF to chitosan hydrogels significantly improved adhesion qualities(shear strength up to 63.4±7.60 kPa) (Fig.9H) [119].This composite also has great antibacterial properties that can further accelerate wound tissue healing.Besides,due to their almost invisible characteristic,these adhesive hydrogel films also could meet the aesthetic requirements.
In addition to the cross-linking materials mentioned above,Singhet al.designed a flexible film platform made by bacterial cellulose (BC) and photoactivated carbene-based bio-adhesives (PDz),which could offer an adhesion strength ranging from 7 kPa to 17 kPa and last for a long time [120] (Fig.9I).
6.2.2.Wound healing in maxillofacial regions
Stomatology is a broad term that encompasses the oral cavity and maxillofacial structures.As the most complex and sophisticated part of the human body,surgical access is frequently limited due to the tissue structure of the maxillofacial region,which complicates wound healing.
In order to create the wet-adhesive materials that are potentially suitable for minimally invasive surgical procedures in maxillofacial applications,a study developed a hyaluronic acid(HA)-based hydrogel modified with methacrylate (MA) and 3,4-dihydroxyphenylalanine (Dopa) groups.during the photoinitiated cross-linking process,MA-HA-Dopa can help build strong hydrogels,allowing it to adhere to tissues and promote maxillofacial tissue repair even in a fully immersed wet environment (Fig.9J) [12].
6.3.Wet-adhesive materials to cure the bone defect
When the oral cavity or cranial-maxillofacial region is subjected to traumatic impact (such as a car accident) [121],chronic bacterial infection [122] or tumor invasion [123],the bone tissue attached to them can be easily destroyed,resulting in the formation of a defect area.Though there are currently a number of therapies available to cure cranial-maxillofacial bone defects [124],it remains a challenge to provide effective treatment for these regions due to their close connection to wet,dynamic oral environments and complex sinuses contained in some special bone regions (such as the maxillary bone).
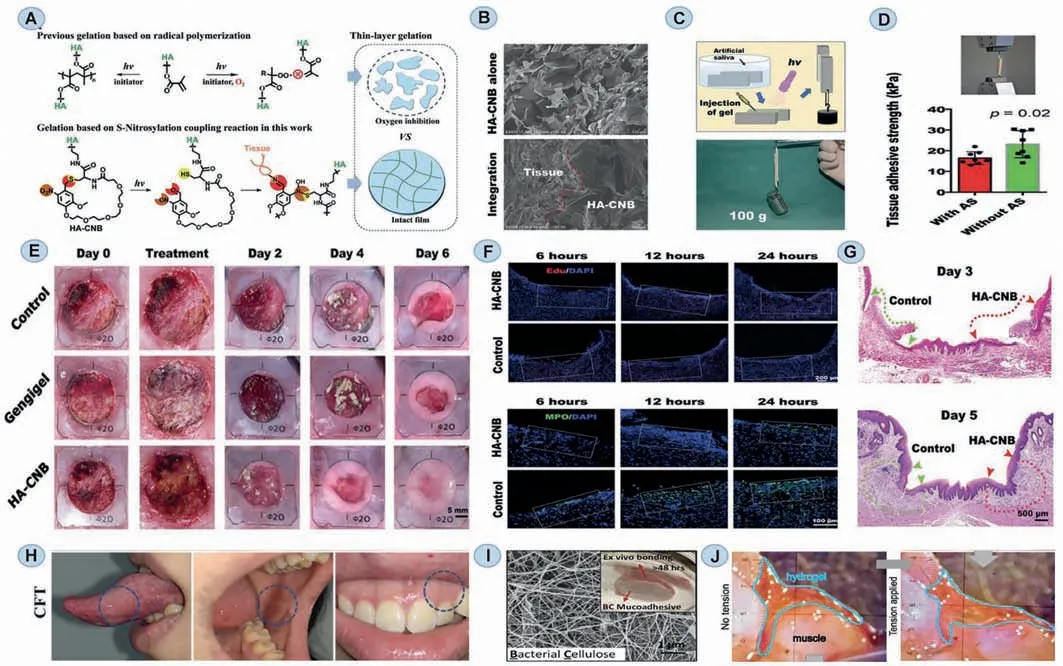
Fig.9.Representative oral and maxillofacial wet-adhesive materials of wound healing.(A) Schematic diagram of the gelation and integration mechanism of an HA-CNB hydrogel adhesive.(B) The HA-CNB gel integrates with rat muscle tissue via SEM image.(C) Coated with HA-CNB (2 wt%) solution,two rat skins were immersed in artificial saliva,and then have a light crosslinked process.(D) Evaluation of adhesive strengths of the glued rat skin on tissue.(E) HA-CNB gel (2 wt%) or a commercial product(Gengigel) was covered on the oral mucosa defect (20 mm diameter) and observe their healing.(F) Proliferating cells stained with Edu in the defect site,as shown in the white dotted box,at different observation times.(G) The healing of rat oral mucosa defects on days 3 and 5 after operation via HE stains.Reproduced with permission[11].Copyright 2021,John Wiley and Sons.(H) Photos of in vitro adhesion properties of hydrogel films.Reproduced with permission [119].Copyright 2021,Elsevier.(I) The structure of bacteria cellulose and its adhesive performance.Reproduced with permission [120].Copyright 2021,Elsevier.(J) Images show adhesive hydrogel adherent to muscle tissue (left).Even when the damaged muscle tissue is pulled apart,the hydrogel still remains adherent to the tissue surfaces (right).Reproduced with permission[12].Copyright 2021,John Wiley and Sons.
