Atramacronoids A-C,three eudesmanolide sesquiterpene-phenol hybrids with an unprecedented C-C linkage from the rhizomes of Atractylodes macrocephala
Hixin Zhng,Jingrong Li,b,Jingung Si,Chengy Dong,Qi Li,Meng Yu,Lingling Qin,Lingyu Li,Chenxu Zho,d,To Zhng,*,Zhongmei Zou,*
a Institute of Medicinal Plant Development,Chinese Academy of Medical Sciences & Peking Union Medical College,Beijing 100193,China
b Medical Sciences,Guizhou Medical University,Guiyang 550000,China
c China National Clinical Research Center for Neurological Diseases,Beijing Tiantan Hospital,Capital Medical University,Beijing 100193,China
d School of Pharmacy,Henan University of Chinese Medicine,Zhengzhou 450000,China
Keywords:Atractylodes macrocephala Compositae Sesquiterpene-phenol hybrids Anti-cancer Neutrophil elastase
ABSTRACT Three eudesmanolide sesquiterpene-phenol hybrids,atramacronoids A-C (1-3),featuring an unusual 6/6/5/5/6 skeleton furnished by forming an unexpected C-8-C-16 linkage,were obtained from the rhizomes of Atractylodes macrocephala.Their structures and absolute configurations were elucidated by spectroscopic data analysis,chemical calculations,combined with X-ray diffractions.The plausible biosynthetic pathways for compounds 1-3 are proposed.Surprisingly,compound 1 exhibited cytotoxicity against SGC-7901 cells by inducing cells apoptosis,which might relate to the promotion of synthesis of neutrophil elastase.
Sesquiterpene lactones,such as eudesmanolide,guaianolide,and germacranolide,have been widely reported from Compositae plants,with theα-methylene-γ-lactone moiety connected to other building blocks to form diverse structurally intriguing and biologically active adducts [1–7].Although the isolated sesquiterpene lactone oligomers present a dramatic increase [8],hybrids of sesquiterpene lactones especially eudesmanolide hybrids,are extraordinary rare.So far,only two types of eudesmanolide hybrids,eudesmanolide-furan [4] and eudesmanolide-alkaloid [9],have been isolated from natural resources.Structurally,both of them possessed the furan or alkaloid moiety linkage to eudesmanolide skeleton onlyviathe methyl at C-11.Due to the fascinating structures,eudesmanolide hybrids have attracted broad interest from natural products and synthetic chemists since 2019 [6,9].
A recently research published inCellrevealed that neutrophil elastase (NE) was a major anticancer protein released by human neutrophils,which specifically activated the cell death pathway in cancer cells.Researchers observed that NE initiated a complex cancer killing program that inhibited cell survival pathways,induced DNA damage,increased the production of mitochondrial reactive oxygen species,and ultimately activated programmed cell death,that is so-called apoptosis [10].It made NE became a hot topic in various scientific communities.However,apart from these inspiring reports,the presence and value of natural products possessing effect on NE remain largely unexplored.
The genusAtractylodes(Compositae) is represented by a relatively limited number of species and mainly distributed in eastern region of Asia.Atractylodes macrocephalaKoidz,is a perennial herb belonging to theAtractylodesgenus,which is widely distributed in China,Korea,and Japan [11].The rhizomes ofA.macrocephalaare the certified plant source for “Bai zhu”,a common traditional Chinese medicine that is often used for the treatment of splenic hypofunction with inappetence,edema,spontaneous sweating,etc.[12].Nowadays,“Bai zhu” is also one of the ingredients inQingfei Paidudecoction,a key traditional Chinese medicine (TCM) formula used for treatment of COVID-19 in China [13].Extensive phytochemical studies fromA.macrocephalarevealed that sesquiterpenes were the main chemical constituents [14].However,there was remained without a significant breakthrough for this plant.
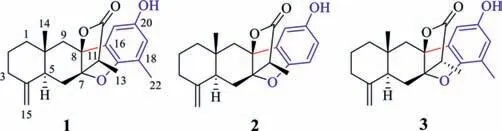
Fig.1.Structures of compounds 1–3.
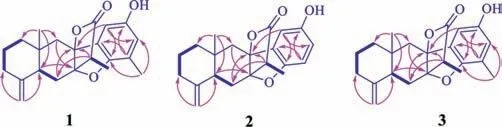
Fig.2.The 1H–1H COSY (thick lines) and key HMBC correlations (pink arrows,from 1H to 13C) of compounds 1-3.
