Oxygen and nitrogen tailoring carbon fiber aerogel with platinum electrocatalysis interfaced lithium/sulfur (Li/S) batteries
Lei Ji,Xi Wng,Yongfeng Ji,Xioxi Qin,Yi Sui,Huizhong Yn,Zhiqing Niu,Jinghi Liu,*,Yuegng Zhng
a State Key Laboratory of Baiyunobo Rare Earth Resource Researches and Comprehensive Utilization,Baotou Research Institute of Rare Earths,Baotou 014030,China
b Inner Mongolia Key Laboratory of Carbon Nanomaterials,Nano Innovation Institute (NII),College of Chemistry and Materials Science,Inner Mongolia MinZu University,Tongliao 028000,China
c Department of Physics,Tsinghua University,Beijing 100084,China
d Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education),Renewable Energy Conversion and Storage Center,College of Chemistry,Nankai University,Tianjin 300071,China
Keywords:Li/S conversion chemistry Ion channels Pt electrocatalysis interface 3D aerogel host (OCNF)Adsorption confinement
ABSTRACT Sluggish kinetics of lithium/sulfur (Li/S) conversion chemistry and the ion channels formation in the cathode is still a bottleneck for developing future Li/S batteries with high-rate,long-cycling and high-energy.Here,a rational cathode structure design of an oxygen (O) and nitrogen (N) tailoring carbon fiber aerogel(OCNF) as a host material integrated with platinum (Pt) electrocatalysis interface is employed to regulate Li/S conversion chemistry and ion channel.The Pt nanoparticles were uniformly sprayed onto the S surface to construct the electrocatalysis interface (Pt/S/OCNF) for generating ion channels to promote the effective penetration of electrolyte into the cathode.This Pt/S/OCNF gives the cathode a high sulfur utilization of 77.5%,an excellent rate capacity of 813.2 mAh/g (2 C),and an outstanding long-cycling performance with a capacitance retention of 82.6% and a decay of 0.086% per cycle after 200 cycles at 0.5 C.Density functional theory (DFT) calculations reveal that the Pt electrocatalysis interface makes the cathode a high density of state (DOS) at Fermi level to facilitate the electrical conductivity,charge transfer kinetics and electrocatalysis to accelerate the lithium polysulfides (LiPSs) electrochemical conversion.Furthermore,the unique chemisorption structure and adsorption ability of Li2Sn (n=1,2,4,6,8) and S8 on OCNF are attributed to the bridging effects of interfacial Pt and the bonding of N-Li.The Pt electrocatalysis interface combined with the unique 3D hierarchical porous structure and abundant functional active sites at OCNF guarantee strong adsorption confinement,fast Li/S electrocatalytic conversion and unblocked ion channels for electrolyte permeation in cathode.
Rational design of cathode structure is an effective means to realize efficient utilization of sulfur in lithium/sulfur (Li/S) batteries.It has been found that a large-scale dissolution and diffusion of polysulfides (LiPSs) in electrolyte correlated to slow electrochemical conversion kinetics directly causes serious shuttle effects [1,2].Moreover,the electronic insulation of S8and Li2S restricts the solid-solid conversion process between S8and Li2S[3–5].More importantly,the heterogeneous aggregation and growth of discharge products Li2S/Li2S2on the cathode surface hinders the contact between electrolyte and active sulfur and the further lithiation of sulfur,resulting in the occurrence of "dead sulfur" and the sharp decline of the specific capacity [6–10].
Three-dimensional (3D) nanoscale carbon material has been reported as the preferred host material to solve the above problems.With its rich hierarchical porous structure and special carbon skeleton,it can realize the instantaneous penetration of electrolytes in the cathodes and accelerate the transfer of electrons and diffusion of ions,e.g.,porous films [11],foams[12–15],papers [16],aerogels [17–21],carbon nanofibers [22–27],biomass carbon material [28–31] and sponges [32–34].Specifically,3D nitrogen-rich mesoporous carbon framework [35],reduced graphite oxide (RGO) [36],porous graphene sponges (S-GS)[37],3D micron-porous graphene foam [38,39],carbon/CNF papers [40,41],3D porous S/graphene@g-C3N4hybrid sponge [42],3D N-enriched CNT-graphene hybrid microtubes [43],3D carbon nanofibers (CNFs) [44–47],carbon-cotton [48] and 3D porous graphitic carbon [49,50] have also been successfully utilized in cathode design.The hierarchical pore structure of these 3D solid carbons can provide sufficient space to absorb a large amount of sulfur to realize high sulfur loading.Moreover,the abundant ion channels and electronic skeletons on the 3D carbon structured cathode ensure the rapid electron/ion conduction pathway,providing convenient channels for active sulfur species transport and sulfur conversion reactions.