As a result of the development of adhesive materials,wetadhesive materials for oral-related bone could also be created in large quantities.For example,one study has already designed a catechol-functionalized chitosan nano-zeolitic imidazolate framework-8 nanoparticle (ZIF-8 NP) composite hydrogel system(Figs.10A and F) [21].It is important to note that in addition to the strong adhesive ability provided by theortho-dihydroxyphenyl(catechol) functional group,ZIF-8 NPs,the material utilized to promote osteogenesis,can also enhance the adhesion (the tensile strengths were up to 0.77 MPa).The result indicates that complexed hydrogels have a promising potential for promoting implantation stability,angiogenesis,and osteogenesis in cranial and maxillofacial bone regeneration applications.Another biomimetic material inspired by mussels was engineered using an alginate-based adhesive,photo-crosslinkable,and osteoconductive hydrogel biomaterial (AdhHG) with customizable mechanical properties.This study put the gingival MSCs (GMSCs) into osteogenic hydroxyapatite microparticles (MPs) and then encapsulated them within the engineered hydrogel.Then this composite system showed excellent crosslinkable adhesion and the ability to guide GMSCs toward osteogenic lineages in the rat peri-implantitis model (Figs.10B–D)[13,125].Besides,a study combined inorganic particles (HAPetc.)with bone morphogenetic protein 2 (BMP-2) into phenolic hydrogels to promote bone formation (Fig.10E) [126].Consequently,the structural and mechanical properties of composite hydrogels with HAP and WKT would be reinforced by additional crosslinkingviacoordination of oxidized phenolic groups with these ions.
In addition to the use of hydrogel as a treatment for cranial and maxillofacial region bone tissue defects,3D-printed technique productions are also a great option [127].Tabatabaeiet al.purposed a 3D-printed functionally-graded porous scaffold to accomplish adhesion of hard-soft tissuesin situ[128].In this work,by introducing amino groups onto the surface of the hard construct,biocompatible macromolecules such as collagen can be further attachedviaa cross-linking agent like tyrosinase,which induces the formation of dopaquinones in collagen.Subsequently,fully-differentiated engineered oral mucosa was formed directly on the surface of hard tissue.The advantage of this scaffold over other wet-adhesive materials is that it does not have a barrier like glue,which can disrupt direct cellular interactions between tissues.
7.Challenges and prospects
Despite the rapid advancements of wet adhesives in stomatology,there are still numerous obstacles and unmet clinical demands remain.Here,we list some of the current limitations of the oral and maxillofacial wet-adhesive materials in this section,and we hope that this part will provide guidance for next-generation oral related wet adhesives to gain better properties.
7.1.Material constraints
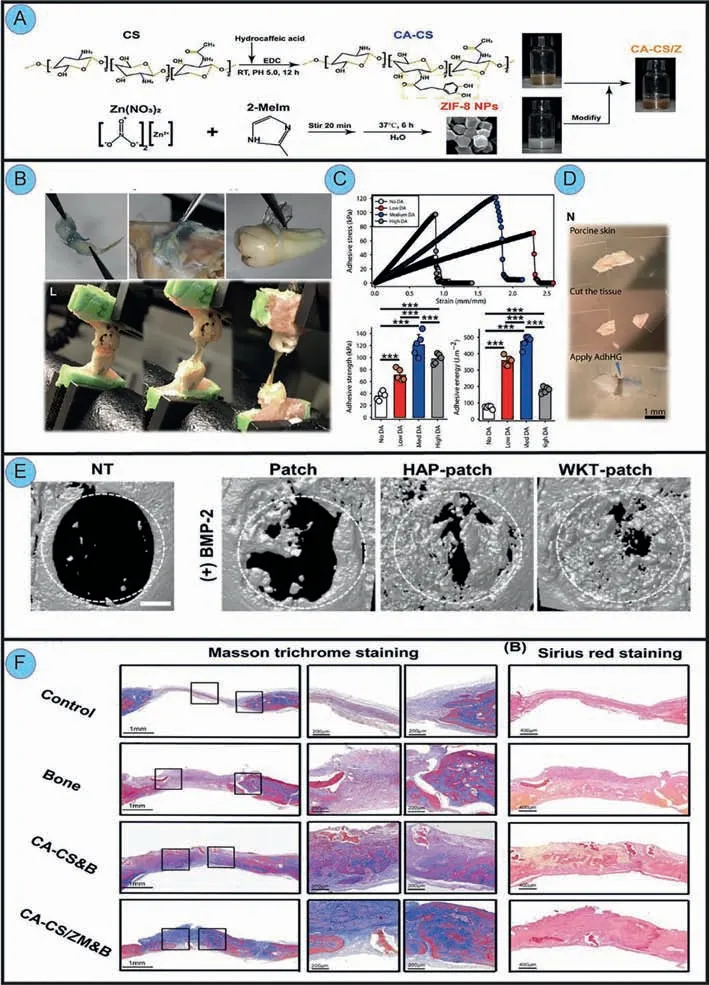
Fig.10.Representative oral and maxillofacial wet-adhesive materials to cure maxillofacial bone defects.(A) Schematic illustrations of the fabrication of osteogenic CACS/Z hydrogels.(F) Masson trichrome staining of bones after implantation at 8 weeks.Reproduced with permission [21].Copyright 2020,ACS Publications.(B) Photographs concerning hydrogel adhesiveness to rat gingiva,rat bone and periosteum,and human tooth root surfaces respectively.(C) Assessment of adhesive strength and stress on rat alveolar bone adhesion.(D) Adhesion test on porcine skin.Reproduced with permission [13].Copyright 2020,AAAS.(E) Micro-CT analysis of bone defects 8 weeks after implantation of patches.Reproduced with permission [111].Copyright 2020,Elsevier.