Our research group has already reported a number of sesquiterpenes fromA.macrocephala[15].Continuing the search for structurally diverse and biologically interesting metabolites from this plant,we turned our focus toward eudesmanolide hybrids.This effort resulted in the isolation of three eudesmanolide sesquiterpene-phenol hybrids with extraordinary C–C linkage,named atramacronoids A-C (1-3).Remarkably,compounds 1-3 represent the first example of naturally occurring unusual hybrids constructed from eudesmanolide sesquiterpene and phenol,possessing pentacyclic 6/6/5/5/6 skeleton by formation of C-8-C-16 and C-7-O-C-17.Compound 2 was 18-demethyl derivative of 1,while 3 was a C-11 epimer of 1 (Fig.1).Herein,the isolation,structure elucidation,possible biosynthetic pathways of these isolates,as well as preliminary mechanism of induced apoptosis through promoting the synthesis of NE,are described.As representative pioneering works,the new linkage pattern of these compounds is not only crucial for the chemical diversity and biosynthesis of sesquiterpene hybrids,but also for the pharmacological studies on anti-cancer.
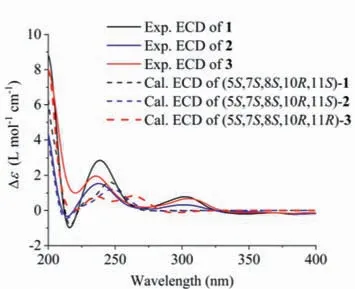
Fig.4.The overlaid experimental ECD (full lines) and calculated ECD (dash lines)spectra of compounds 1–3.
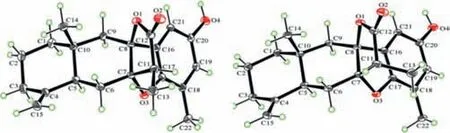
Fig.5.The X-ray ORTEP diagrams of 1 (left) and 3 (right).The thermal ellipsoid is scaled to the 30% probability level.
Atramacronoid A (1) was obtained as colorless needle crystals.Its molecular formula was deduced as C22H26O4from the protonated molecular ion peak atm/z355.1900 [M+H]+(calcd.for C22H27O4,355.1904) in HRESIMS,which corresponded to 10 indices of hydrogen deficiency.The IR spectrum clearly exhibited absorption bands of hydroxyl (3421 cm–1),γ-lactone (1778 cm–1),and unsubstituted double bond (1644 cm–1) functional groups.The1H NMR data (Table S1 in Supporting information) displayed the diagnostic signals of an exocyclic double bond atδH4.86 (1H,br d,J=1.6 Hz,Ha-15) and 4.63 (1H,br d,J=1.6 Hz,Hb-15),three methyl protons atδH2.13 (3H,s,H-22),1.25 (3H,d,J=7.1 Hz,H-13) and 0.76 (3H,s,H-14),two downshielded aromatic protons atδH6.56(1H,d,J=2.5 Hz,H-19) and 6.54 (1H,d,J=2.5 Hz,H-21),indicating the existence of a 1,2,3,5-tetrasubstituted phenyl ring.In accordance with the molecular formula,analysis of the13C NMR and DEPT data (Table S1) displayed 22 carbon resonances that could be attributed to three methyl groups atδC17.0 (C-14),15.1 (C-22) and 8.2 (C-13),five aliphatic methylene groups atδC49.4 (C-9),43.0 (C-1),38.0 (C-3),26.7 (C-6) and 24.0 (C-2),one exocyclic methylene atδC107.5 (C-15),two aliphatic methines atδC45.8(C-11) and 43.6 (C-5),two aromatic methines atδC119.3 (C-19)and 108.1 (C-21),five olefinic quaternary carbons atδC153.9 (C-20),150.3 (C-4),149.0 (C-17),132.9 (C-16) and 124.0 (C-18),one aliphatic quaternary carbon atδC35.0 (C-10),one ester carbonyl group atδC177.7 (C-12),and two oxygenated quaternary carbons atδC92.2 (C-7) and 90.4 (C-8).The assignments of all hydrogen and carbon signals were further achieved by its HSQC spectrum.Further analysis of the1H-1H COSY and HMBC spectra (Fig.2) led to the elucidation of the planar structure of 1.The1H-1H COSY spectrum revealed three spin systems,including H2-1/H2-2/H2-3,H-5/H2-6 and H-11/H3-13.The three coupling networks were connected by use of HMBC correlations of H3-14/C-1,C-5,C-9,C-10;H2-15/C-3,C-4,C-5; H-5/C-7,C-14; H2-6/ C-5,C-7,C-8,C-10,C-11; H3-13/C-7,C-11,C-12; H2-9/C-1,C-7,C-8.Above evidence made for the construction of the eudesmanolide moiety.The position of the remaining methyl group was located at C-18,as confirmed by HMBC correlation from H3-22 to C-17,C-18 and C-19.Correlations from H-21 to C-16,C-17 and C-19,and from H-19 to C-17,C-18 and C-21 also resulted the assignment of substituent position of methyl on phenyl ring.In addition,key correlation from H-21 to C-8 unequivocally indicated the C–C connection between C-8 and C-16.To satisfy molecular formula of unsaturation,it was deduced that C-7 was joined with C-17 by sharing oxygen atom,forming a pentacyclic 6/6/5/5/6 skeleton.Hence,the planar structure of 1 was determined as shown in Fig.1.