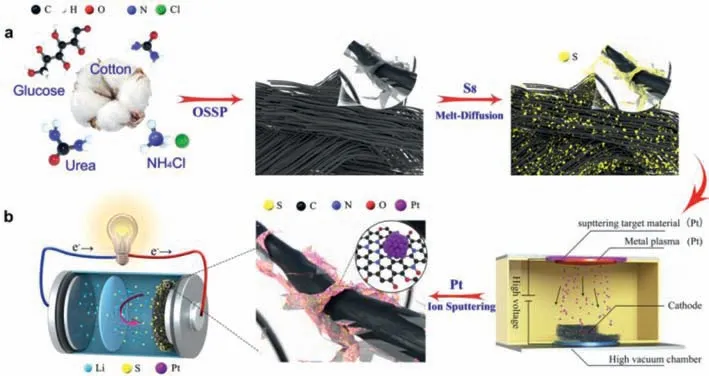
Scheme 1.Conceptual illustration for preparation process of OCNF as a model of O and N tailoring carbon fiber aerogel (a) and Pt electrocatalysis (Pt/S/OCNF) interfaced Li/S conversion chemistry (b).
Metal active site is an effective approach to restrain the shuttling and to facilitate efficient utilization of active sulfur,attributed to its excellent electrocatalysis to accelerate the direct bidirectional conversion of lithium polysulfides (LiPSs) [51–53],high electrical conductivity to promote electron transfer [54–61],and strong affinity for LiPSs to enhance the immobilization of sulfur [62–66].These metal atoms as active sites for electrocatalysis and chemisorption,activate the transformation and phase transition of S8,LiPSs and Li2S,promote significant changes in the bond lengths and angles of Li-S and S-S,and accelerate the chain breaking and bonding of Li-S and S-S to obtain fast redox kinetics and strong physico-chemical adsorption [67–70].For TiN-Ti4O7nanofiber membrane reactor [70],the excellent electrocatalysis and chemical confinement of the active Ti atomic site plays a crucial role in suppressing the LiPSs shuttling.Moreover,the cathode module design of the membrane reactor-mediated LiPSs chemical conversion enables the Li/S cell to display efficient sulfur utilization as well as excellent rate performances and long-cycling capability.
Thus,benefiting from the inspiration of these research progress,a unique sulfur cathode integrating oxygen (O) and nitrogen (N)tailoring carbon fiber aerogel (OCNF),electroactive sulfur,and interfacial platinum nanoparticles (Pt/S/OCNF) is designed,aiming to construct ion channels and accelerate the kinetics of Li/S conversion chemistry.The achievement of high-performance Li/S batteries is attributed to three key advantages of this unique cathode structure.Firstly,the electrocatalytic interface composed of Pt nanoparticles accelerates the bi-directional solid-liquid conversion between S8,Li2S/Li2S2and Li2Sn(n=3–8).Secondly,the fast phase transformation and redox kinetics alleviates the blocks of “dead sulfur” to promote the formation of ion channels,the penetration of electrolyte and diffusion of ions.Lastly,strong LiPSs immobilization suppresses the shuttle effect.The DFT theoretical calculations elucidate that the stable chemisorption structure and large adsorption energy restricts the massive diffusion of soluble LiPSs in the electrolyte.Moreover,an increase in the density of states (DOS) at the Fermi level and decrease in the energy barrier facilitates the charge transfer and chemical conversion kinetics.With these advanced features,this Pt/S/OCNF gives the cathode a high sulfur utilization of 77.5%,an excellent rate capacity of 813.2 mAh/g at 2 C,and an outstanding long-cycling performance with the capacitance retention of 82.6% and a decay of 0.086% per cycle after 200 cycles at 0.5 C.
