The cycloaddition reaction of ethylene and methane mediated by Ir+to generate a half-sandwich structure IrHCp+
Wei Li,Hechen Wu,Xunlei Ding,*,Xiaonan Wu,*
a School of Mathematics and Physics,North China Electric Power University,Beijing 102206,China
b Department of Chemistry,Fudan University,Shanghai 200433,China
c Institute of Clusters and Low Dimensional Nanomaterials,North China Electric Power University,Beijing 102206,China
Keywords:Cycloaddition reaction Mass spectrometry Theoretical calculation Half-sandwich structure
ABSTRACT The cycloaddition reactions of methane and ethylene mediated by Ir+ have been designed and studied by the techniques of mass spectrometry in conjunction with theoretical calculations.Studies have shown that Ir+ can mediate the cycloaddition reaction of CH4 and two C2H4 to generate a half-sandwich structure IrHCp+ (Cp=η5-C5H5) including pentamethylcyclopentadienyl ligand by continuous dehydrogenation reaction with the forming of three C-C bonds and seven C-H bonds.The orbital analysis indicates the mechanism of the cyclization reaction to generation of pentamethylcyclopentadienyl ligand with odd number carbon atom depends on the overlap of π orbitals in -C2H2 and carbene,which is more difficult than the forming of cyclobutadiene ligand and benzene.This study may help to understand the reaction mechanism in the cycloaddition reactions of organic compounds,which will be useful to guide the rational design of new catalysts with tailored selectivity and increased efficiency.
The cycloaddition reactions of small organic molecules involving alkane,alkene and alkyne have been a topic for intense investigation during the past decades as one of the most straightforward methods to produce useful carbocyclic systems [1–4].Due to the thermodynamic stability and kinetic inertness of small organic molecules,the cycloaddition reactions for them are difficult to occur,which always need extremely high temperature or high pressure [5–8].Therefore,the catalyst is essential for the cycloaddition reactions.Transition metal compounds are effective in catalyzing the cycloaddition reactions by changing its spin state due to the empty or half-filled d orbitals of transition metals,which have become the most abundant industrial catalysts for cycloaddition reactions [9–13].
In the past decades,the researchers applied different forms of transition metal catalysts including the bare transition metal atoms (Co,Ti,Y,Zr,Nb,Ni,Ru,Rh and Mo,etc.) [14–18],metal oxides (TiO2,VO2) [19–21],neutral metal clusters (Pdn),ionic metal clusters (Fen+) [22] and so forth to study the reaction of acetylene cyclization through experiments and theoretical calculations[23–29].The intermediate products M(η2-C2H2)+,M(η2-C4H4)+,M(η4-C4H4)+,M(η2-C6H6)+and M(η6-C6H6)+are generated.Their structures have been characterized in the gas phase experiments with theoretical calculations.M(η2-C2H2)+,M(η2-C4H4)+and M((η2-C6H6)+are metal-ligand ring structures which can be described by the Dewar-Chatt-Duncanson (DCD) complexation model [30–35].According to this model,the interaction of metals and ligands can be described asσdonation andπback donation [30–35].M(η4-C4H4)+and M((η6-C6H6)+with metal cationπstructure contain cyclobutadiene and benzene,which are wellknown in organometallic chemistry,and even been employed in gas-phase ion chemistry [30–35].
In addition to the reactions to generate the cyclobutadiene ligand and benzene with even numbers of carbon atom,the cycloaddition reaction to form pentamethylcyclopentadienyl (Cp) ligand with odd numbers of carbon atom is also important type of reaction in the fields of organic synthesis,medicinal chemistry,pesticides,and chemical industry [36,37].As far as we know,the reaction to obtain a ring with odd numbers of carbon atom has not been obtained in the gas phase.Because the ground state of Ir+is 5d76s1,which has the enough empty valence orbital (of suitable symmetry) for the cycloaddition reaction.The iridium and iridium complexes have been applied to mediate the different cyclization reaction of small organic molecules [38–41].Therefore,in order to obtain the five-membered pentamethylcyclopentadienyl ring ligand,in this work,the cycloaddition reactions of methane and ethylene mediated by Ir+have been designed and studied by the techniques of mass spectrometry in conjunction with theoretical calculations.We will clearly investigate the generation of the halfsandwich structure IrHCp+(Cp=η5-C5H5) including pentamethylcyclopentadienyl ligand by high selectivity,the potential energy surfaces of consecutive reactions in different electronic states and the bond analysis of the products to further understand the special cycloaddition reaction and guide the rational design of new catalysts.