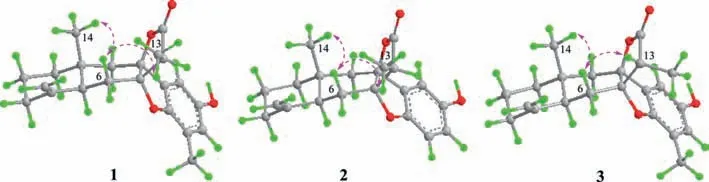
Fig.3.Main NOE correlations (pink dashed double arrows) of 1–3.
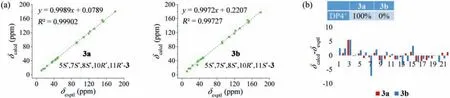
Fig.6.The 13C NMR calculation results of two plausible stereoisomers of compound 3.(a) Linear correlation plots of calculated vs. experimental 13C NMR chemical shift values of 3a and 3b.(b) Relative errors between the calculated 13C NMR chemical shifts the recorded data and DP4+ probability analysis.
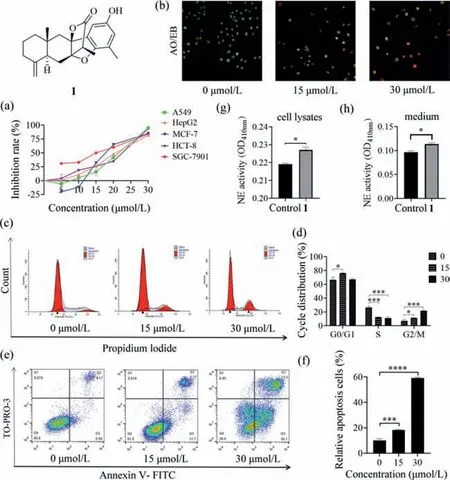
Fig.7.(a) Effects of 1 on the growth of various cell lines (48 h),with the IC50 values ranging from 13 μmol/L to 25 μmol/L,etoposide as positive.(b) 1 induced SGC-7901 cells apoptosis detected by AO/EB staining.(c,d) Representative histograms depicting cell-cycle distribution as analyzed by flow cytometry in SGC-7901 cells treated with indicated concentrations of 1 for 24 h.Counts of G2/M phase cells increased remarkably in the treated cell in a concentration-dependent manner.(e,f) SGC-7901 cells were incubated with 1 at concentrations of 0,15,and 30 μmol/L for 24 h.Apoptosis was analyzed by annexin V-FITC/TO-PRO-3 staining.(g) NE synthesis concentration in conditioned medium treated with 1.(h) NE secretion concentration in lysate treated with 1.Values are presented as mean ± SD for three individual experiments.*P<0.05,***P<0.001,****P<0.0001 vs. control.