Almost every experiment involving the mechanical properties of wet-adhesive oral and maxillofacial materials includes classical experiments such as the lap-shear test,tensile test,and peel test.However,such tests can only certify the adhesive ability from a unidirectional perspective,ignoring the effect of cyclic stress.Because oral movement is cyclic rather than moving towards a single direction,wet adhesives’fatigue resistance should not be overlooked.
While it is true that recently developed wet oral and maxillofacial adhesives have attained enough adhesive strength,there are still two major issues with adhesive performance that need to be addressed.On the one hand,only a few materials ensure that the adhesion was limited to the layer that adhered to the mucosa surfaces.Existing wet adhesives,on the other hand,are either strong but irreversible or invertible but weak [129,130].As a result,creating a wet adhesive substance with both strong adherence and reversibility remains a difficult issue.
Various than marine mussels,other species in nature can inspire biomimetic wet adhesives either.Sandcastle worms,for example,can bind strongly to reefs in turbulent underwater conditions [131],whereas octopuses show incredible adhesive abilities of attaching to rocks stably with their cups [132].Among terrestrial organisms,spiders and snails also rely on their unique physiological qualities to adhere successfully in damp environments[133,134].Although some researchers have created biomimetic materials based on the above creatures’adhesion mechanisms,however,they are not specifically tailored for oral and maxillofacial region.Therefore,designing more biomimetic materials with wetadhesive properties for stomatology is a promising future direction.
7.2.Biological constraints
Since the potential cytotoxicity that materials may cause have a negative impact on adjacent cells and possibly the entire body,determining their biocompatibility is critical.Furthermore,researchers often utilize standardized cell modeling to examine the relevant features of wet-adhesive materialsin vitrotests with few designs for specific disease tissues,which is mostly discordant with the actual situation.Actually,once the tissue is under pathological or inflamed conditions,it is definitely different from the normal tissue in terms of physical,chemical,and even immunological microenvironment features [135–137].To put it another way,employing just healthy cells to generalize the adhesive properties of materials in all instances is far from sufficient.As for the establishment of animal modelsin vivo,most current studies focus on the related effects over a comparatively short time,which lacks sufficient long-term persuasive evidence [138].
8.Conclusion
In the past,the bulk of adhesive materials was intended for ordinary skin and few researches aimed at the peculiarity of the wet environment in the oral cavity and maxillofacial regions.However,because of the evident differences between oral-related regions and normal skin tissues,such as the comparably wet environment,constant temperature and continuous oral movement,it is not advisable to transplant the design strategies of skin adhesives on oral and maxillofacial region directly.Recently,some materials with wet-adhesive properties have been developed,involving naturally derived polymer-based adhesives,mussel biomimetic materials and adhesive electrospun fiber membranes.These materials have given rise to new optimism for the treatment of oral illnesses.This review summarizes the recent progress of oral and maxillofacial wetadhesive materials in stomatology from multifaceted classification,core design mechanisms to emerging applications.Besides,we also provide guidelines for the rational design and future development of the next generation adhesives.To sum up,wet-adhesive biomaterials are doomed to be one of the promising focuses in biomaterial engineering field.As environmental characteristics in the oral and maxillofacial region are more understood,and related material design theories are continually enriched,the wet-adhesive materials employed in stomatology are bound to be further developed in the near future.And we hope that this review is conducive to profound investigation into wet-adhesive biomaterials in the application oral field.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.82101076,81771122,81970985),Key Research Program of Sichuan Science and Technology Department(No.2019YJ0147),Postdoctoral Research Foundation of China (No.2020M683334).
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
- Boron: A key functional component for designing high-performance heterogeneous catalysts