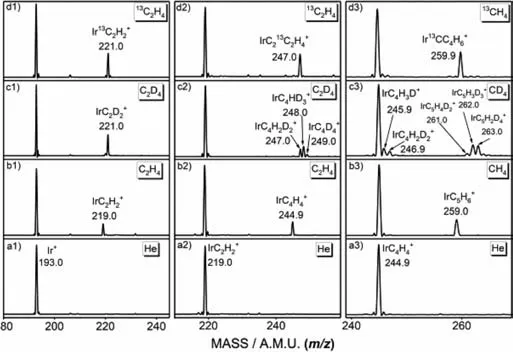
Fig.1.Mass spectra from the reactions of [Ir]+ and [IrC2H2]+ with (a1,a2) He,(b1,b2) C2H4,(c1,c2) C2D4 and (d1,d2) 13C2H4,as well as the reactions of [IrC4H4]+with (a3) He,(b3) CH4,(c3) CD4 and (d3) 13CH4,the specific m/z value for each of peaks of mass spectra are given,respectively.
The experiments are performed by an ion trap mass spectrometer equipped with a laser vaporization-supersonic expansion ion source coupled with a flow tube reactor,which has been reported previously [42,43].For getting pentamethylcyclopentadienyl ligand,the cycloaddition reaction routes Ir+→IrC2H2+→IrC4H4+→IrC5H6+are designed.The metal ions Ir+are generated by pulsed laser ablation of a rotating and translating metal Ir target.The 532 nm second harmonic of a Nd:YAG laser with an energy of 5–12 mJ/pulse is used.The nascent ablated plasma is entrained by the helium carrier gas (99.999%) expanded from a pulsed valve (General Valve,series 9) at a backing pressure of about 0.3–0.5 MPa.The generated ions are guided and mass-selected by the quadrupole,and then sent into a quadrupole linear ion trap.The mass spectra from the reactions of Ir+ions with He,C2H4,C2D4and13C2H4in the ion trap are shown in Fig.1 (panels a1,b1,c1,d1).The results indicate that only one product with chemical formula of IrC2H2+is generated by reaction 1.
Isotopic-labeling experiments by using the C2D4and13C2H4(panels c1 and d1) confirm this result with the generation of IrC2D2+and Ir13C2H2+.Similarly,the reaction of mass-selected IrC2H2+with C2H4is studied,and the mass spectra from the reactions with He,C2H4,C2D4,and13C2H4are shown in Fig.1 (panels a2,b2,c2,d2).The observation of only product IrC4H4+suggests that the reaction 2 takes place:
Isotopic-labeling experiments by using the C2D4and13C2H4(panels c2 and d2) confirm that the generation of the IrC213C2H4+and IrC4H2D2+/IrC4HD3+/IrC4D4+.
For getting the five-membered ring ligand,the mass spectra from the reactions of IrC4H4+with He,CH4,CD4,and13CH4are studied and shown in Fig.1 (panels a3,b3,c3,d3).One peak that can be assigned to the product ions with chemical formula of [IrC5H6]+is observed in the spectra,reaction 3.
Isotopic-labeling experiments using the13CH4sample confirm that the four carbon atoms in the [IrC5H6]+product ion are originated from the C2H4reactant and one carbon atom is originated from the CH4reactant.However,we point out that when Ar is added for cooling before the reaction gasses are inlet,no products are found for the reactions of [IrC4H4]+with CH4.For the reactions of IrC2H2+or Ir+with C2H4,no similar phenomenon is found.
In order to gain insight into the reaction mechanisms,the geometry optimization and frequency calculations for reactants,products,reaction intermediates (IMs) and transition states (TSs)were carried out by using the TPSS method and the Def2TZVP basis sets by Gaussian 09 software package [44–47].All the stationary point structures were characterized on the Potential Energy Surface(PES) by performing vibrational frequencies analysis,which aimed at identifying the nature of the stationary point (minima or saddle point).In predicting the reaction pathways,the intrinsic reaction coordinate (IRC) [48,49] calculations were performed to confirm the correctness of the transition states.All energies are reported by zero-point vibrational energy (ZPE) correction.The analyses of the quantum theory of atoms in molecules (QTAIM) [50,51],charge decomposition analysis (CAD) [52] and orbital interaction diagram are generated using the Multiwfn package [53].