The relative configuration of 1 was established by ROESY spectrum (Fig.3).The CH3-14 was arbitrarily assigned asβ-orientation.The key correlation of H3-14/H-6b indicated the junction of the eudesmane rings wastrans-fused and H-5 wasα-oriented.Meanwhile,theβ-orientation of CH3-13 was deduced from the crosspeak between H3-13 and H-6b.Furthermore,the relative configurations of the chiral centers of C-7 and C-8 were deduced because of the rigid structure of 1.Consequently,the established relative configuration of 1 was determined as shown in Fig.3.The calculated ECD curve for 1 matched well with the experimental one(Fig.4),suggesting its absolute configuration to be 5S,7S,8S,10R,11S.Subsequent X-ray diffraction using Cu Kαradiation with the Flack parameter of 0.02(7) (Fig.5) verified the planner structure as well as the absolute configuration of compound 1.
Atramacronoid B (2) was isolated as a yellow oil and had the molecular formula C21H24O4,with 14 mass unit lower than that of 1,which was deduced from HRESIMS atm/z341.1746 [M+H]+(calcd.for C21H25O4,341.1747).The NMR spectroscopic data of 2 closely resembled those of 1 (Table S1),except for the absence of resonances for the methyl unit,which suggested that 2 was 18-demethyl derivative of 1.The deduction was verified by 2D NMR experimental data analysis (Fig.2),especially by the1H-1H COSY cross-peak of H-18/H-19 and the HMBC correlations from H-21 to C-8,C-17 and C-19,from H-19 to C-17 and C-21,and from H-18 to C-16 and C-20 as well as by the chemical shifts of these hydrogen and carbon resonances.Finally,the intact planner structure of 2 was determined.Compared with 1,similar NOE correlations(Fig.3) of H3-14/H-6b and H3-13/H-6b of 2 indicated that both compounds shared identical relative configurations.Thus,the absolute configuration of 2 was determined as 5S,7S,8S,10R,11Sby comparison of the experimentally measured ECD and theoretically calculated ECD spectra.
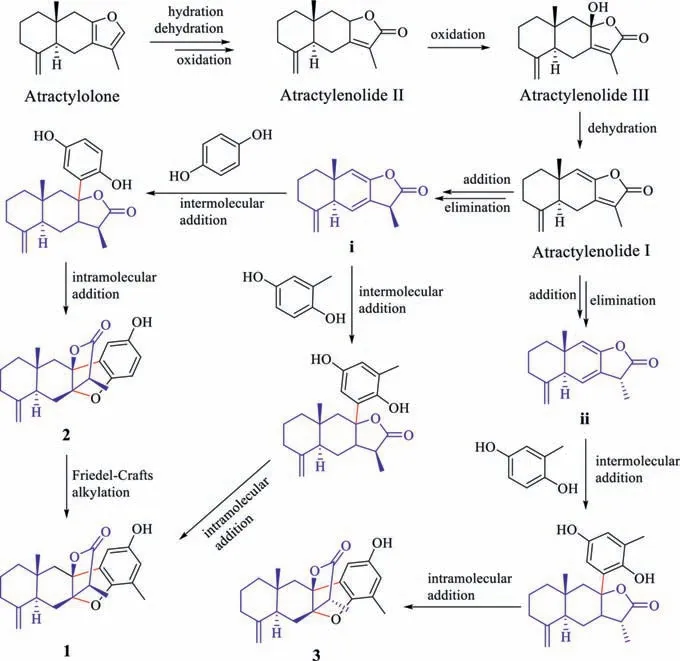
Scheme 1.The plausible biosynthetic pathways of compounds 1-3.
Atramacronoid C (3) was isolated as colorless needle crystals,and possessed the same molecular formula as 1 by the HRESIMS ions atm/z355.1900 [M+H]+(calcd.for C22H27O4,355.1904).Compound 3 was an isomer of 1,which could be preliminary concluded through analyzing detailed 1D NMR (Table S1).In ROESY spectrum of 3,the correlation between H3-14 and H-6b implied that CH3-14 and H-5 wereβ- andα-oriented,respectively.In addition,correlation of H-11 with H-6b (Fig.3) suggested that 3 was 11-epimer of 1.Taken together,the relative configuration of 3 should be fixed as 5S*,7S*,8S*,10R*,11R*.To further consolidate the above deduction,the13C NMR chemical shifts of two isomers (5S*,7S*,8S*,10R*,11R*)-3 (3a) and (5S*,7S*,8S*,10R*,11S*)-3(3b) were calculated.Comparison between the experimental and the calculated13C NMR data allowed the determination of relative configuration of 3 to be 5S*,7S*,8S*,10R*,11R*,with a DP4+probability of approximately 100% (Fig.6).Based on the agreement between the experimental and calculated ECD spectra (Fig.4),finally,the absolute configuration of 3 was assigned as 5S,7S,8S,10R,11R.After repeated recrystallization in different solvent systems,we successfully obtained a crystal of 3 at CH2Cl2-CH3CN (1:1).Single crystal X-ray diffraction (Fig.5) using Cu Kαradiation with the Flack parameter of 0.04 (8) unequivocally confirmed the absolute configuration of 3.