Scheme 1 gives a conceptual illustration for preparing oxygen (O) and nitrogen (N) tailoring carbon fiber aerogel (OCNF)by one-step solid-state pyrolysis (OSSP) chemistry,where a cotton natural biomass fiber works as a building block for carbon fiber aerogel.The O and N tailoring is realized by spatially grafting the oxygenated carbon nitride (OCN) nanosheets on the carbon fiber during the OSSP process.Pyrolysis of urea and glucose produced OCN nanosheets in-situ growing on the cotton fibers,which were converted into O and N tailoring carbon fiber aerogel under the pyrolysis atmosphere.Ammonium chloride (NH4Cl) plays a role in regulating the pore structure and the alignment of OCN on carbon fiber.Then,four OCNF model materials,named OCNF,CCF-1000,CCF-1100 and CCF-1200 were obtained.The detailed preparation process of the materials is shown in the Experimental Section in Supporting information.
The solid sulfur powders were directly deposited on the OCNF host by one-step thermal melt-diffusion method,where the obtained OCNF/S composite was used to fabricate the sulfur cathode.Subsequently,the Pt nanoparticles were uniformly coated onto the sulfur cathode by ion sputtering.Therefore,an electrocatalysis interface composed of Pt nanoparticles was formed on the surface of sulfur cathode (Pt/S/OCNF),which will realize the efficient conversion of active sulfur,the significant inhibition of LiPSs shuttling and excellent long-cycling stability.
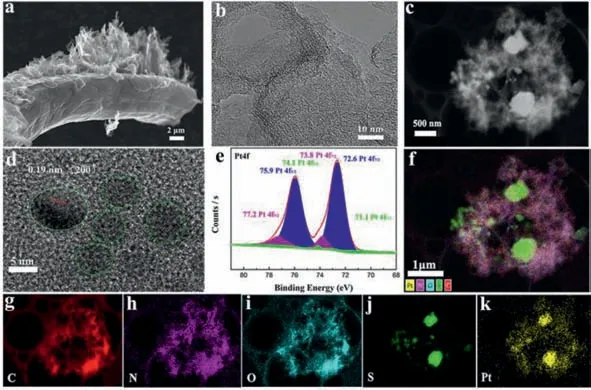
Fig.1.Morphology and structure of OCNF and Pt nanoparticle interfaced sulfur cathode (Pt/S/OCNF).(a) SEM and (b) TEM image for OCNF.(c) SEM image for sulfur cathode.(d) HRTEM image for Pt nanoparticles.(e) High-resolution Pt4f XPS spectrum.(f) Elementary mapping for merging Pt,N,O,S and C.Elementary mappings of (g) C,(h) N,(i)O,(j) S and (k) Pt.Scale bar: 1 μm.
Representatively,the OCNF exhibits a 3D carbon fiber aerogel structure (Fig.S1c in Supporting information) similar to the raw cotton precursor (Fig.S1a in Supporting information).The scanning electron microscopy (SEM) images show that the OCNF exhibits a disordered interlaced network structure with a large amount of graphene-like OCN grafted on the smooth surface of carbon fiber(Fig.1a and Fig.S1f in Supporting information).Transmission electron microscopy (TEM) images along with STEM images clearly exhibit thein-situgrowth of OCN with a typical folded wrinkled 2D lamellar morphology (Fig.1b and Fig.S2a in Supporting information).The edges of these folds would form a considerable channel and pore structure,providing a high specific surface area.Therefore,from the N2adsorption/desorption curves (Fig.S4 in Supporting information) and pore volume analysis (Table S1 in Supporting information),the typical type I isothermal curves indicate the characteristic microporous/mesoporous structure for OCNF.Moreover,the thermal decomposition of the precursor ammonium chloride (NH4Cl) working as gaseous pore-making agent makes the OCNF with a large specific surface area of 1305.2 m2/g and pore volume of 0.71 cm3/g,especially with a large micropore volume of 0.47 cm3/g.Correspondingly,the high nitrogen (4.6%)and oxygen content (6.88%) calculated from XPS (Table S3 in Supporting information) also demonstrates the critical role of ammonium chloride.