The various possible structures of dehydrogenation products IrC2H2+,IrC4H4+and IrC5H6+are obtained by calculations at the TPSS/def2-TZVP level and shown in Figs.S1-S3 (Supporting information).For IrC2H2+,the most stable structure is metal cation-πcomplex3Ir(η2-C2H2)+.The corresponding singlet state structure is predicted to lie 0.09 eV higher in energy than the triplet state.The most stable structure of IrC4H4+is metallacycle structure1Ir(η2-C4H4)+of coupling by -C2H2(from IrC2H2+) and -C2H2(activated products of ethylene) which has the ground state1A1.The1Ir(η4-C4H4)+and corresponding triplet state structure3Ir(η2-C4H4)+are predicted to lie 0.35 eV and 0.58 eV higher in energy than the most stable structure.The most stable structure of IrC5H6+is1IrH(η5-C5H5)+,which is half-sandwich structure with IrH moiety as the center and coordinated with Cp ligand that is obtained from the coupling by -C2H2,-C2H2and -CH (activated products of methane).The isomers1Ir(η5-C5H6)+and1IrH(η2-C5H5)+are predicted to lie 0.43 eV and 0.45 eV higher in energy than the most stable structure.
The pathway for the first dehydrogenation reaction Ir++C2H4starting with the quintet state as ground reactants is shown in Fig.2 and the details are shown in Fig.S4 (Supporting information).An ethene adduct,5I1 is formed upon initial approach.The efficient dehydrogenation at the thermal energies cannot occur along the quintet state surface because the transition state5TS2 lies 0.68 eV above the reactants,so further calculations along the quintet state surface are not conducted.A surface crossing must occur from the quintet state to the singlet state or triplet state surface for dehydrogenation.Along the triplet state surface,the ethene adduct,3I1,lies 3.82 eV lower in energy than the ground state reactants.From3I1,the first oxidative addition process with the transfer of the first H atom from ethene to the Ir+occurs through3TS1 (-2.58 eV) resulting in the formation of the intermediate3I2 (-3.24 eV).The second oxidative addition process,with C–H bond cleavage from -C2H to the Ir+center leads to generate3I3 (-2.70 eV) by a transition state3TS2 (-2.24 eV).Then,intermediate3I4 (-2.95 eV) in which H2is adsorbed by Ir+through weak interactions is formed with the decrease of H-H distance.The final triplet state product3Ir(η2-C2H2)+with metal cation-πstructure is generated with the reductive elimination process of H2.Along the singlet state surface,similar to the triplet state surface,this crossing takes place in the entrance channel because intermediate1I1 lies 3.28 eV below5I1.Then the reaction occurs through the oxidative addition and reductive elimination processes of C-H bonds.It requires 0.09 eV more energy than the triplet state dehydrogenation process,but still lies 2.24 eV below ground state reactants.Therefore,an effective dehydrogenation reaction can not only occur along the triplet state surface but also along the singlet state surface,the singlet and triplet state products1Ir(η2-C2H2)+and3Ir(η2-C2H2)+may be coexisting.
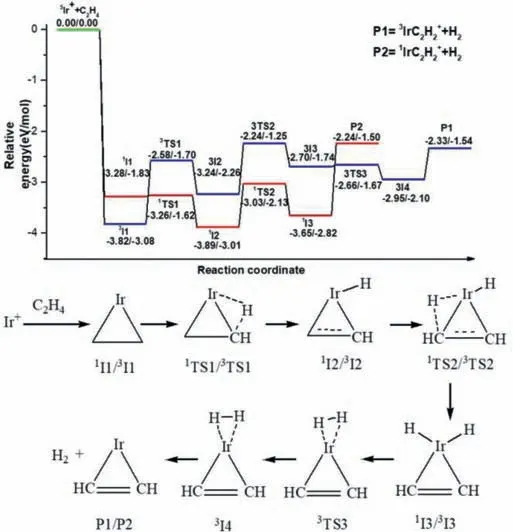
Fig.2.The dehydrogenation pathways for the cycloaddition reaction of Ir++C2H4.Relative energies of the reaction intermediates,transition states,and products with respect to the separated ground state reactants are given by TPSS/CCSD(T) (the unit is eV).