To the best of our knowledge,atramacronoids A-C (1-3) are the first example of eudesmanolide sesquiterpene with phenol forming C–C bondviacyclization.The hypothetical biosynthetic pathways for 1-3 are proposed as shown in Scheme 1.Biogenetically,their biogenetic precursor is traced to be atractylolone,which is biosynthesized through mevalonate pathway and abundantly occurs in this plant.Atractylolone undergoes hydration,dehydration and oxidation to produce atractylenolide II.Afterwards,sequential oxidation and dehydration reactions to afford atractylenolide III and atractylenolide I.Atractylenolide I then yield i and ii through addition and elimination reaction,which is followed by the intermolecular addition of the C–H bond and intramolecular addition of the phenol O–H bond [16] involving the formation of C–C bond between C-8 and C-16 and C–O bond between C-7 and C-17,respectively.The cyclization of i with hydroquinone or methylhydroquinone finally give rise to compounds 2 and 1,respectively.In addition,compound 2 also can be converted to 1 by Friedel-Crafts alkylation,while ii is cyclized with methylhydroquinone to produce compound 3.
In the bioactivity assays,compound 1 exhibited stronger growth inhibitory effects on SGC-7901 cells than other human cancer lines of A549,HCT-8,HepG2,and MCF-7 (Fig.7a),with the IC50value of 13 μmol/L.Thus,SGC-7901 cells were used for further study.Morphological analysis is one of the considerations in process of apoptosis [17].To determine whether 1 could induce cell death by apoptosis,the AO/EB straining assay was performed.As shown in Fig.7b,with increasing the concentration of 1,more apoptotic cells exhibited apoptotic characteristic such as nuclear shrinkage and chromatin condensation.
The propidium iodide (PI) and annexin V-FITC/TO-PRO-3 double staining were applied further to examine cell cycle distribution and cell apoptosis induced by 1,respectively.Cell cycle arrest is considered a critical control point for the management of cancer cell growth [18].As shown in Figs.7c and d,1 caused G2/M cell cycle arrest in SGC-7901 cells.In the apoptosis assay (Figs.7e and f),the increase of early and late apoptosis was significantly observed in SGC-7901 cells treated with 1 when compared to the control,with the percentage running up to 59.6%,and the rate of apoptosis increased in a dose-dependent manner,indicating that compound 1 could induce apoptosis in SGC-7901 cells.Amazingly,we found the synthesis and secretion of NE increased after treating cell with 1 (Figs.7g and h).This suggested that 1 could promote inflammation.From another perspective,we preliminarily inferred 1 induced apoptosis by promoting the synthesis of NE.While in contrast to 1,compound 2 showed no cytotoxicity against SGC-7901 cells at 50 μmol/L,and 3 showed moderate cytotoxicity against SGC-7901 cells with the IC50value of 27 μmol/L.However,surprisingly,compound 2 inhibited NE synthesis (Fig.S6 in Supporting information),which further backup our aforementioned result for 1.Compound 3 could also inhibit the synthesis of NE,but the effect was weaker than 2 (Fig.S6).Basing on this,we speculated that 3 showing moderate cytotoxic activity may ascribe to another mechanism,which needed to be further researched.
In summary,the first discovery of atramacronoids A-C(1-3),featuring unprecedented eudesmanolide sesquiterpenephenol skeleton furnished by forming an unexpected C-8-C-16 linkage,represents a milestone work after decades of the research onA.macrocephala.Predictably,the discovery of fascinating compounds 1-3 enriches the structural diversity of sesquiterpene hybrids and would attract much interest of synthetic chemists for total synthetic and biosynthetic purposes.In addition,mechanistic study revealed compound 1 showed cytotoxicity against SGC-7901 cells by inducing cell apoptosis,which might through promoting the synthesis of NE.These results could provide a new insight for further pharmacological investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.82073992) and the CAMS Innovation Fund for Medical Sciences (CIFMS,No.2021-I2M-1-071).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.107743.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