We then investigated the deposition and localization of platinum (Pt) nanoparticles obtained by ion sputtering,and the corresponding chemical local micro-environment and valence state (Fig.1).The diameter of Pt nanoparticle about 5–7 nm withd-spacing of 0.19 nm was detected by high-resolution TEM,consistent with the (200) plane lattice of high crystalline Pt (Fig.1d).As shown in Fig.1e,the chemical state of Pt nanoparticles was confirmed by XPS spectra.The high-resolution Pt4fXPS spectrum shows three chemical valence states,where the predominant bond energies of 72.6 eV and 75.9 eV are assigned to the Pt2+4f7/2and Pt2+4f5/2.The STEM image (Fig.1c) and elementary mappings(Fig.1f) clearly show the localization of Pt nanoparticles and the distribution of nitrogen (N),oxygen (O) and carbon (C) elements(Figs.1g-i).The uniform distribution of Pt with C,N and O elements was observed intuitively.Moreover,the Pt on the surface of sulfur (S) particles is obviously observable,demonstrating the directly intimate contact between S and Pt,and laying the foundation for the Li/S chemical conversion by Pt electrocatalysis(Figs.1j and k).
Electrochemical performances of the as-designed 3D OCNF sulfur cathode (Fig.2) and Pt nanoparticle interfaced sulfur cathode(Pt/S/OCNF,denoted as OCNF-Pt),were comprehensively investigated to confirm the advantages of Pt-activated interfacial electrocatalysis in enhancing the Li/S chemical conversion kinetics.Firstly,cyclic voltammetry (CV) within the voltage range of 1.6–2.8 V at a scan rate of 0.1 mV/s was employed to explain the Li/S electrochemical transformation.Fig.2a shows the CV curves of OCNF-Pt and OCNF cathodes,presenting the typical oxidation and reduction peaks of Li/S cell.In the reduction process,the first reduction peak(C1 at 2.29 V,OCNF cathode) represents the initial transformation of solid cyclic S8into soluble long-chain Li2S8and Li2S6in the liquid electrolyte,and the long-chain Li2S6is further transformed into short-chain Li2S4,delivering 25% of total capacity (1675 mAh/g).Meanwhile,the second reduction peak (C2 at 1.97 V) indicated that soluble Li2S4was finally converted into solid Li2S2and Li2S,releasing a high theoretical specific capacity of 1275 mAh/g.Conversely,in the oxidation process,the first oxidation peak (A1) at 2.40 V means that solid Li2S2/Li2S are converted into soluble Li2S4,then into long-chain soluble Li2S6and Li2S8at another oxidation reaction (A2 at 2.45 V),and finally into solid cyclo-S8.This electrochemical process involves the breaking and bonding of long/short chain S-S bonds and the phase transformation between solid-state and liquid-state.
From the oxidation–reduction curves of OCNF and OCNF-Pt cathodes,we found that the Pt nanoparticles with electrocatalysis significantly reduced the overpotential of sulfur chemical conversion with a sharp increase in the reduction current.OCNFPt exhibits a reduced overpotential of 49.5 mV (C2),12.5 mV (C1)and 39.5 mV (A1),compared to that for OCNF respectively.Meanwhile,the reduction current (C2) increases significantly in the process of transforming soluble Li2S4into solid Li2S2and Li2S,demonstrating outstanding chemical conversion kinetics.Noteworthy,mediated by Pt electrocatalysis interface,the oxidation current (A1) of the OCNF-Pt cathode increases significantly.And,the value of A1 higher than that of A2 indicates the conversion from Li2S to Li2S4with excellent electrocatalytic kinetics,which solves the aggregation of Li2S on the cathode surface to reduce the occurrence of "dead sulfur" and realize the efficient application of short-chain LiPSs.At the same time,after 20 stabilization cycles,the lowest oxidation and reduction overpotential shift (8.17 mV for C2,24.9 mV for A1) further indicates the importance of Pt interfacial catalysis.Furthermore,the catalytic conversion of Pt electrocatalysis interface for polysulfides redox reactions can be further investigated by the symmetrical cells assembled by OCNFPt and 0.1 mol/L Li2S6at the fixed voltage of -1~1 V (Fig.2b).The CV with OCNF-Pt cathode reveals a remarkable redox symmetry pair,which is located at 0.75 V and -0.75 V,demonstrating facile Li2S6conversion reaction in comparison to that of OCNF cathode.