The pathway for the second dehydrogenation reaction,which begins with the singlet state or triplet state Ir(η2-C2H2)++C2H4is shown in Fig.3 and the details are shown in Fig.S5 (Supporting information).The single state1Ir(η2-C2H2)++C2H4is determined as ground reactants for discussion.Along the singlet state surface,the adsorption complex,1I1 lies 2.85 eV lower in energy than the ground state reactants.The first oxidative addition process with the transfer of the first H atom from ethene to the Ir+occurs through1TS1 (-2.08 eV) resulting in the formation of the intermediate1I2 (-2.70 eV).The second oxidative addition process,with C–H bond cleavage from -C2H to the Ir+center leads to intermediate1I3 (-2.83 eV) by a transition state1TS2 (-2.67 eV).The cycloaddition reaction with the coupling of -C2H2from reactant Ir(η2-C2H2)+and -C2H2from the activated product of reactant C2H4forms a metallacycle structure1I4 (-1.98 eV) through a transition state1TS3 (-0.60 eV).Then,intermediate1I5 (-2.60 eV)in which H2is adsorbed by the metal cation Ir+through weak interactions is formed with the decrease of H-H distance.The final metallacycle structure1Ir(η2-C4H4)+with singlet state is generated with the reductive elimination process of H2.In addition,the1Ir((η4-C4H4)+is also generated through the potential energy surface (1I4–1TS5–1I6).The IRC calculations of the pathways1I3–1TS3–1I4 and1I4–1TS5–1I6 are performed and the results are shown in Figs.S6 and S7 (Supporting information).The reaction pathway to generate3Ir(η2-C4H4)+for the triplet surface is similar to that of the singlet state.It requires 0.58 eV more energy than the singlet state dehydrogenation process,but still lies 1.65 eV below ground state reactants,so the reaction can proceed along the singlet state and the triplet state surface,the1Ir(η4-C4H4)+,triplet and singlet products Ir(η2-C4H4)+are generated.But the energy of3TS4,1TS5 is higher than that of1TS4,and the energy of P4,P5 is higher than that of P3,we predict the main product of IrC4H4+is1Ir(η2-C4H4)+.
The pathway for the third dehydrogenation reaction is shown in Fig.4 and the details are shown in Figs.S8 and S9 (Supporting information).The reaction leading to the IrC5H6++H2products is predicted to proceedviaoxidative addition,reductive elimination,ring-forming and dehydrogenation processes.If the reaction begins with the singlet state1Ir(η2-C4H4)++CH4or1Ir(η4-C4H4)++CH4as the ground reactants,the efficient dehydrogenation at the thermal energies cannot occur because the energy of transition state is higher than the reactants (Figs.S6 and S7),this is consistent of experimental results that no product is observed when ions are cooled by Ar gas.The experimental results can be explained that the part of the release energy in the reactions of IrC2H2+or Ir+with C2H4is not transferred away and the product IrC4H4+owns the extra energy to overcome the barrier.So the reaction can begin with the triplet state3Ir(η2-C4H4)++CH4as ground reactants or singlet state with the extra energy [54].Along the triplet state surface,the ethene adsorption complex,3I1 lies 0.47 eV lower in energy than the ground state reactants.When the activation of the first C-H bond of CH4occurs,the second transfer of hydrogen atom leads to the intermediate3I3 by the transition state3TS2 which lies 0.05 eV above the reactants.Because of this,efficient dehydrogenation at thermal energies cannot occur along the triplet state surface,so further calculations along the triplet state surface are not conducted.The triplet state Ir(η2-C4H4)+is directly coordinated to CH4for forming the ethene