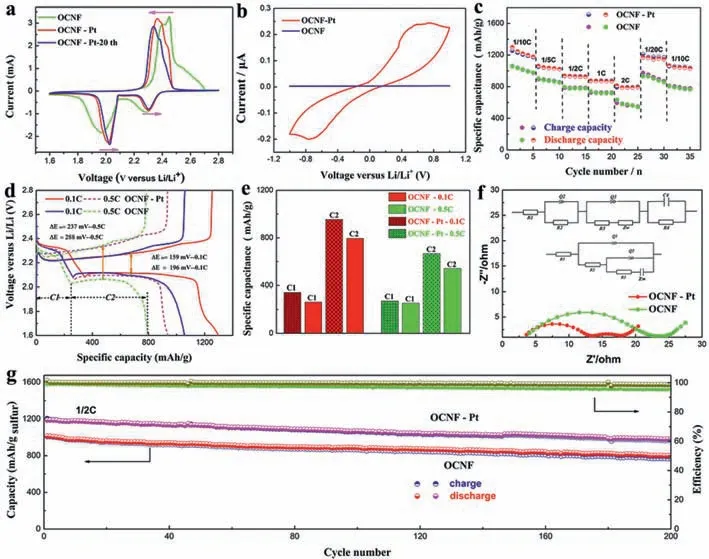
Fig.2.Electrochemical performances of OCNF and Pt nanoparticles interfaced Li/S cell.(a) CV curves,0.1 mV/s.(b) CV curves of symmetrical cells with OCNF or OCNF-Pt cathodes.(c) Rate capability.(d) Galvanostatic charge-discharge profiles.(e) Comparison of discharge plateaus specific capacitance at 0.1 C and 0.5 C.(f) Electrochemical impedance spectroscopy (EIS) and its equivalent circuit diagrams.(g) Cycling performances.
The electrocatalysis at the Pt interface promotes the LiPSs chemical conversion,endowing the OCNF-Pt cathode with excellent rate performances at high current density.As displayed in Fig.2c,the OCNF-Pt cathode releases a high discharge capacity of 1298.2 mAh/g with an average coulombic efficiency (C.E.) of 99.1%at 0.1 C,exhibiting an effective utilization of 77.5%,which is 1.2 and 1.3 times higher than OCNF (C.E.97.9%) and CCF-1100 (C.E.97.6%),1.1 and 1.3 times higher than CCF-1200 (C.E.98.6%) and CCF-1000(C.E.96.5%) (Fig.S11b in Supporting information),respectively.As the rate current increase to 1 C,the capacity remains 874.3 mAh/g(C.E.99.7%).A capacity of 813.2 mAh/g still maintains at a high rate of 2 C (C.E.99.9%),1.36 times bigger than that of OCNF (C.E.97.9%) and 1.70 times CCF-1100 (C.E.97.4%).After 5 cycles at 2 C,the capacity recovers to 1171.8 mAh/g at 0.1 C,90.26% of the initial capacity.Accordingly,the galvanostatic charge/discharge curves at 0.1 C and 0.5 C were further investigated to elucidate the modulation of Pt electrocatalytic interface on the activation overpotential(ΔE) and discharge capacity at different stages (C1 and C2).As shown in Fig.2d,the OCNF-Pt cathode exhibits a relatively lower ΔEof 159 mV at 0.1 C and 237 mV at 0.5 C than that of OCNF with 196 mV at 0.1 C and 288 mV at 0.5 C.Correspondingly,the two typical discharge plateaus increase by 81.2 mAh/g and 160 mAh/g at 0.1 C,and by 17.7 mAh/g and 125.3 mAh/g at 0.5 C in contrast to that of OCNF (Fig.2e).And the C2/C1 ratio of OCNF-Pt cathode at 0.5 C is 2.4,which is larger than that of OCNF (2.1),demonstrating outstanding Li/S conversion kinetics and high sulfur utilization.Hence,the excellent Pt electrocatalytic interface makes Pt/S/OCNF a reducing electrochemical overpotential and improving coulombic efficiency and specific capacity,correlated to the fast Li/S chemical conversion kinetics.
The electrochemical impedance (EIS) and its corresponding equivalent circuit diagrams were used to investigate the effects of Pt electrocatalysis interface on the charge transfer impedance and ion diffusion resistance.As shown in Fig.2f,the OCNF-Pt cathode exhibits a lower charge transfer impedanceRctof 9.7Ωand internal electrical resistanceR1of 3.6Ωthan those of OCNF (Rctof 19.8ΩandR1of 4.0Ω) at high frequency,demonstrating a faster charge transfer and sulfur conversion.The charge transfer impedanceRct’of 4.3Ωappears at the intermediate frequency,indicating a new interface,which is attributed to the redox reactions between Pt and LiPSs on the cathode surface.
Additionally,the cycling performance was examined at 0.5 C to evaluate the improvement of chemical conversion kinetics and suppression of shuttle effect by Pt electrocatalysis interface.As shown in Fig.2g,OCNF-Pt cathode presents a high capacitance retention of 82.6%,decaying from the initial specific capacity of 1188.1 mAh/g to 982.3 mAh/g after 200 cycles at 0.5 C with an average coulomb efficiency of 99.4% and a decay of 0.086%per cycle,in comparison to the OCNF with capacitance retention of 76.1% and average coulomb efficiency of 97.5%,and CCF-1000 (79.3%,97.7%),CCF-1100 (64.6%,98.7%) and CCF-1200 (69.4%,96.4%) (Figs.S11d-f in Supporting information).The increase in discharge capacity,coulomb efficiency and capacitance retention confirms the contribution of Pt electrocatalysis interface to suppressing the LiPSs shuttling.The Pt electrocatalysis interface improves the electrochemical performances of the cathode in sulfur electrochemical conversion kinetics,ion diffusion and electron transfer kinetics.This results prove the key roles of Pt/S/OCNF in effectively suppressing the shuttle effect and "dead sulfur" to realize highefficiency sulfur utilization,and further verify the importance of this cathode design.
We further investigated the mechanism of interfacial Pt nanoparticle in electrocatalytically mediating the Li/S conversion chemistry and in inhibiting LiPSs shuttling.Firstly,a visualized adsorption experiment and corresponding UV–vis absorption spectroscopy provide visual evidence to verify the adsorption of soluble LiPSs by OCNF.In the four groups of simulated electrolyte Li2S6solution (6.0 mL,10.0 mmol/L),15 mg of OCNFs and control(CCF-1000,CCF-1100 and CCF-1200) were added respectively.After 15 min,the solution with OCNF gradually became colorless and transparent,but the others showed a slight change (Fig.3a).The disappearance of corresponding wide absorption from 400 nm to 600 nm in the visible spectra further demonstrates the strong adsorption of OCNF towards soluble LiPSs (Fig.3b).The electronic structure calculations were applied to elaborate the excellent electrical conductivity of the Pt interface.The results indicate that the Pt electrocatalysis interface allows the OCNF-Pt cathode to exhibit larger total density of state (DOS) at Fermi level compared to the one of OCNF (Figs.3c and d),giving the OCNF-Pt with capability of high electrical conductivity and fast electron transfer.Then,density functional theory (DFT) calculations were used to understand the mechanism for the Li/S conversion chemistry and chemisorption for LiPSs.The optimized bond structure diagram of the OCNFPtcluster-PS chemisorption conformation demonstrates that the interfacial Pt nanoparticles bridge between Li2Sn(n=1,2,4,6,8 and S8) and OCNF to form a stable chemisorption structure (Fig.S12 in Supporting information).The pyridine nitrogen atom in OCNF as the active site binds to Li atom in Li2Sn(n=1,2,4,6,8,without S8) to form another adsorption structure of OCNF-PS(Fig.S13 in Supporting information).The adsorption energy data shows that OCNF-Ptclusterexhibits higher adsorption energy than OCNF during the first discharge plateau stage from solid-phase S8to liquid-phase Li2S6,which were -3.64 eV (S8),-4.11 eV (Li2S8)and -3.85 eV (Li2S6) respectively.And the increasing trend of adsorption energy is also reflected in the transformation process from soluble Li2S4to insoluble Li2S/Li2S2.During the charging transformation process from Li2S to Li2S4,the adsorption energy reveals an upward trend along with the propagation of S-S bond,by 0.45 eV increase from Li2S to Li2S2and 0.55 eV from Li2S2to Li2S4,respectively.These results indicate that the Pt electrocatalysis interface has a more favorable bonding interaction and chemical anchoring,which restrains the diffusion of soluble Li2S8,Li2S6and Li2S4in the electrolyte.On the other hand,the gradual accumulation of adsorption energy during the oxidation of short-chain Li2S to long-chain Li2S4promotes the solid-liquid conversion kinetics,enhancing the kinetics of insoluble Li2S being oxidized and reducing the aggregation of "dead sulfur" on the cathode surface.