adduct,1I1,lies 1.13 eV lower in energy than the ground state reactants.From1I1,the first oxidative addition process with the transfer of the first H atom from CH4to the Ir+occurs through1TS1 (-0.11 eV) resulting in the formation of the intermediate1I2 (-0.64 eV).The first reductive elimination process,with the transfer of H atom from Ir+to adjacent C atom leads to intermediate1I3 (-1.74 eV) by a transition state1TS2(-0.24 eV).The second oxidative addition process with the transfer of the H atom from -CH3to the Ir+occurs through1TS3 (-0.89 eV)resulting in the formation of the intermediate1I4 (-1.36 eV).Then the first ring-forming reaction with the coupling of -C4H5and-CH2from the activated product of reactant CH4forms a metallacycle structure1I5 (-1.54 eV) through a transition state1TS4(-0.41 eV).The third oxidative addition process,with the transfer of H atom from -CH2(the activated product of reactant CH4)to Ir+atom leads to intermediate1I6 (-1.20 eV) by a transition state1TS5 (-0.87 eV).Then the H atom is transferred between the two C atoms to form intermediate1I7 (-1.65 eV) by a transition state1TS6 (-0.43 eV).The second ring-forming reaction with the coupling of two C atoms adjacent to Ir+through1TS7 (-0.30 eV)results in the formation of the intermediate1I8 (-0.43 eV) with a like-half-sandwich structure that IrH2+connects to the fivemembered ring -C5H6.The final oxidative addition process,with the transfer of the H atom from -CH2to the Ir+center takes place to form the intermediate1I9,in which H2is adsorbed by the metal Ir through weak interactions.Finally,the half-sandwich structure IrHCp+is generated with the reductive elimination process of H2,which is consistent with the experimental results.The generated pathways of isomers Ir(η5-C5H6)+and IrH(η2-C5H5)+are exothermic processes which lie 0.98 eV and 0.96 eV below ground state reactants.But the energies of Ir(η5-C5H6)+and IrH(η2-C5H5)+are higher than the IrHCp+,Therefore,the IrHCp+is major product,Ir(η5-C5H6)+and IrH (η2-C5H5)+may be also coexisting,which is similar with the isomers of IrC4H4+.The IRC calculations of the pathways1I3–1TS3–1I4 and1I4–1TS5–1I6 are performed and the results are shown in Figs.S10 and S11 (Supporting information).
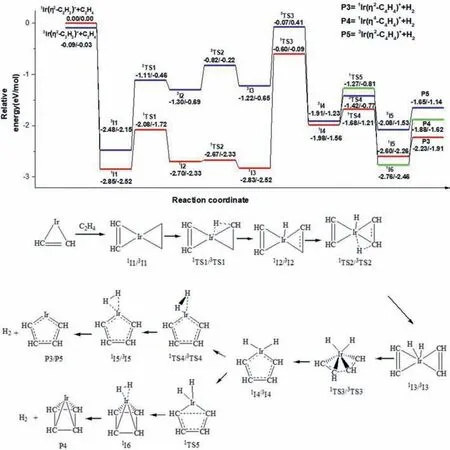
Fig.3.The dehydrogenation pathways for the cycloaddition reaction of Ir(η2-C2H2)++C2H4.Relative energies of the reaction intermediates,transition states,and products with respect to the separated ground state reactants are given by TPSS/CCSD(T) (the unit is eV).
In addition,the single-point energy of dispersion correction,the entropy and Gibbs free energy are calculated and the comparison diagrams are shown in Figs.S12–14 (Supporting information) and the values are given in Tables S1-S3 (Supporting information).The energy trends of dispersion correction and the entropy are consistent with the calculation of the TPSS function used.The singlepoint calculations using high-level CCSD(T) methods on the reactants,products,reaction intermediates and transition states are shown in Figs.2-4.The reaction pathways are consistent with the TPSS function except for the3TS3 of the cycloaddition reaction of Ir(η2-C2H2)+with C2H4.The relative energy of transition states3TS3 is higher 0.41 eV than the ground state reactants,but the energy singlet state1TS3 is lower than the reactant.So the cycloaddition reaction of Ir(η2-C2H2)+with C2H4might have a cross point(CP) in the singlet and triplet potential energy surfaces [55].The reaction products are same as the results of TPSS function,so the calculation of TPSS function is accurate.