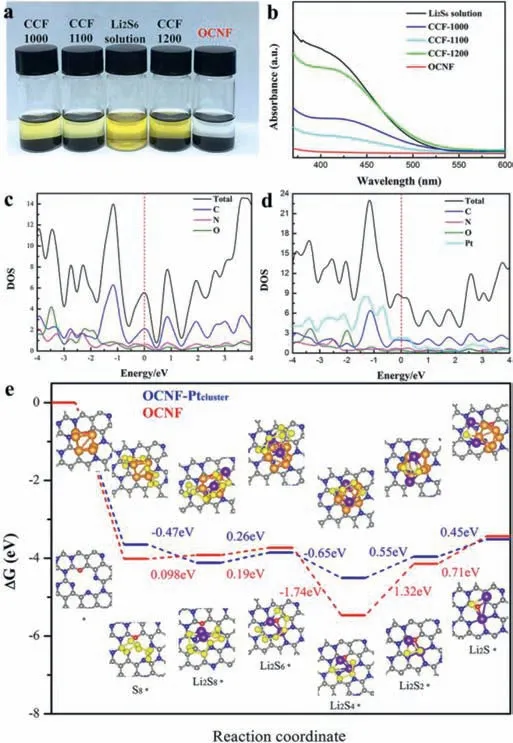
Fig.3.Mechanism understanding of OCNF and Pt nanoparticles interfaced Li/S conversion chemistry.(a) Optical photographs for adsorption of sulfur hosts towards Li2S6.(b) UV–vis spectra of Li2S6 solution after adsorption.Density of states (DOS)for (c) OCNF and (d) OCNF coupled Pt cluster.(e) Gibbs energy profiles of Li/S conversion chemistry.
Besides,the S-reduction pathways of OCNF-Ptcluster(blue line)and OCNF (red line) cathodes were investigated to reveal the improvement of the Pt electrocatalytic interface in the thermodynamics and kinetics of LiPSs conversion.As shown in Fig.3e,during the reduction process from Li2S4to Li2S2,the maximum endothermic Gibbs free energy is 1.32 eV (OCNF) and 0.55 eV (OCNF-Ptcluster),respectively,indicating the rate-limiting step in the discharge process.In addition,the whole process of the second plateau discharge from Li2S4to Li2S2shows a significant endothermic,and the Gibbs free energy of the OCNF-Ptcluster(0.55 eV and 0.45 eV) is obviously lower than that of the OCNF (1.32 eV and 0.71 eV),indicating the low energy barrier to obtain fast redox and chemical conversion kinetics.Therefore,the reduction of S on an OCNF cathode with Pt electrocatalytic interface presents more thermodynamic advantages.
Traditionally,the effective utilization of sulfur is attributed to direct contact of the electrolyte with sulfur.However,the diffi-culty in achieving complete contact between electrolyte and sulfur mainly includes the limited diffusion and penetration of electrolyte into the interior of the cathode,and the massive layer of insoluble Li2S on the cathode surface shielding the contact of electrolyte.Therefore,it is critical to understand the manner and capability of the electrolyte to diffuse and penetrate into the interior of the cathode.As shown in Fig.4a,3D AFM pattern of the OCNFPt cathode surface was realistically restored,exhibiting a bumpy and rugged shape similar to a ridge.The diffusion and penetration of the electrolyte on the cathode surface were observed visually by contact angle test.The first drop of electrolyte was observed to achieve rapid diffusion or penetration into the cathode (Fig.4b).However,the subsequent third drop gives the surface obvious saturation (Fig.S14 in Supporting information).A schematic diagram shows the mechanism of the OCNF-Pt cathode,where the Pt electrocatalytic interface achieves the rapid Li/S chemical conversion kinetics and fast transformation of solid-phase S8to soluble LiPSs to generate ionic channels,and the 3D OCNF inhibits the massive aggregation of insoluble Li2S on the cathode and accelerates the effective penetration of electrolyte into the cathode interior(Fig.4c).
To further explore the permeability of the electrolyte into the interior of the OCNF-Pt cathode,XPS argon etching technique was used to analyze the changes of high-resolution C 1s and S 2p spectrum of OCNF-Pt cathode (after 200 cycles) at different etching times (depths).From the high-resolution C 1s spectrum,the peak area of C-F3(293.1 eV) originating from the electrolyte is gradually decreasing,with C-F3/C=C changing from the initial 32.1% to 3.1%after 12 min etching (Figs.4d-f and Fig.S21a in Supporting information).From the high-resolution S 2p spectrum,the peak area of the S-S bond originating from the S8molecule presents an increasing tendency,while the one of the sulfate produced by the redox process gives a weakening trend (Figs.4g-i).The ratio of SS/sulfate increases from 24.2% to 123.3% after 12 min etching (Fig.S21b in Supporting information).These results provide direct evidence for the diffusion and penetration resistance of electrolytes into the interior of the sulfur cathode,denoting the significance of ionic channels in sulfur cathode to promote the Li/S chemical conversion and to maintain the long-cycling capacity retention.It is noteworthy that no significant signal of Li2S/Li2S2was detected on the surface and inside of the OCNF-Pt cathode after charging.
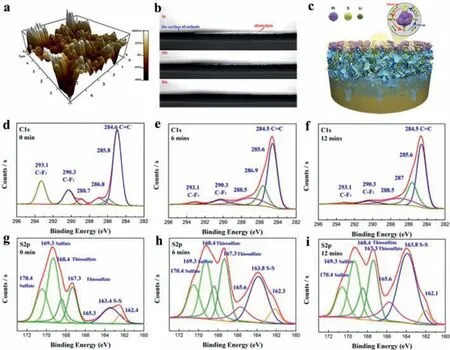
Fig.4.Mechanism of electrolyte diffusion and penetration on sulfur cathode.(a) 3D AFM simulation diagram of OCNF-Pt cathode surface.(b) Contact angle for electrolyte diffusion and penetration.(c) Schematic diagram of the mechanism of Pt nanoparticles interfaced sulfur cathode in the redox process.High resolution C 1s and S 2p spectra etched at various times: (d) C 1s and (g) S 2p without etching; (e) C 1s and (h) S 2p at 6 min (min); (f) C 1s and (i) S 2p at 12 min.
In summary,we have developed a cathode structure design to regulate Li/S conversion chemistry and ion channel.The Pt electrocatalysis interface combined with the unique 3D hierarchical porous structure and abundant functional active sites at OCNF guarantees strong adsorption confinement,fast Li/S conversion and unblocked ion channels for electrolyte permeation in the cathode.The exploring of sulfur cathode with electrocatalysis interface and ion channel for feasible electrolyte diffusion and Li/S transformation kinetics provides an efficient pathway towards high-capacity,high-rate and long-cycling lifetime Li/S batteries.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
We thank funding support from National Key R&D Program of China (No.2016YFB0100100),The National Natural Science Foundation of China (Nos.21961024,21961025,21433013,U1832218),Inner Mongolia Natural Science Foundation (No.2018JQ05).Supported by Incentive Funding from Nano Innovation Institute (NII)of Inner Mongolia University for Nationalities (IMUN).Inner Mongolia Autonomous Region Funding Project for Science & Technology Achievement Transformation (No.CGZH2018156).Inner Mongolia Autonomous Region Incentive Funding Guided Project for Science& Technology Innovation (2016).Inner Mongolia Autonomous Region Science & Technology Planning Project for Applied Technology Research and Development (No.2019GG261).Tongliao Funding Project for Application Technology Research & Development (2017).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.016.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