In order to explain the mechanism of the cyclization reaction,we analyze the localized molecular orbital (LMO) of the cyclization process and the results are shown in Fig.S15 (Supporting information).For the cyclization process of reaction1Ir(η2-C2H2)++C2H4,from1I3 to1I4 through the transition state1TS3,with the distance between the carbon atoms from1Ir(η2-C2H2)+and C2H4gradually decreases,the twoπorbitals of -C2H2from Ir(η2-C2H2)2+approach,and then overlap to form a multi-center bond (1TS3),and further overlap to form a new C-Cσbond,theπorbitals of carbons in ring overlap result in forming the four-membered cyclobutadiene ring ligand C4H4.The LMO of the cyclization process above is the same to the that of the reaction3Ir(η2-C2H2)++C2H4to generate3Ir(η4-C4H4)+.For the first ring formation process of reaction1Ir(η2-C4H4)++CH4,the two overlappingπorbitals are from the -IrCH2with carbene structure and -C2H2of ringη2-C4H4,which leads to form another new C-Cσbond.Finally,theπorbitals of carbons in ring overlap to form the pentamethylcyclopentadienyl ligand (Cp ring).Therefore,the mechanism of the cyclization reaction depends on the overlap ofπorbitals which is consistent with the statement of previous paper [13].
In order to classify bond types,characterize bond nature,and distinguish bond strength of the special product IrH(Cp)+containing pentamethylcyclopentadienyl ligand.The bond analysis is applied by the topological parameters at the bond critical point(BCP) between two atoms based on the quantum theory of atoms in molecules (QTAIM) theory [56-59].From Table S4 (Supporting information),for the topological parameters the Ir-C bonds of IrH(Cp)+,ρband ∇2ρbare positive,Hbis negative,and -Gb/Vbis greater than 0.5 and less than 1,which illustrate that IrH(Cp)+is formed through the dative bond between IrH+and Cp ligand[56–59].For the topological parameters the C-C bond of Cp,ρbis positive,∇2ρbandHbare negative,and -Gb/Vbis less than 0.5,which illustrate the C-C bond of Cp is covalent bonds [56–59].The analyses of orbital interaction diagram and charge decomposition analysis (CDA) [60,61] are applied to understand how orbitals of fragments are mixed to form the dative bond of IrH(Cp)+(Figs.S16 and S17 in Supporting information).For IrH(Cp)+,the LUMO is mainly composed of the dxzorbital (44%) of1Ir+and theπorbital (41%) of Cp,the all occupied molecular orbitals are formed by the s+dz2orbital (68%) of IrH+and theπorbital (20%) of Cp,the s+dz2orbital (25%) of IrH+and theπorbital (26%) of Cp,the dxzorbital (34%) of1Ir+and theπorbital (47%) of Cp,the dxyorbital (34%) of1Ir+and theπorbital (14%) of Cp,the s+dz2orbital(16%) of IrH+and theπorbital (64%) of Cp.The calculated data of CDA of IrH(Cp)+indicate that the dative bond of IrH+and Cp is formed between the donation from IrH+to Cp and back-donation from Cp to IrH+by above mixed orbitals.The numbers of donation electron and back-donation electron are 0.1164 and 0.1361 and the back-donation interaction is greater than donation.
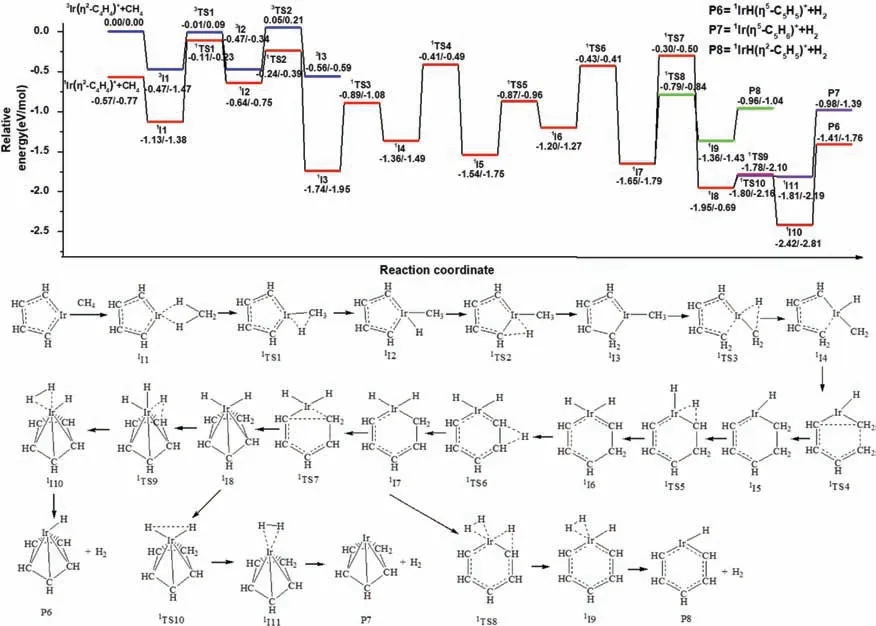
Fig.4.The dehydrogenation pathways for the cycloaddition reaction of Ir(η2-C4H4)++CH4.Relative energies of the reaction intermediates,transition states,and products with respect to the separated ground state reactants are given by TPSS/CCSD(T) (the unit is eV).
Previous studies have shown that catalysts containing transition metals can effectively promote the reaction of acetylene cyclization to form cyclobutadiene ligand and benzene [23–29].Cp is important ligand of sandwich structure compound whose importance has been shown in the fields of organic synthesis,catalysis,medicinal chemistry,pesticides,and chemical industry [36,37].Normally,due to the thermodynamic stability and kinetic inertness,the cycloaddition reaction of ethylene and methane to form pentamethylcyclopentadienyl ligand with the activation and forming of C-C bond and C-H bond does not happen at room temperature [5–8].Besides,the ring with odd numbers of carbon atom is more difficult to generate than the ring with even numbers of carbon atom,because -C2H2has the sameπorbitals and is easier to overlap to form the ring with even numbers of carbon atom,while the ring with odd numbers of carbon atom have to overlap with carbene,which is a dehydrogenation product methane with differentπorbital than that of the -C2H2.Therefore,despite a lot of work on synthesis of cyclobutadiene ligand and benzene,there is no work on the generation of Cp in the gas phase.In this paper,the cycloaddition reaction of two ethylene and methane by the “catalyst” Ir+can happen to generate the Cp ring ligand by high selectivity,which proceeds by the activation/formation of three C-C bonds and seven C-H bonds and only one products IrC2H2+,IrC4H4+and IrC5H6+are found for Ir+/C2H4,IrC2H2+/C2H4and IrC4H4+/CH4reactions in this process.The metal ions Ir+plays a key role,which decreases the energy for C-H and C-C bond activation and promotes the overlap of differentπorbital.In addition,our study shows that the products Ir(η2-C2H2)+,Ir(η2-C4H4)+,Ir(η4-C4H4)+and IrH(Cp)+are formed by the dative bonds.These may help to understand the reaction cycloaddition mechanism and guide the rational design of new half-sandwich and sandwich catalysts with tailored selectivity and increased efficiency.
In conclusion,the cycloaddition reactions of methane and ethylene mediated by Ir+are designed,which have been studied by gas-phase experiments with theoretical calculations.Experimental results found the high selectivity reactions of Ir+/C2H4,IrC2H2+/C2H4and IrC4H4+/CH4,and only one products IrC2H2+,IrC4H4+and IrC5H6+are confirmed,respectively.Calculations have shown that Ir+can mediate the cycloaddition reaction of CH4and two C2H4to generate the half-sandwich structure IrHCp+containing the pentamethylcyclopentadienyl ligand by continuous dehydrogenation reaction with the forming of three C-C bonds and seven C-H bonds.The orbital analysis indicates the mechanism of the cyclization reaction to generation of pentamethylcyclopentadienyl ligand depends on the overlap of -C2H2and carbeneπorbitals,which is more difficult than the overlap of same -C2H2πorbitals to form cyclobutadiene ligand and benzene.The calculated QTAIM and CDA data of IrH(Cp)+indicate that the dative bond of IrH+and Cp is formed between the donation from IrH+to Cp and back-donation from Cp to IrH+.The back-donation interaction is greater than donation.This study may help to understand the reaction mechanism and metal-mediated ability in cycloaddition reaction of organic compounds,which will be useful to guide the rational design of new catalysts with tailored selectivity and increased efficiency.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by Beijing Natural Science Foundation (No.2214064),the National Natural Science Foundation of China (Nos.21603037,21688102,92161115,21973016,91545122),the Fundamental Research Funds for the Central Universities (Nos.JB2015RCY03,JB2019MS052) supported by the fund of North China Electric Power University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.02.002.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
